Abstract
Many parasitic species of insects complete their entire development in seeds. They feed off storage reserves within the ovule. These reserves only normally accumulate in fertilized ovules. Consequently, female insects that oviposit their eggs directly into the plant ovule need to be able to select correctly, as unfertilized ovules of conifers normally become so-called empty seed. We provide clear evidence that in conifers, seed-parasitizing insects do not need to discriminate between fertilized and unfertilized plant ovules when ovipositing their eggs. A host-specific insect, the chalcid Megastigmus spermotrophus Wachtl (Hymenoptera: Torymidae), lays its eggs in ovules of Douglas fir (Pseudotsuga menziesii (Mirbel) Franco) before fertilization has taken place in the plant. Oviposition not only prevents the expected degeneration and death of unfertilized ovules, but it induces energy reserve accumulation. Ovules that would otherwise develop as empty seed are redirected in their development by the insect to provide food for the developing larvae. Instead of the insect exploiting normal events during seed development, the insect manipulates seed development for its own reproductive advantage.
Keywords: seed parasite, seed development, Pseudotsuga, Megastigmus
1. Introduction
Sexual reproductive success in plants requires male and female development to be highly coordinated (Herrero 2003). Equally, the reproductive fitness of insects that parasitize seeds and seed cones may depend on the coordination of oviposition and plant development (McClure et al. 1998). This is partly dictated by the type of seed plant. The two extant types—angiosperms and gymnosperms—present different opportunities. Pollination and fertilization occur more closely to one another in flowering plants (angiosperms) than in gymnosperms such as conifers. In the latter, the time from pollen arrival to male gamete delivery into the plant egg is a few days in some species, but up to a year in others (Singh 1978). In Douglas fir, it is a gap of six to ten weeks depending on the season (Owens et al. 1991). Female receptivity is delayed while the haploid megagametophyte, which eventually houses the plant eggs, grows and differentiates. Megagametophytes are polarized, with one end producing the gametes and the other end developing a large mass of prothallial cells, the site of future storage reserve accumulation. Megagametophytes are large even before fertilization and it would be surprising if they were not targets for parasites. Although there are many ovules, not every ovule is fertilized, and in some species, such as Douglas fir, seed yield can be very low in some years (Owens et al. 1991). The problem for a seed parasite at the time of oviposition is how to select as many fertilized ovules as possible, as significant storage accumulation only begins in the megagametophyte if fertilization has taken place. Unfertilized ovules are, in theory, undesirable targets because megagametophytes undergo apoptosis a few weeks after plant egg death has occurred.
Parasites are known to influence pollination of flowering plants, thereby improving their chances of ovipositing in or near fertilized seed that will amass nutrient reserves; this is the case of fig wasps (Weiblen 2002). Gymnosperms are evolutionarily more plesiomorphic seed plants than flowering plants; wind-pollination is the rule, with the notable exception of cycads, and with the rare exception of inadvertent, accidental insect pollination. As in the case of the chalcid Megastigmus spermotrophus, insects are left with the choice of ovipositing eggs either in fertilized or unfertilized plant ovules (Hussey 1955). The fact that infestation levels of this species exceeded the expected amount of filled seed led Niwa & Overhulser (1992) and Rappaport et al. (1993) to suggest that the insect must also be choosing unfertilized plant ovules, but no known mechanism could account for this observation. We tested whether female insects were ovipositing in sexually immature (=prefertilization) ovules, in sexually mature but unfertilized ovules, or in successfully fertilized ovules. We studied storage tissue development in both uninfested and infested ovules. We also set out to test whether the interaction between host and parasite was the same. We carried out extensive histological studies on various stages of seed development in uninfested and infested seed collected in France, where this insect is an invasive pest, as well as in its native habitat in western North America to see if the host/insect relationship was similar.
2. Materials and methods
(a) Study system
(i) The host
Douglas fir trees were sampled from two locations. Four flowering trees from clones 3782 and 3788 of Pseudotsuga menziesii were randomly selected from the breeding orchard at the Institut National de la Recherche Agronomique (INRA) in Orléans, France. The same experiment was repeated at the British Columbia Ministry of Forests breeding orchard at Puckle Road Research Station (BCMF) in Saanichton, BC, Canada. The clones utilised were 63-8L, 101-3L and 134-10L. Before pollination occurred, branches bearing from 5 to 20 female buds were enclosed in exclusion bags to prevent pollen and other organisms from entering the cones. Pollen cones had been manually removed from these branches. The bags were randomly assigned to the following four treatments: (i) no pollination, no seed chalcid; (ii) no pollination, introduction of M. spermotrophus seed chalcids; (iii) manual pollination, no seed chalcids; (iv) manual pollination, introduction of M. spermotrophus seed chalcids. For the pollination treatments, a polymix—a mixture of many Douglas fir pollen genotypes collected from a breeding orchard—was applied during the period of female receptivity either by brush (INRA) or by syringe injection (BCMF). To ensure success, each female cone was pollinated twice. Paper exclusion bags were replaced two weeks later with woven bags to allow air flow but prevent any natural attack of chalcids. Over the course of the spring, female cones were monitored weekly. Stages were categorized according to Allen & Owens (1972). An independent study on the effect of bagging cones was carried out. Ovules from open-pollinated cones were compared with ovules from bagged cones. As we had artificially introduced the insects to the cones in situ, we also compared our results with open-pollinated seed cones, which had been infested by native populations of M. spermotrophus. Ovules from bagged cones were approximately one week in advance of open-pollinated cones on the same tree by the time of plant egg maturation, that is, eight weeks after initial bagging. The effect of bagging was therefore considered to be minor.
(ii) The parasite
About 1000 seeds infested by M. spermotrophus were collected at a seed orchard in Lavercantière, located in southwestern France. In the BCMF portion, the insects were raised from seed collected from local natural stands on Vancouver Island. The seeds were stored at outdoor conditions until adult emergence. Insects that emerged in the closed bag of seed were collected and fed for 1 day on sugared water. Ten males and 10 females were introduced per bag. Two additional studies were carried out: the first tabulated the location of oviposited eggs (n=235), and the second counted egg and larvae number per megagametophyte at three different stages of development (50 samples per stage).
(b) Developmental study
At INRA, cones taken weekly from trees were dissected in the laboratory and selected ovules removed. The weekly collections were begun at prior to meiosis and included all developmental stages of the female gametophyte. At BCMF, ovules were similarly sampled weekly from just prior to meiosis onward, but only those removed for the period from cellularization to megagametophyte maturity were processed for closer investigation. In each study, a minimum of five ovules per stage were sectioned, and at least a dozen per stage were sectioned from the more important stages and treatments.
Ovules were processed and stained differently in the two studies. At INRA, ovules were isolated and fixed in FAA (formalin, acetic acid, alcohol). These were processed and embedded in paraffin, cut into 7 μm sections, and affixed to gelatin-coated slides. Material was stained with Safranin-Fast Green (general stain). Ovules removed from trees at BCMF were fixed in 2.5% gluteraldehyde in 0.075 M phosphate buffer at pH 7.2. The samples were stored at 4°C until required. Samples were processed and embedded in glycol methacrylate (Technovit 7100, Marivac, Canada), then sectioned at 5 μm using a Leica SM 2400 sledge microtome with a tungsten carbide knife. Sections were stained with Ponceau Red 2R/Azure Blue for proteins and cell walls, respectively, IKI, or Toluidine Blue O as a general metachromatic stain. All methods followed Gutmann (1995). To verify lipids, material was processed for electron microscopy using previously described methods (Rohr et al. 1989). Thin sections were contrasted with Reynolds lead citrate and viewed with either a Hitachi HU12A or a Philips CM120 transmission electron microscope. Thick sections (1 μm) were stained with Toluidine Blue O in 2.5% aqueous sodium carbonate, pH 8.0.
(c) X-ray analysis
X-rays of extracted seed were made with a Faxitron N 43855A (Hewlett Packard). Seed was exposed to 15 kV for 30 s for samples taken from dry cones, and for 6 min at the same exposure if taken from fresh cones.
3. Results
(a) Timing of insect oviposition in relation to megagametophyte development
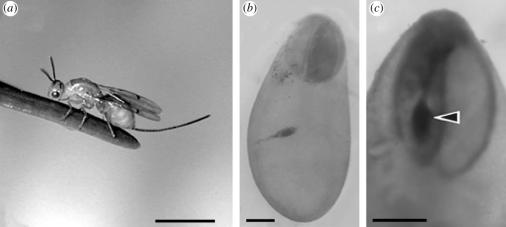
To determine the target and timing of insect oviposition, we dissected ovules to look for insect eggs. Megastigmus spermotrophus females (figure 1a) pushed their ovipositor through the cone bract, across the outer layer of the ovule and into the megagametophyte. Of 235 parasitized ovules dissected, only 0.9% had insect eggs that were located elsewhere; these were found between the outer layer and the megagametophyte. Insect eggs were deposited in the prothallial cells of the central and basal portions of 74.5% of megagametophytes (figure 1b). The remaining ovules had insect eggs in the apical portion, of which half of these were laid directly in central cells (figure 1c); these were the very large cells that after a further division gave rise to the plant egg cells. Insect eggs destroyed plant central cells, but as there were four or five central cells per megagametophyte, death of one central cell did not prevent eventual fertilization in the remaining gametes. The presence of insect eggs and then larvae was not detrimental to the megagametophyte, which continued to grow and differentiate. Megastigmus spermotrophus eggs were usually laid one per megagametophyte (74%), although two insect eggs per megagametophyte were found in 26% of the samples. In another sample taken when insect eggs had hatched, we observed two or more young larvae per megagametophyte in 30% of megagametophytes sampled. This proportion changed with time, as only single mature larvae were found in seed at the end of the season (50/50).
Figure 1.
Oviposition of insect eggs into Douglas fir megagametophytes. (a) Female Megastigmus spermotrophus with ovipositor. Bar, 3 mm. (b) Insect eggs deposited in central portion of megagametophyte and (c) in a central cell (arrow), the precursor to the plant egg cell (stage 6). Bars, 250 μm
Oviposition was restricted to sexually immature megagametophytes. Insect eggs were found almost exclusively in megagametophytes in which central cells were differentiating (figure 2—stage 6). Only rarely were insect eggs found in late stage 5 megagametophytes in which cellularization was nearing completion. Cellularization, also known as alveolation, is the stage during which a syncytial megagametophyte develops walls between its hundreds of free nuclei. Although a number of megagametophyte developmental stages apparently overlap with the period in which insects oviposited (figure 2), this variation is due to differences in development between sampled trees.
Figure 2.
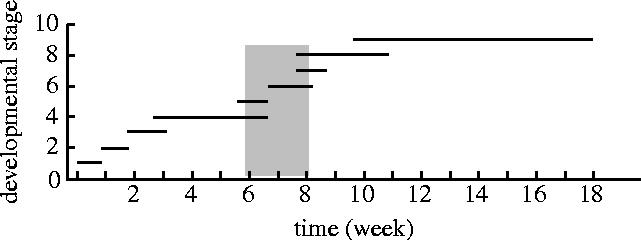
Developmental stages of Douglas fir megagametophytes against time, emphasizing the period of Megastigmus spermotrophus oviposition. Shading indicates period of insect oviposition. The length of line indicates the range in length of stage. Stages are according to standard description (Allen & Owens 1972) of unparasitized Douglas fir ovules: 1. megaspore mother cell, 2. meiosis, 3. megaspore, 4. free-nuclear stage, 5. cellularization, 6. central cell differentiation, 7. plant egg maturation and fertilization, 8. megagametophyte storage product accumulation, 9. embryo maturation.
(b) Effect of insect on megagametophyte development
The influence of the presence of insect larvae on megagametophyte development was investigated by a histological comparison of parasitized and unparasitized ovules in both pollinated and unpollinated ovules. Control megagametophytes of unparasitized, unpollinated ovules produced mature plant eggs that were not fertilized and consequently degenerated within a week, leaving a dry, empty seed. This was the only treatment that resulted in empty seed.
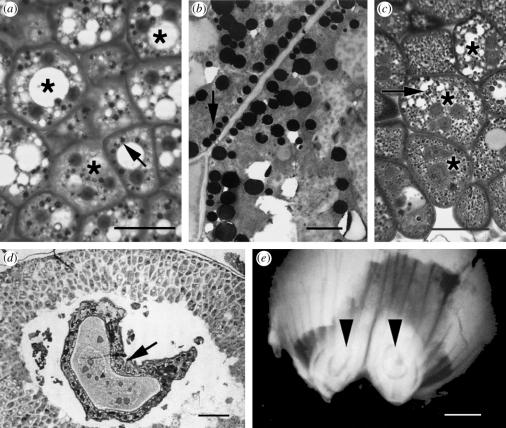
The presence of larvae in unfertilized ovules prevented such degeneration. Unpollinated ovules produced mature plant eggs that were not fertilized and which subsequently broke down, but instead of the entire megagametophyte degenerating within a few weeks, the prothallial cells began to accumulate starch, then lipid and protein. The storage reserves available to the larva changed over the course of its development. The megagametophyte's prothallial cells at the time of oviposition were characteristically highly vacuolated and did not have any lipid bodies, starch grains, or protein bodies. After plant egg abortion in unfertilized ovules, the megagametophyte built up starch grains in the central zone. This zone quickly degenerated spontaneously, forming a corrosion cavity, which is the lacuna that normally forms in a megagametophyte. At its margins, multinucleate storage cells developed (figure 3a), in contrast to earlier stages of megagametophyte development, in which mostly uninucleate cells and the occasional binucleate cell were to be found. Cells began to build up reserves in the form of lipid bodies (figure 3b), protein bodies (figure 3c), and starch (not shown) until all the remaining cells of the megagametophyte were no longer vacuolated but filled with storage reserves. Reserve accumulation continued unabated even as the larva continued to grow. Cells closest to the insect cavity, even if not directly consumed, were less full, indicating that reserves were being broken down. Larvae preferred to eat cells from the margins of the cavity (figure 3d), steadily working their way to the edge of the megagametophyte until they filled most of the seed (figure 3e). There was still megagametophyte tissue surrounding the insect at the time of the X-ray, indicating that feeding does not trigger further degeneration. A schematic summary of the differences between parasitized and unparasitized ovules at two different stages is given in figure 4.
Figure 3.
Mature storage tissue in parasitized, unpollinated Pseudotsuga menziesii ovules. (a) Eleven week old megagametophyte cells with granular protein accumulation (arrow) and multinucleate cells (asterisk). Bar, 25 μm (b) Electron micrograph of similar cell as in a: lipid bodies indicated by arrow (bar, 2 μm). (c) Cells in 13-week old megagametophyte. Numerous small dark granules are protein bodies (arrow). Multinucleate cells shown with asterisks. Bar, 20 μm (d) Paraffin section of Megastigmus spermotrophus larva (arrow) surrounded by megagametophyte storage cells. Crystals of protein are visible in the stomach. Bar, 50 μm (e) X-ray of two ovules each of which has a mature insect larva (arrows). Bar, 1 mm
Figure 4.
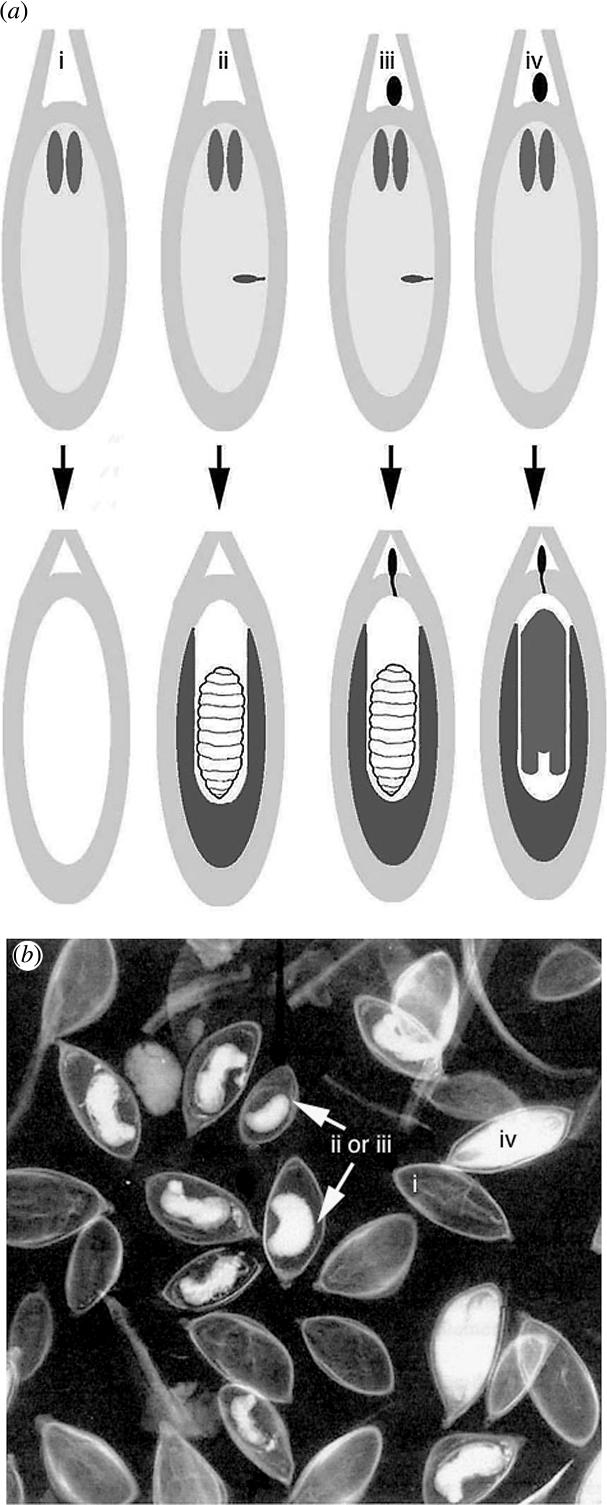
Types of Douglas fir seed in the four treatments. (a) The schematic developmental scheme shows ovules at stage 6, week 7 in the top row, and those at week 13 in the bottom, when megagametophyte developmental differences were most apparent. (i) Unpollinated, unfertilized ovule (above) produced empty seed (below). Megagametophyte with two apical central cells surrounded by an integument. (ii) Unpollinated ovule with insect egg in central megagametophyte. Larva shown in bottom row is surrounded by storage tissue. (iii) Megagametophyte with insect egg and pollen (black) on nucellus. Although the larva consumed the embryo, storage product accumulation resulted in a situation similar to (ii) above. (iv) Pollination of unparasitized ovule resulted in embryo surrounded by storage tissue. (b) X-ray of seed (week 18). Seed types corresponding to above figures are indicated by small roman numerals. Parasitized seed that was either unpollinated (ii) or pollinated (iii) was indistinguishable by this time.
Another aspect of insect influence was found in the pollinated ovules. As larvae growing in the megagametophyte did not prevent fertilization, the resulting plant embryos were eaten by larvae. The loss of these early stage embryos had no effect on megagametophyte development, which, as in the previous treatment, resulted in multinucleate storage cells with abundant reserves of lipid, protein and starch that served as energy stores for the insect larvae.
In unparasitized fertilized ovules, megagametophyte development and embryogenesis resulted in full seed. Lipid bodies and protein bodies began to fill prothallial cells until the entire megagametophyte was composed of cytoplasmically dense, multinucleate cells filled with nutrient reserves. These megagametophytes developed identically to the two previous treatments.
X-rays showed that seed removed from cones could be segregated according to full seed, empty seed, and parasitized seed (figure 4b). In spring, before insect emergence, it was possible to further segregate male or female adult and larvae still in diapause.
These studies carried out in both France and Canada gave identical results. At some stages, phenology varied by a week, but the staged histological investigations of parasitized and unparasitized ovules, that were either pollinated or not pollinated, did not differ in even minor details.
4. Discussion
Insect parasitism of Douglas fir megagametophytes alters seed development in two different ways. The first effect is on unpollinated megagametophytes that normally die within a few weeks of the unfertilized plant egg degenerating (Owens et al. 1991), but when parasitized, fail to abort. Instead, they build up storage reserves as if they had been fertilized. The second effect of insect parasites is on ovules that have been fertilized but whose embryos die at an early stage of development because they are eaten by the larvae. Normally, an immediate consequence of embryos dying at an early stage is the breakdown and death of the surrounding megagametophyte (Orr-Ewing 1957). However, after the larva consumes the young embryo, the megagametophyte does not abort, but continues to build up reserves, feeding the larva instead. In both situations, the mechanisms that trees use to avoid investing resources in failing seed have been co-opted by the insect to its own advantage.
Insect–plant interactions are known to involve insect influence of signal transduction in plants, often by manipulation of hormonal signals (Schultz & Appel 2004). How such factors may be influencing programmed cell death in megagametophytes is unknown, as apoptosis in megagametophytes during seed development has only been studied in relation to germination (He & Kermode 2003a,b). During normal Douglas fir megagametophyte development, concentrations of hormones change predictably (Chiwocha & von Aderkas 2002), but the influence of parasitism remains to be determined.
Manipulation of megagametophytes by insects may not be unique to the host–insect pair of Douglas fir and M. spermotrophus. Similar parasitism by species of Megastigmus is probably to be discovered in hosts whose phenology is similar to Douglas fir, such as Larix (Kosinski 1987). In a comparison of published observations of Megastigmus–seed interactions in two of the largest families of conifers, Cupressaceae and Pinaceae, it was found that Megastigmus oviposit in a manner characteristic to each family. Developing megagametophytes were preferred in pinaceaous conifers, but mature megagametophytes in the case of cupressaceaeous ones (Rouault et al. 2004). It is probably that insects may also override abortion in some of these conifers, but this needs to be verified with histological study.
Grissell (1999) categorized a number of Megastigmus species as gall-formers; seed-infesting species were not included, because the morphology and physiology of galls appears, at least on initial inspection, to be different from what occurs in infested seed. Gall inducers cause host-plant tissues to differentiate into novel structures, ranging in complexity from relatively open pits or folds to structures in which the galler is enclosed entirely by plant tissues (Stone & Schönrogge 2003). Recent phylogenetic studies of different groups of gall insects all support galler control for the major aspects of gall morphology (Cook et al. 2002), gall structures representing the extended phenotypes of galler genes (Stone & Cook 1998). Although the overall morphology of insect galls is variable, the inner organization of gall tissues appears quite similar (Shorthouse & Rohfritsch 1992). The gall-former alters the physiological state of plant tissue, particularly that of cells nearest to the feeding larva, which constitute the nutritive tissue, which is maintained in a metabolically active state by the gall-former (Bronner 1992). For example, in virtually all cynipid galls the larval chamber is lined with nutritive cells and bounded externally by a layer of vacuolate parenchyma and a thin layer of sclerenchyma (Stone et al. 2002). Another way to consider gallers is in the context of the nutrition hypothesis, which states that galls provide enhanced nutrition over other feeding modes (Stone & Schönrogge 2003). Using this criterion, is M. spermotrophus perhaps a galler in Douglas-fir seed? The answer is more ambiguous. On the one hand, at least from a histological point of view, the presence of a chalcid larva does not induce a novel structure, because the development of female gametophyte in unfertilized infested seeds is similar to that observed in fertilized ovules without insects. On the other hand, the chalcid larva controls megagametophyte development, preventing the degeneration process, even inducing differentiation of plant storage tissues. A key criterion for answering the question would be to compare proteins found in the gametophyte during normal development with those induced by the gall insect.
This relationship between M. spermotrophus and Douglas fir differs from other tightly linked insect–plant systems. Yucca moths (Pellmyr 2003), senita moths (Fleming & Holland 1998) and agaonid wasps on figs (Weiblen 2002) benefit their hosts by pollinating flowers in which they have oviposited. These plants produce seeds, a certain percentage of which are lost to developing larvae, which are assured of nutrients from the accumulating seed storage reserves. Douglas fir receives no such advantage as oviposition occurs before fertilization. Megastigmus spermotrophus induces seed storage reserve formation, freeing the insect from a dependence on pollination. As a result, such induced ovules only feed developing larvae. Although this is the first report of this type of insect–host relationship in seed plants, it is probably more widespread. It also provides an excellent system in which to study the insect-mediated delay of apoptosis in another organism.
Acknowledgments
We acknowledge the help of Drs R. Bennett, J. Turgeon, J.-N. Candau, M. El Maâtaoui, B. Anholt and J.-P. Raimbault, as well as Andrea Coulter, Chris Dewdney, Jenny Robb, Stephen O'Leary and Guy Chanteloup. The following agencies provided support for this research: Fonds France–Canada pour la Recherche, Conseil Général de la Région Centre, Canadian Forestry Service, British Columbia Ministry of Forests and the Natural Sciences and Engineering Research Council of Canada.
References
- Allen G.S, Owens J.N. Environment Canada, Forestry Service; Ottawa: 1972. The life history of Douglas fir. [Google Scholar]
- Bronner R. The role of nutritive cells in the nutrition of cynipids and cecidomyiids. In: Shorthouse J.D, Rohfritsch O, editors. Biology of insect-induced galls. Oxford University Press; Oxford: 1992. pp. 118–140. [Google Scholar]
- Chiwocha S, von Aderkas P. Endogenous levels of free and conjugated forms of auxin, cytokinins and abscisic acid during seed development in Douglas fir. Plant Growth Regul. 2002;36:191–200. [Google Scholar]
- Cook J.M, Rokas A, Pagel M, Stone G.N. Evolutionary shifts between host oak sections and host-plant organs in Andricus gallwasps. Evolution. 2002;56:1821–1830. doi: 10.1111/j.0014-3820.2002.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Fleming T.H, Holland J.N. The evolution of obligate pollination mutualisms: senita cactus and senita moth. Oecologia. 1998;114:368–375. doi: 10.1007/s004420050459. [DOI] [PubMed] [Google Scholar]
- Grissell E. American Entomological Institute; Gainesville: 1999. An annotated catalogue of world Megastigminae (Hymenoptera: Chalcidoidea: Torymidae) [Google Scholar]
- Gutmann M. Improved staining procedures for photographic documentation of phenolic deposits in semi-thin sections of plant tissue. J. Microsc. 1995;179:277–281. [Google Scholar]
- He X, Kermode A.R. Nuclease activities and DNA fragmentation during programmed cell death of megagametophyte cells of white spruce (Picea glauca) seeds. Plant Mol. Biol. 2003a;51:509–521. doi: 10.1023/a:1022319821591. [DOI] [PubMed] [Google Scholar]
- He X, Kermode A.R. Proteases associated with programmed cell death of megagametophyte cells after germination of white spruce (Picea glauca) seeds. Plant Mol. Biol. 2003;52:729–744. doi: 10.1023/a:1025008117046. [DOI] [PubMed] [Google Scholar]
- Herrero M. Male and female synchrony and the regulation of mating in flowering plants. Phil. Trans. R. Soc. B. 2003;358:1019–1024. doi: 10.1098/rstb.2003.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey N.W. The life histories of Megastigmus spermotrophus Wachtl (Hym. Chalcidoidea) and its principal parasite, with descriptions of the developmental stages. Trans. R. Entomol. Soc. 1955;106:133–151. [Google Scholar]
- Kosinski G. Empty seed production in European larch (Larix decidua L.) For. Ecol. Manage. 1987;19:241–246. [Google Scholar]
- McClure M, Quiring D.T, Turgeon J.J. Proximate and ultimate factors influencing oviposition site selection by endoparasites on conifer seed cones: two sympatric dipteran species on larch. Entomol. Exp. Appl. 1998;87:1–13. [Google Scholar]
- Niwa C.A.G, Overhulser D.L. Oviposition and development of Megastigmus spermotrophus in unfertilized Douglas-fir seed. J. Econ. Entomol. 1992;85:2323–2328. [Google Scholar]
- Orr-Ewing A.L. A cytological study of the effects of self-pollination on Pseudotsuga menziesii (Mirb.) Franco. Silvae Genet. 1957a;6:179–185. [Google Scholar]
- Owens J.N, Colangeli A.M, Morris S.J. Factors affecting seed set in Douglas-fir (Pseudotsuga menziesii) Can. J. Bot. 1991;69:229–238. [Google Scholar]
- Pellmyr O. Yuccas, yucca moths, and coevolution: a review. Ann. Mo. Bot. Garden. 2003;90:35–55. [Google Scholar]
- Rappaport N, Mori S, Roques A. Estimating impact of a seed chalcid, Megastigmus spermotrophus (Hymenoptera: Torymidae) on Douglas-fir seed production: the new paradigm. J. Econ. Entomol. 1993;83:845–849. [Google Scholar]
- Rohr R, von Aderkas P, Bonga J.M. Ultrastructural changes in haploid embryoids of Larix decidua during early embryogenesis. Am. J. Bot. 1989;76:1460–1467. [Google Scholar]
- Rouault G, Turgeon J.J, Candau J.-N, Roques A, von Aderkas P. Oviposition strategies of conifer seed chalcids in relation to host phenology. Naturwissenschaften. 2004;91:472–480. doi: 10.1007/s00114-004-0554-4. [DOI] [PubMed] [Google Scholar]
- Schultz J.C.A, Appel H.M. Cross-kingdom cross-talk: hormones shared by plants and their insect herbivores. Ecology. 2004;85:70–77. [Google Scholar]
- Shorthouse J.D, Rohfritsch O. Oxford University Press; New York: 1992. Biology of insect-induced galls. [Google Scholar]
- Singh H. Gebrüder Borntraeger; Stuttgart: 1978. Embryology of gymnosperms. [Google Scholar]
- Stone G.N, Cook J.M. The structure of cynipid oak galls: patterns in the evolution of an extended phenotype. Proc. R. Soc. B. 1998;265:979–988. [Google Scholar]
- Stone G.N, Schönrogge K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003;18:512–522. [Google Scholar]
- Stone G.N, Schönrogge K, Atkinson R.J, Bellido D, Pujade-Villar J. The population biology of oak gall wasps (Hymenoptera: Cynipidae) Ann. Rev. Entomol. 2002;47:633–668. doi: 10.1146/annurev.ento.47.091201.145247. [DOI] [PubMed] [Google Scholar]
- Weiblen G.D. How to be a fig wasp. Ann. Rev. Entomol. 2002;47:299–330. doi: 10.1146/annurev.ento.47.091201.145213. [DOI] [PubMed] [Google Scholar]