Abstract
Genes which provide resistance to novel challenges such as pesticides, toxins or pathogens often impose fitness costs on individuals with a resistant phenotype. Studies of resistance to Bacillus thuringiensis and its insecticidal Cry toxins indicate that fitness costs may be variable and cryptic. Using two field populations (Karak and Serd4) of the diamondback moth, Plutella xylostella, we tested the hypothesis that the costs associated with resistance to the B. thuringiensis toxin Cry1Ac would be evident when insects were grown under poor environmental conditions, namely limited or poor quality resources. On a poor quality resource, a cultivar of Brassica oleracea var. capitata with varietal resistance to P. xylostella, only one resistant population, Karak, showed reduced fitness. Conversely, when we limited a high quality resource, Brassica pekinensis, by imposing larval competition, only resistant Serd4 insects had reduced survival at high larval densities. Furthermore, Cry1Ac resistance in Serd4 insects declined when reared at high larval densities while resistance at low densities fluctuated but did not decline significantly. These results confirm the hypothesis that resistance costs can appear under stressful conditions and demonstrate that the fitness cost of resistance to Bacillus thuringiensis can depend on the particular interaction between genes and the environment.
Keywords: biopesticide, crystal toxin, gene X environment interaction, Plutella xylostella, resistance management
1. Introduction
Bacillus thuringiensis (Bt) is a spore-forming bacterium that produces proteinaceous toxins with narrow host activity against members of several insect orders. It is widely sprayed as a microbial pesticide and the genes encoding crystal toxins have now been engineered into insect-resistant genetically modified crops, including commercially grown cotton and maize (Shelton et al. 2002). A continuing threat to the sustainability of these applications is the evolution of resistance to Bt toxins. Many insect species have evolved resistance to Bt strains or specific toxins in the laboratory although only two species, one of which is the diamondback moth, Plutella xylostella, have evolved substantial resistance to Bt sprays outside the laboratory (Tabashnik et al. 1997; Ferré & Van Rie 2002; Janmaat & Myers 2003).
Studies of the impact of pesticide resistance genes on individuals have provided classic case-studies in ecological genetics as well as informing pesticide resistance management strategies (Gould 1998; Coustau et al. 2000). Selection for any novel trait can produce correlated changes with other characters, the major cause of which is pleiotropy, i.e. the ability of a single gene to affect more than one trait. Selection for resistance to pesticides, at least initially, generally produces genetic correlations that result in a reduction in relative fitness in the absence of the pesticide (Gould 1998; Coustau et al. 2000; Bourguet et al. 2004). The antagonistic trade-off in fitness caused by the presence of resistance genes will be referred to as a ‘fitness cost’ for brevity. Evidence for these fitness costs is, in part, provided by the decline or instability of resistance in the laboratory in the absence of selection (Ferré & Van Rie 2002; Bourguet et al. 2004). However, stable and high levels of resistance to Bt products or toxins in culture have been observed in some cases (McGaughey & Beeman 1988; Liu et al. 1996; Tang et al. 1997; Sayyed et al. 2004). It is unknown whether differences in environmental or culture conditions between laboratories can, in part, explain this variation (Cerda et al. 2003). Alternatively, resistance costs may be cryptic, i.e. laboratory measures of performance are indistinguishable between resistant and susceptible insects but the instability of resistance implies the presence of an unknown fitness cost (Sayyed & Wright 2001b).
In general, costs of adaptations to overcome challenges such as parasitoids, bacterial toxins or pesticides may be detectable under stressful conditions (Kraaijeveld & Godfray 1997; Carrière et al. 2001; Gazave et al. 2001; Bourguet et al. 2004) and may explain some of the variability in the strength of the fitness costs observed hitherto. In the present study, two strains of P. xylostella, one of which (Karak) has shown a high stability of resistance in culture (Sayyed et al. 2004) and a second strain (Serd4) that has shown cryptic costs of resistance (Sayyed & Wright 2001b), were investigated to determine whether fitness costs imposed by resistance genes would be detected under stressful conditions. These conditions were either poor quality food resources or resource limitation imposed by high larval competition. Following results of initial competition experiments we determined, for one strain, how differing levels of larval competition would affect the stability of resistance in the laboratory.
2. Methods
(a) Insects and selection protocols
Two populations of P. xylostella from Malaysia, Serd4 and Karak, with high levels of field-evolved resistance to B. t. kurstaki (Btk) and one of its constituent toxins, Cry1Ac were used in this study (Sayyed & Wright 2001a; Sayyed et al. 2004). These populations have distinct and independently evolved mechanisms of resistance to Cry1Ac (Sayyed et al. 2001; Sayyed & Wright 2001a). The Serd4 population had been in culture for at least 80 generations, the Karak population for 30 generations. Resistance was maintained by repeated selection every 2–3 generations with leaf discs dipped in Cry1Ac solution (20–50 μg ml−1), although insects were not exposed to toxin in the generation immediately prior to experiments. The population size of selected lines did not fall below 75. Revertant Cry1Ac susceptible lines of Karak and Serd4 were produced by maintaining a subpopulation of each culture in the absence of Btk or toxin on Chinese cabbage, Brassica pekinensis var. ‘One Kilo S.B.’, for more than one year. Prior to experiments resistance levels to Cry1Ac were checked using standard leaf-dip bioassays (Sayyed et al. 2000).
Cry1Ac toxin was produced in recombinant Escerichia coli (strain ECE 53, Bacillus Genetic Stock Centre, Dept of Biochemistry, Ohio State University). Bacteria were grown in LB medium supplemented with 10 μg ml−1 of ampicillin at 30 °C with continuous shaking for 2–3 days. Toxin crystals were recovered by centrifugation at 9700 g for 20 min at 4 °C. The pellet was re-suspended in ice-cold 1 M NaCl–5 mM EDTA and sonicated for 30 s to break the crystals. The crystals were washed twice in 1 M NaCl–5 mM EDTA and then twice in sterile distilled water by resuspension and centrifugation. The final pellet was dissolved in carbonate buffer (10 mM Na2CO3, pH 9.5) and kept at −20 °C.
(b) Performance experiment
Poor quality food resources were produced by growing Brassica oleracea var. captitata cv. ‘Wheeler's Imperial’ in 12 cm diameter pots for more than 140 days in an unheated greenhouse. Plants were fed with an NPK fertilizer (15:30:15%, 0.9 g l−1) every two weeks in the last month of growth. This cabbage variety has partial resistance to attack by P. xylostella; this resistance increases substantially in plants older than 100 days (Verkerk & Wright 1994). All plants had thick waxy leaves at the end of their growth period and were randomly allocated to the different population treatments (n=8 per subpopulation). Forty newly-emerged, active neonates were transferred to the central four leaves of each plant using a fine brush; plants were then bagged and maintained in a controlled-environment culture room (25 °C, 50–60% R.H., 16 h photophase). This experiment was set up over 2 days. Six days after insects had been feeding, larvae were recovered from plants and the total mass and number of larvae alive recorded. At this time no insects had pupated and the fastest growing larvae had reached mid-late fourth instar. After 7 days plants were checked daily and insects that had formed pupae were removed and weighed. The means of data from each plant were used in analyses to avoid pseudo-replication.
(c) Competition experiment
We tested whether increased costs of resistance would become evident under more intensive levels of larval competition by rearing P. xylostella larvae from early third instar until pupation on 4.5 cm diam. leaf discs (cut from 5-week-old Chinese cabbage plants) within 5 cm diam. Petri dishes. Three treatment densities of 1, 3 and 7 larvae per dish were used for each subpopulation. Insects were kept at 25 °C, 50–60% R.H., 16 h photophase. Leaf discs were not changed during the course of the experiments. Experiments for Karak and Serd4 were run separately. For Karak, the sample sizes of susceptible and resistant subpopulations had minima of 52, 69 and 217 larvae at treatment densities of 1, 3 and 7 larvae per dish, respectively; for Serd4, sample sizes per subpopulation were 90, 120 and 210 larvae, respectively. The survival of larvae until pupation was analysed using the means (total surviving/total number of larvae) for each treatment. Larvae were weighed from a random subset of Petri dishes 1, 3 and 7 days after setting up trials. Relative growth rate ((ln(final mass/initial mass))/no. of days, with mass in mg) was calculated for each Petri dish from day 1 until the time point at which the maximum mass was reached (hereafter referred to as RGRmax). The fecundity of survivors was measured by selecting the first emerging female from each Petri dish and crossing her with a randomly selected single male from within that treatment. Adults were maintained in 500 ml glass jars without a sugar source. Females laid eggs on Parafilm dipped in cabbage extract, which was changed every 2 days until females died.
(d) Stability experiment
Our final experiment investigated the stability of Cry1Ac resistance in the Serd4 population at two larval densities. To minimize the potentially confounding effects of genetic background the Serd4 susceptible sub-population used in the previous two experiments was selected for resistance to Cry1Ac over three generations. This reselected strain was then crossed with the original revertant subpopulation and the F1 adult progeny of this mass cross mixed at a 1:1 ratio with re-selected adults to make up our initial (G0) population in order to guarantee some heterogeneity at resistance loci. Replicate lines were started with 25 G0 pupae and four lines were randomly allocated to each density treatment. Each replicate was reared in an individual cage at 25 °C, 85% R.H. and 16 h photophase. In the low density treatment, competition was minimized by transferring 25 second instar larvae to each of four plants from initial oviposition plants on which adults had laid eggs for 48 h. In the high density treatment, adults were allowed to oviposit freely on two plants for 4 days. These plants were then transferred to rearing cages. When the insects had consumed nearly the whole of the two oviposition plants two additional plants were added to each cage. Larval density in this treatment was in excess of 250 larvae per plant at third instar. Bioassays took place at G0, G1, G3, G4 and G5 using third instar larvae from oviposition plants. Assays used a minimum of four doses plus surfactant only controls with 30 insects per doses, final assays at G6 used five doses.
Statistical analysis was carried out in R (http://www.r-project.org) using analysis of variance and general linear modelling. When binomial errors were used in the analysis of proportional data F-tests were applied to correct for moderate over-dispersion when appropriate. The stability experiment was analysed using a mixed effect model that accounted for repeated measures. Log-transformed dose and density treatment were fixed effects; cage and generation were treated as random effects and arc-sine transformed bioassay data used as the response variable. Confidence intervals for LC50s in the stability experiment were calculated using Fieller's theorem and binomial errors in GLIM 3.77 after fitting minimal adequate models individually to each treatment in each generation; cage and dose were used as factors in the maximal model (Crawley 1993). The assumptions of all statistical models were checked using standard techniques.
3. Results
(a) Relative resistance of resistant and revertant susceptible sub-populations
All Karak and Serd4 sub-populations were assayed in June and September 2003 prior to performance and competition experiments, respectively. There was a twenty-two fold difference in susceptibility to Cry1Ac in the resistant and susceptible Karak sub-populations (F1,18=20.1, p<0.001), the LC50 of the resistant and susceptible Karak sub-populations being 62.8 and 2.9 μg ml−1, respectively. Date of assay did not interact with sub-population (F1,15=2.16, p>0.05) nor affect mean mortality (F1,17=0.27, p>0.05). Similarly Serd4 was assayed in June, September and in October prior to the stability experiment and there was a forty-two fold difference in susceptibility to Cry1Ac between resistant (or reselected) and the revertant susceptible sub-population (F1,35=75.2, p≪0.0001), the LC50s being 39.2 and 0.94 μg ml−1, respectively. Date of assay did not significantly affect results (date * sub-population interaction F2,29=1.03, p>0.05; main effect F1,32=1.21, p>0.05).
(b) Performance experiment
Differences in performance between resistant and susceptible subpopulations cultured on poor quality ‘Wheeler's Imperial’ cabbage plants were only evident for the Karak population (figure 1a,b). Thus, there was a significant interaction between population and resistance for larval growth rate (F1,29=6.35, p<0.05) and for survival till pupation (F1,28=23.6, p≪0.0001). There was also a significant effect on survival of day on which replicates were set up (F1,28=9.00, p<0.01). Since larvae did not successfully pupate on several plants there were fewer pupal weight data available. However, mean pupal weights were indistinguishable to two significant figures (4.4 mg) for all four subpopulations.
Figure 1.
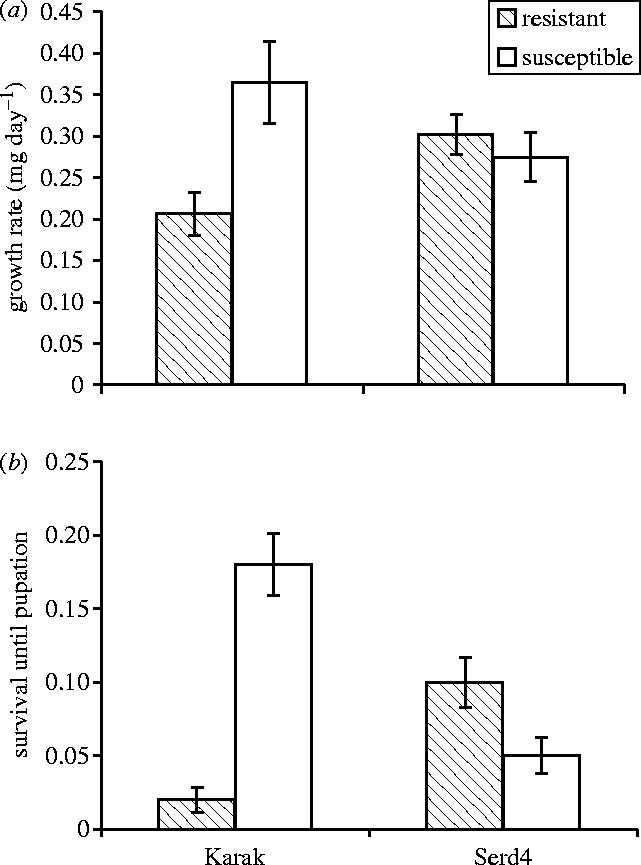
Fitness parameters of P. xylostella larvae on Brassica oleracea var. capitata (Serd4 and Karak strains) resistant and susceptible to the Bt toxin Cry1Ac (n=8 plants per treatment, data are means±s.e.). There was a significant interaction between population and resistance treatments for both parameters: (a) larval growth rate; (b) survival until pupation.
(c) Competition experiment
For the Karak population survival decreased with increasing larval densities but was unaffected by resistance to Cry1Ac (table 1, figure 2). Only the resistant Serd4 population showed a fitness cost, in terms of reduced survival, as larval density increased (table 2, figure 3). The number of eggs produced by surviving females was unaffected by Cry 1Ac resistance and varied significantly only with density for both the Karak and Serd4 populations (tables 1–3). RGRmax did not significantly vary with either density or Cry 1Ac resistance for either Karak or Serd4) (tables 1–3).
Table 1.
Summary of statistical analyses for performance parameters for competition experiment using the Karak population.
| parameter | source of variation | SS | d.f. | MS | F-ratio |
|---|---|---|---|---|---|
| RGRmax | density | 0.0095 | 1 | 0.0095 | 0.89 NS |
| resistance | 0.0168 | 1 | 0.01678 | 1.57 NS | |
| resistance×density | 0.0001 | 1 | 0.0001 | 0.005 NS | |
| residuals | 1.172 | 110 | 0.0107 | ||
| fecundity | density | 15524 | 1 | 15524 | 16.18*** |
| resistance | 1791 | 1 | 1791 | 1.87 NS | |
| resistance×density | 1564 | 1 | 1564 | 1.63 NS | |
| residuals | 92084 | 96 | 959 |
| d.f. | deviance | resid. d.f. | residual deviance | p (χ2) | ||
|---|---|---|---|---|---|---|
| survival | null model | 5 | 85.6 | |||
| density | 1 | 79.97 | 4 | 5.59 | **** | |
| resistance | 1 | 2.06 | 3 | 3.53 | NS | |
| resistance×density | 1 | 0.15 | 2 | 3.37 | NS |
In the analysis of deviance, significance testing is carried out by using the change in deviance as a χ2 value. ***p<0.001.
Figure 2.
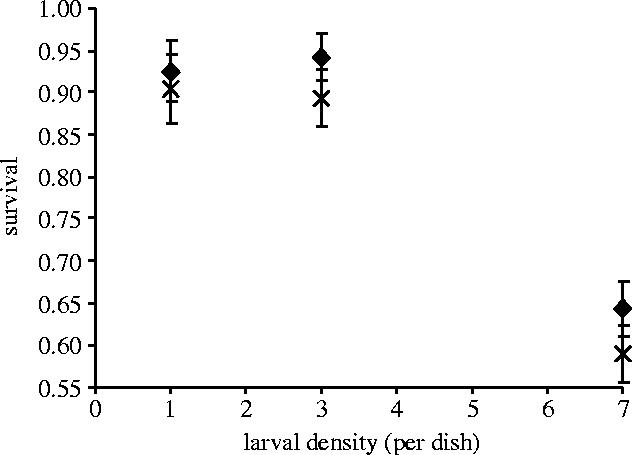
Effect of rearing density and resistance to the Bt toxin Cry1Ac on the survival of P. xylostella larvae from the Karak population in a competition experiment (data are means±s.e.). Filled diamonds represent Cry1Ac resistant subpopulation and crosses represent Cry1Ac susceptible revertant subpopulation.
Table 2.
Summary of statistical analyses for performance parameters for competition experiment using the Serd4 population.
| parameter | source of variation | SS | d.f. | MS | F-ratio |
|---|---|---|---|---|---|
| RGRmax | density | 0.0026 | 1 | 0.29 | 0.29 NS |
| resistance | 0.0010 | 1 | 0.0010 | 0.11 NS | |
| resistance×density | 0.024 | 1 | 0.024 | 2.76 NS | |
| residuals | 1.02 | 115 | 0.0089 | ||
| fecundity | density | 16349 | 1 | 16349 | 15.14*** |
| resistance | 529 | 1 | 529 | 0.49 NS | |
| resistance×density | 599 | 1 | 599 | 0.46 NS | |
| residuals | 145778 | 135 | 1080 |
| d.f. | deviance | resid. d.f. | residual deviance | p (χ2) | ||
|---|---|---|---|---|---|---|
| survival | null model | 5 | 61.13 | |||
| density | 1 | 48.73 | 4 | 12.40 | **** | |
| resistance | 1 | 4.31 | 3 | 8.09 | * | |
| resistance×density | 1 | 6.20 | 2 | 1.88 | * |
In the analysis of deviance, significance testing is carried out by using the change in deviance as a χ2 value. *p<0.05, ***p<0.001.
Figure 3.
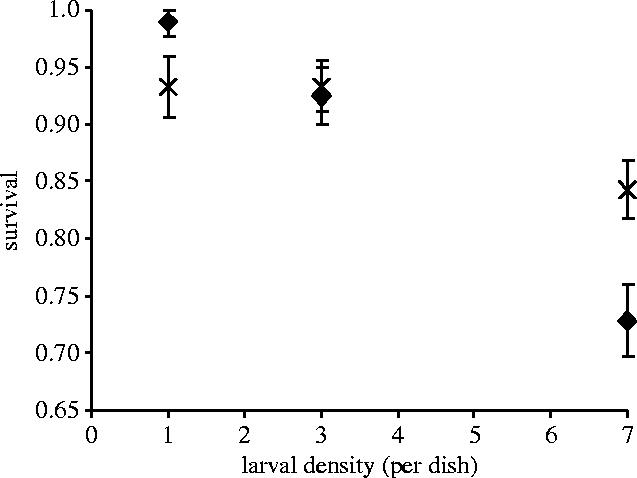
Effect of rearing density and resistance to the Bt toxin Cry1Ac on the survival of P. xylostella larvae from the Serd4 population in a competition experiment (data are means±s.e.). Symbols are as in figure 2 legend.
Table 3.
The fecundity and relative growth rate (RGRmax) of Cry1Ac resistant and susceptible P. xylostella from two populations in larval competition experiments.
| subpopulation | density (larvae per dish) | fecundity | RGRmax mean (s.e.) n=20 | |
|---|---|---|---|---|
| mean (s.e.) | n | |||
| Karak population | ||||
| resistant | 1 | 65.7 (8.5) | 20 | 0.27 (0.02) |
| 3 | 42.1 (7.3) | 18 | 0.33 (0.03) | |
| 7 | 33.4 (6.4) | 19 | 0.30 (0.02) | |
| susceptible | 1 | 57.3 (9.7) | 21 | 0.28 (0.02) |
| 3 | 56.0 (8.5) | 20 | 0.25 (0.02) | |
| 7 | 45.1 (7.2) | 15 | 0.29 (0.02) | |
| Serd4 population | ||||
| resistant | 1 | 63.7 (13.4) | 16 | 0.26 (0.02) |
| 3 | 46.7 (7.3) | 29 | 0.27 (0.01) | |
| 7 | 43.6 (4.6) | 37 | 0.28 (0.03) | |
| susceptible | 1 | 52.7 (10.1) | 21 | 0.31 (0.03) |
| 3 | 53.4 (7.0) | 28 | 0.26 (0.02) | |
| 7 | 38.9 (4.9) | 33 | 0.26 (0.02) | |
(d) Stability experiment
This experiment was analysed in two ways. Firstly, calculation of LC50s and their 95% confidence limits was carried out for each treatment at each time point. Inspection of confidence limits indicated that susceptibility to Cry1Ac was diverging in the two density treatments as the experiment progressed, although there was some variation in mean LC50s between bioassays (figure 4). Secondly, mortality data from generations 3–5 was analysed in a mixed effects ANOVA. Comparison of maximum likelihood models showed that larval density did significantly affect susceptibility to Cry1Ac over that time period (likelihood ratio 5.75; d.f.=2,6; p<0.05). Data collected after one generation of competition could not be included in the above repeated measures ANOVA because of missing values. However, a separate mixed effect ANOVA (likelihood ratio 1.85; d.f.=2,6; p=0.16) and a comparison of the mean and confidence limits of the LC50s indicated that there was no significant effect of treatment at generation 1 (figure 4).
Figure 4.
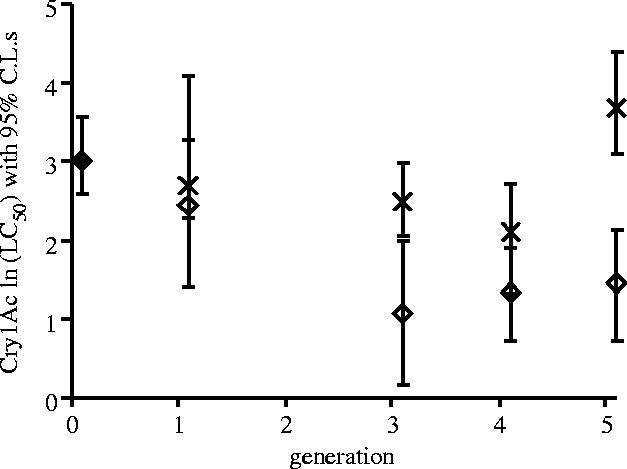
The stability of resistance to the Bt toxin Cry1Ac in the P. xylostella population Serd4 reared at two different densities (n=4 cages per treatment). Data are loge transformed LC50 values with 95% confidence intervals calculated using Fieller's theorem. Filled diamonds represent initial population, crosses represent low density (25 larvae per plant) and open circles high density (>250 larvae per plant).
Furthermore, while bioassays of the low density treatment were variable, in part because of variation in leaf discs between assays, there was no evidence that resistance to Cry1Ac was consistently declining in the low density treatment over the course of this experiment. Susceptibility to toxin in this treatment at generation 4 and 5 was not significantly different from the susceptibility to toxin in the G0 generation at the beginning of this experiment (F1,42=1.48, p>0.05) and the confidence limits of LC50s in assays from generations 1–5 always overlapped those of initial assays at generation 0 (figure 4).
4. Discussion
It has been proposed that an understanding of the physiological basis of resistance can predict whether or not particular resistance genes will impose a fitness cost (Coustau et al. 2000). In the present case, while Serd4 and Karak share one mechanism of resistance, reduced binding of toxin to the midgut, Serd4 possesses at least two other resistance mechanisms, one of them being reduced activation of the Cry1Ac protoxin (Sayyed et al. 2001; Sayyed & Wright 2001a; Sayyed et al. 2004). Here, we have shown that two different resistant strains exhibited fitness costs under different environmental conditions indicating that the interaction between genes and environment rather than the physiological basis of resistance determined the consequences of a novel adaptation for individual insects. Experimental evidence for gene×environment interactions are rare in animals, although genes and environment have been shown to affect both the inheritance and strength of fitness costs associated with resistance to Bt in Pectinophora gossypiella (Carrière et al. 2004) and have been found in bacteria resistant to phage attack and lettuces resistant to aphids or fungi (Bergelson 1994; Bohannan et al. 1999).
Resistance to parasitoid attack has been shown to be traded-off against successful competitive ability in Drosophila melanogaster (Kraaijeveld & Godfray 1997). Since Drosophila encounter variable and significant levels of mortality in the field when competing for ephemeral resources (Santos et al. 1999) this trade-off can explain some of the observed genetic variation in resistance to parasitoids (Kraaijeveld & Van Alphen 1995). Although it is difficult to extrapolate our data to field conditions, for the Serd4 P. xylostella population, reduced competitive ability is unlikely to affect survival of resistant insects in the field since the larval density on cabbage crops in Malaysia very rarely exceeds 25 individuals per plant (R. Butcher, J. Cook and D.J. Wright unpublished data) and resistance to Cry1Ac did not consistently decline at this larval density in our laboratory experiment. While it is not possible to exclude any other pleiotropic costs of resistance acting outside the laboratory, our data suggest that those particular fitness costs that are mediated by larval competition may not be imposed at larval densities typical of field-grown cabbages.
Trade-offs in resistance to pesticides are a vital component of resistance management and can substantially reduce the frequency of resistance genes during periods such as over-wintering (Foster et al. 1997; Carrière et al. 2001; Bourguet et al. 2004). Pleiotropic fitness costs of resistance are especially effective at slowing or reversing the evolution of resistance when partially dominant (Carrière & Tabashnik 2001). The cost of resistance to pesticides can be subsequently reduced by the evolution of fitness modifiers (Clarke & McKenzie 1987; Lenormand et al. 1998). Preliminary complementation studies have indicated that the loss of binding resistance mechanism shared by the Karak and the Serd4 strain may be the result of mutations on the same gene (A. Sayyed unpublished data). Given the large difference in performance of these two resistant individuals from these populations on B. oleracea, there is the possibility that fitness modifiers may have evolved to reduce costs of resistance in the Serd4 population. Without doubt, the results of our stability experiment and performance experiments from this and previous studies (Sayyed & Wright 2001b) demonstrate that the resistance to Cry1Ac in Serd4 imposes few costs in the laboratory. Thus, while fitness costs can be detected by artificially imposing unusually high stress on resistant individuals, these stresses and their concomitant costs may be reduced in the conditions in which resistance evolved. Resistance management, particularly the reversal of resistance, may be more difficult to apply following the evolution of resistance genes that impose few costs on their hosts (e.g. Carrière & Tabashnik 2001). The potential for the evolution of fitness modifiers, or low cost resistance genes, emphasizes the need for effective preemptive strategies that will maintain the frequency of resistance genes at a low level and reduce the selection pressure for genes that ameliorate the costs of resistance.
Acknowledgments
The Biotechnology and Biological Sciences Research Council (grant D15960) funded this study.
Footnotes
Present address: Department of Zoology, South Parks Road, Oxford OX1 3PS, UK.
References
- Bergelson J. The effects of genotype and the environment on costs of resistance in lettuce. Am. Nat. 1994;143:349–359. [Google Scholar]
- Bohannan B.J.M, Travisano M, Lenski R.E. Epistatic interactions can lower the cost of resistance to multiple consumers. Evolution. 1999;53:292–295. doi: 10.1111/j.1558-5646.1999.tb05355.x. [DOI] [PubMed] [Google Scholar]
- Bourguet D, Guillemaud T, Chevillon C, Raymond M. Fitness costs of insecticide resistance in natural breeding sites of the mosquito Culex pipiens. Evolution. 2004;58:128–135. doi: 10.1111/j.0014-3820.2004.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Tabashnik B.E. Reversing insect adaptation to transgenic insecticidal plants. Proc. R. Soc. B. 2001;268:1475–1480. doi: 10.1098/rspb.2001.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Patin A.L, Sims M.A, Meyer S, Liu Y.B, Dennehy T.J, Tabashnik B.E. Overwintering cost associated with resistance to transgenic cotton in the pink bollworm (Lepidoptera: Gelechiidae) J. Econ. Entomol. 2001;94:935–941. doi: 10.1603/0022-0493-94.4.935. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Biggs R, Higginson D.M, Dennehy T.J, Tabashnik B.E. Effects of gossypol on fitness costs associated with resistance to Bt in pink bollworm. J. Econ. Entomol. 2004;97:1710–1718. doi: 10.1603/0022-0493-97.5.1710. [DOI] [PubMed] [Google Scholar]
- Cerda H, Sayyed A.H, Wright D.J. Laboratory culture conditions affect stability of resistance to Bacillus thuringiensis Cry1Ac in Plutella xylostella (Lep., Plutellidae) J. Appl. Entomol.-Z. Angew. Entomol. 2003;127:142–145. [Google Scholar]
- Clarke G.M, McKenzie J.A. Developmental stability of insecticide resistant phenotypes in blowfly—a result of canalizing natural-selection. Nature. 1987;325:345–346. [Google Scholar]
- Coustau C, Chevillon C, French-Constant R. Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol. Evol. 2000;15:378–383. doi: 10.1016/s0169-5347(00)01929-7. [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Methods in Ecology. Blackwell; Oxford: 1993. GLIM for Ecologists. [Google Scholar]
- Ferré J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- Foster S.P, Harrington R, Devonshire A.L, Denholm I, Clark S.J, Mugglestone M.A. Evidence for a possible fitness trade-off between insecticide resistance and the low temperature movement that is essential for survival of UK populations of Myzus persicae (Hemiptera: Aphididae) Bull. Ent. Res. 1997;87:573–579. [Google Scholar]
- Gazave L, Chevillon C, Lenormand T, Marquine M, Raymond M. Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity. 2001;87:441–448. doi: 10.1046/j.1365-2540.2001.00926.x. [DOI] [PubMed] [Google Scholar]
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- Janmaat A.F, Myers J. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc. R. Soc. B. 2003;270:2263–2270. doi: 10.1098/rspb.2003.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Van Alphen J.J.M. Geographical variation in encapsulation ability of Drosophila melanogaster larvae and evidence for parasitoid-specific components. Evol. Ecol. 1995;9:10–17. [Google Scholar]
- Lenormand T, Guillemaud T, Bourguet D, Raymond M. Appearance and sweep of a gene duplication: adaptive response and potential for new functions in the mosquito Culex pipiens. Evolution. 1998;52:1705–1712. doi: 10.1111/j.1558-5646.1998.tb02250.x. [DOI] [PubMed] [Google Scholar]
- Liu Y.B, Tabashnik B.E, Pusztai-Carey M. Field-evolved resistance to Bacillus thuringiensis toxin CryIC in diamondback moth (Lepidoptera: Plutellidae) J. Econ. Entomol. 1996;89:798–804. [Google Scholar]
- McGaughey W.H, Beeman R.W. Resistance to Bacillus thuringiensis in colonies of Indian meal moth and almond moth (Lepidoptera, Pyralidae) J. Econ. Entomol. 1988;81:28–33. [Google Scholar]
- Santos M, Eisses K.T, Fontdevila A. Competition and genotype-by-environment interaction in natural breeding substrates of Drosophila. Evolution. 1999;53:175–186. doi: 10.1111/j.1558-5646.1999.tb05343.x. [DOI] [PubMed] [Google Scholar]
- Sayyed A.H, Wright D.J. Cross-resistance and inheritance of resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth (Plutella xylostella L) from lowland Malaysia. Pest Manag. Sci. 2001a;57:413–421. doi: 10.1002/ps.313. [DOI] [PubMed] [Google Scholar]
- Sayyed A.H, Wright D.J. Fitness costs and stability of resistance to Bacillus thuringiensis in a field population of the diamondback moth Plutella xylostella L. Ecol. Entomol. 2001b;26:502–508. [Google Scholar]
- Sayyed A.H, Haward R, Herrero S, Ferré J, Wright D.J. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 2000;66:1509–1516. doi: 10.1128/aem.66.4.1509-1516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed A.H, Gatsi R, Kouskoura T, Wright D.J, Crickmore N. Susceptibility of a field-derived, Bacillus thuringiensis-resistant strain of diamondback moth to in vitro-activated Cry1Ac toxin. Appl. Environ. Microbiol. 2001;67:4372–4373. doi: 10.1128/AEM.67.9.4372-4373.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed A.H, Raymond B, Ibiza-Palacios M.S, Escriche B, Wright D.J. Genetic and biochemical characterization of field evolved resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 2004;70:7010–7017. doi: 10.1128/AEM.70.12.7010-7017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton A.M, Zhao J.Z, Roush R.T. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 2002;47:845–881. doi: 10.1146/annurev.ento.47.091201.145309. [DOI] [PubMed] [Google Scholar]
- Tabashnik B.E, Liu Y.B, Malvar T, Heckel D.G, Masson L, Ballester V, Granero F, Mensua J.L, Ferrè J. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA. 1997;94:12780–12785. doi: 10.1073/pnas.94.24.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.D, Gilboa S, Roush R.T, Shelton A.M. Inheritance, stability, and lack-of-fitness costs of field-selected resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae) from Florida. J. Econ. Entomol. 1997;90:732–741. [Google Scholar]
- Verkerk R.H.J, Wright D.J. Interactions between the diamondback moth Plutella xylostella L. and glasshouse and outdoor-grown cabbage cultivars. Ann. Appl. Biol. 1994;125:477–488. [Google Scholar]


