Abstract
Recently, there has been increasing evidence of species' range shifts due to changes in climate. Whereas most of these shifts relate ground truth biogeographic data to a general warming trend in regional or global climate data, we here present a reanalysis of both biogeographic and bioclimatic data of equal spatio-temporal resolution, covering a time span of more than 50 years. Our results reveal a coherent and synchronous shift in both species' distribution and climate. They show not only a shift in the northern margin of a species, which is in concert with gradually increasing winter temperatures in the area, they also confirm the simulated species' distribution changes expected from a bioclimatic model under the recent, relatively moderate climate change.
Keywords: range shift, global warming, bioindicator, bioclimatic model, evergreen broad-leaved species, Ilex aquifolium
1. Introduction
Despite an increasing number of ecological ‘fingerprints’ of climate change (Walther et al. 2001; Parmesan & Yohe 2003; Root et al. 2003; see also Hughes 2000; Walther et al. 2002), consensus on the ecological impacts of global warming still remains elusive (see Jensen 2003). One reason for the lack of consensus may relate to the fact that case studies on species' range shifts (e.g. Grabherr et al. 1994; Parmesan et al. 1999; Thomas & Lennon 1999; Hill et al. 2002; Crozier 2003) often associate local changes in the distribution of species at small scales to large-scale climatic changes on the regional to global level. This is mainly due to the lack of historical biogeographic data on the local and regional distribution of a species, coupled with concurrent climatic data on the same spatio-temporal resolution. One of the exceptions, and to our knowledge the only one, is provided by Iversen (1944), who closely linked the occurrence of some evergreen broad-leaved species to measurements from nearby climate stations. Thus it has been used as the classical example to illustrate a climatically limited species' northern distribution and continues to be regularly featured in standard textbooks of ecology in general (e.g. Begon et al. 1996), and botany in particular (Sitte et al. 2002; Larcher 2003).
A particular feature of the Iversen (1944) study is that it provides a detailed synchronous record for the bioclimatic and biogeographic situation of climatically limited species before the recent rise in global average temperature. In recent decades, the regional climate of the Iversen study area has warmed, especially in the winter season (Folland & Karl 2001). Given this warming trend, one would expect to see a truly climatically limited species response to the changes in climate in these areas.
In this paper, we compare both detailed historic climatic and biogeographic records with updates of the same parameters at the same localities in the northern fringe area of the distribution of Ilex aquifolium, applying the same methodology as used in the original study (Iversen 1944). We also compare our field data to predictions from simulations of Ilex distributions using a purely climate-driven bioclimatic model. Our aim is, therefore, to verify whether a potential shift in the local and regional distribution of a species is in synchrony with concurrent changes in climatic data on the same spatio-temporal resolution.
2. Material and methods
From among the subset of evergreen species described by Iversen (1944) we selected holly (I. aquifolium), the only true shrub and smaller tree species (Peterken & Lloyd 1967; Callauch 1983). The northern margin of the Ilex distribution in Europe is often shown related to the 0 °C-isoline (Holmboe 1913; Loesener 1919; Enquist 1924; Walter & Straka 1970; Sitte et al. 2002). Although the mean temperatures are not themselves considered to be physiologically effective, they correlate with absolute minimum, and therefore are used as a surrogate for the frequency of lethal extreme events (Sitte et al. 2002; Woodward et al. 2004; see also Runge 1950; Fischer 1965; Peterken & Lloyd 1967; Sakai 1982; Prentice et al. 1992; Sykes et al. 1996). The other evergreen species studied by Iversen (1944) either depend on appropriate host trees such as the epiphyte Viscum album, or, in the case of the climbing ivy (Hedera helix), have two separate and different growth forms. Ivy, when creeping on the ground, is protected from winter cold by snow and is thus, in this growth form, a less reliable climate indicator than when it is climbing trees (see also Andergassen & Bauer 2002). Further, these two different growth forms have not been clearly separated in the original data (Iversen 1944).
Our biogeographic field data for holly were obtained from several unpublished recent records of I. aquifolium scattered through various local field surveys and monitoring programmes (see §4 and acknowledgements below). We verified these records in the field in the northern fringe area of the distribution of holly in northern Germany, Denmark, southern Norway and southern Sweden in 2003, and updated the same climatic parameters of the same local climate stations as used in the original study (Iversen 1944). As the study covers more than a half century, not all the climate stations can provide data to the present day. For the abandoned stations we used data from nearby stations or extrapolated surrogate records based upon overlapping periods (see Electronic Appendix A).
The static shell (STASH) bioclimatic model is a simple model which uses a minimum set of bioclimatic parameters (mean temperature of the coldest month, growing season warmth, drought index and degree of chilling required before budburst) to describe a species range. The assumption is that these parameters represent responses to physiologically important mechanisms for plants, for example, accumulated temperature during the growing season (growing degree days) is an index of the presence of energy suitable for the completion of the plant's life cycle. Some of these parameters act directly as on–off switches, for example, if the minimum mean coldest month temperature (as a surrogate for the absolute minimum) in a grid cell falls below the species limit, the species is excluded. Some parameters, however, also directly affect net assimilation and respiration, and thus growth rate, and this is reflected in the degree of establishment success within a grid cell (see Sykes et al. 1996 for further details). We used STASH in a data-model comparison for current and recent past occurrences of holly. We used 12 monthly values for each of monthly mean temperature, precipitation and percentage sunshine from the ATEAM European gridded 10′ climate dataset (http://www.pik-potsdam.de/ateam/) based on the Climate Research Unit dataset (New et al. 1999, 2000). The recent past was modelled as the 30 year normal for the 1931–1960 period, and the current period as the mean of 1981–2000.
Statistical analyses of the simulated and actual distributions of holly in the area between 51–70° N and 4–25° E were based on Cohen's kappa coefficient of agreement (Cohen 1960) providing an estimate for the overlap of spatial data (cf. Fielding & Bell 1997).
3. Results
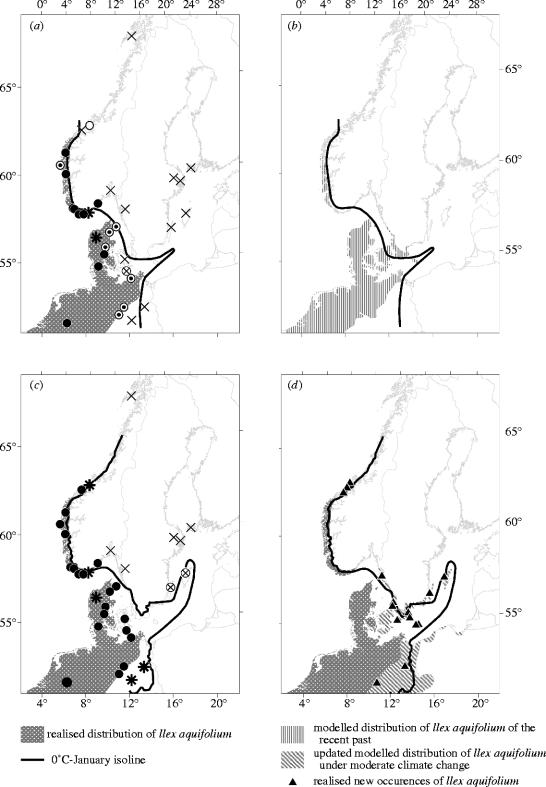
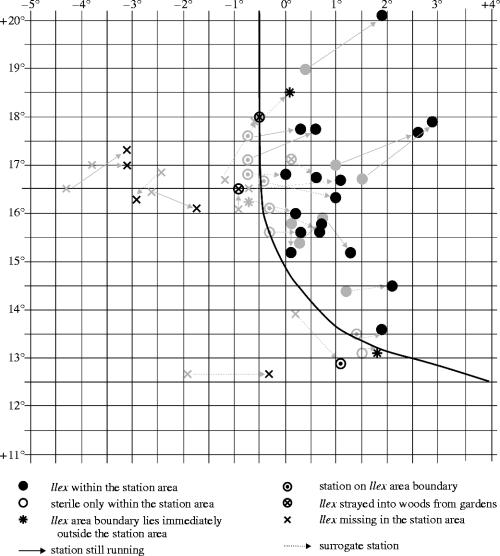
At all the localities where Iversen reported the occurrence of I. aquifolium, we confirmed that the species is still present. In addition, we found new occurrences of holly at locations that were reported Ilex-free at the time of Iversen's investigation (cf. figure 1a,c). The growth form and size, date of first notification, and at some places tree ring analyses allowed an estimation of the approximate age of these individuals (data not shown, but cf. Berger 2003). Based on this information we conclude that these occurrences of Ilex are in fact new, and that Ilex was not present at the time of Iversen's investigation. These new occurrences represent a geographical shift in the distribution of holly towards the north in Norway and northeast in Germany, Denmark (cf. also Banuelos et al. 2004) and especially southern Sweden, where I. aquifolium expanded into new areas along the southern Swedish coast (figure 1d). In addition, the results of the field survey have been plotted in a thermal correlation diagram (figure 2), that is, a coordinate system where the ordinate represents the mean temperature for the warmest month (July/August, depending on the individual station), and the abscissa the mean temperature for the coldest month (January/February, same as for warmest month; cf. Iversen 1944). In this thermal correlation diagram (figure 2), not only the status but also the position of the related climatic stations changed in accordance with the findings of the field survey. Whenever a station, previously designated holly-free, advanced to, or even crossed, the thermal limit curve in the thermal correlation diagram, field observations revealed that the station's surrounding area has been colonized by holly in the time since Iversen's investigation. Consequently, the change in the position of these stations is in synchrony with the change in their biogeographic status, indicated by different symbols in figure 2. Furthermore, while the position and status of several stations have changed, the position of the thermal limit curve has remained stable compared with the outline given by Iversen (1944; cf. figure 2). In the past, the 0 °C January-isoline ran parallel with the northern margin of Ilex distribution (figure 1a), and with the recent shift of both climate isoline and species' distribution change, this relationship remains consistent (figure 1c). Last but not least, the bioclimatic model based on purely bioclimatic response factors simulates a potential Ilex distribution of the recent past (based on 1931–60 climate data) that matches the actual area with a kappa value (K) of 0.82, which is well in the range of ‘excellent’ agreement (K>0.75 according to Fielding & Bell 1997; see also Landis & Koch 1977). The model also predicts new areas that will be colonized by holly under a warming scenario based on the 1981–2000 climate data (figure 1d). In this case, the kappa value for the simulated range shift and the observed change (the latter is the area delimited by the new occurrences; see triangles in figure 1d; excluding the actual area of the recent past; see figure 1a) is 0.50, which, though lower, is in the range of a ‘moderate’ kappa agreement (Landis & Koch 1977).
Figure 1.
Distribution of Ilex aquifolium and the 0 °C-January isoline at different times. (a) Former range of I. aquifolium (dark grey shading) based on Enquist (1924) and Meusel et al. (1965), isoline based upon Walter & Straka (1970), symbols based upon Iversen (1944); for symbol legends see figure 2. (b) Modelled range of I. aquifolium in the recent past (1931–60; vertical shading), isoline as in (a). (c) Former range of I. aquifolium (dark grey shading) as in (a); isoline updated for 1981–2000 based on Mitchell et al. (2004), symbols updated; for symbol legends see figure 2. (d) Former range of I. aquifolium (dark grey shading) complemented with the simulated species' distribution under a moderate climate change based on 1981–2000 climate data (diagonal shading), isoline as in (c); triangles represent locations with new actual occurrences of I. aquifolium.
Figure 2.
Thermal correlation diagram with Iversen (1944) values (grey symbols) and updated values (black symbols); the arrows link the values of corresponding stations. For details see text and Electronic Appendix A.
4. Discussion
In the original work, the climatic stations were critically selected in order to ensure that they sufficiently represented the surrounding areas (defined by Iversen (1944) as the area within a circle of 20 km and within a vertical distance of 40 m). Because of the long period of this study (ca. 50 years) it was not possible to update all the climate data from all the original stations. However, due to the large geographical distances between the climatic stations used in this study, some minor variation arising from the use of nearby surrogate climate stations instead of abandoned original stations is relatively insignificant, especially when considering the potential differences in proportion to changes in microhabitats within the 20 km circle considered representative for the thermosphere of the plants (see Iversen 1944). Furthermore, the data used in the thermal correlation diagram revealed no systematic discrepancy in terms of magnitude, or direction of change in status or position, between the subset of original and surrogate stations (see Electronic Appendix A). Therefore, although not all the original climate stations survived the time span covered in this study, the dataset is considered sufficiently reliable for the purpose of the study. Also, the observed change, especially at winter temperatures, accords well with the latest IPCC findings reporting a 0.6–1.0 °C per decade warming for the period between 1976–2000 in the area of southern Scandinavia (Folland & Karl 2001).
Although we could have based our resurvey on records from several local vegetation monitoring programmes, it was necessary to verify all notifications of (potential) Ilex-occurrences in the field, because, for example, in some places we identified Mahonia aquifolium instead of the expected I. aquifolium. All the localities with previous occurrences of Ilex reported by Iversen (1944) were confirmed. In addition, we also found new areas with holly that were reported Ilex-free at the time of Iversen's investigation. In some cases, new individuals were considered to be escapees from planted garden individuals. However, in that regard, Iversen in his study also included a category named ‘Ilex strayed into woods from gardens’ (cf. Iversen 1944, p. 471). Such subspontaneous occurrences are not in opposition to our approach, rather, they are in agreement with the methodology and findings of the original study. Such events provide opportunities for Ilex to keep pace with the rate of climate change by reducing the time-lag that may be due to, for example, dispersal limitations (Svenning & Skov 2004; cf. also Pott 1990; Leemans 1996) and/or interrupted migration routes (Skov & Svenning 2004), thus allowing a species the chance to occupy its new potential range immediately.
The observed north- and northeastward range expansion tracks the increased warming measured at local climate stations. Whenever a station which previously was reported Ilex-free advanced to or crossed the thermal limit curve in the thermal correlation diagram, a new occurrence of holly was found in the field in the surrounding area. Furthermore, both the historic and the present northern margin of Ilex distribution in Europe remains related to the 0 °C-isoline (figure 1a,c). STASH model output showed very clearly that, in the past, Ilex has filled a great portion of its potential range (figure 1b). Furthermore, the new occurrences of I. aquifolium overlap with the potential range of this species under the recent moderate climate change predicted by the model using climate data of the last two decades only (see figure 1d; cf. also Banuelos et al. 2004 for Denmark). However, the lower kappa value resulting from the comparison of the expected and the actual shift in distribution of the last two decades suggests that Ilex, probably for chorological reasons, has not yet fully occupied its climatically determined potential new range predicted by the model (cf. also Svenning & Skov 2004).
Northern range limitation by climatic parameters has not only been reported from plant species (e.g. Woodward 1987; Graves & Reavey 1996; Woodward et al. 2004; see also Walther 2004), it is also of importance for other taxa such as insects (e.g. Hill et al. 1999; Addo-Bediako et al. 2000; Thomas et al. 2001), birds (e.g. Thomas & Lennon 1999; Forsman & Monkkonen 2003; Brommer 2004) and mammals (e.g. Humphries et al. 2002), and thus is an important ecological feature in temperate regions. The present resurvey of the distribution of a climatically limited species and the reanalysis of closely related local climatic measurements revealed a coherent shift in both species' distribution and climate with the same spatio-temporal resolution. This gives high confidence to the conclusion that the changing climate is the responsible driver for the observed northward range expansion. Consequently, this reported species' shift is more than just an ecological ‘fingerprint’, it is an ecological ‘footprint’ of recent climate change.
Acknowledgments
Floristic data were kindly provided by T. Tyler (Projekt Skånes Flora, Sweden), L. Jonsell (Projekt Upplands Flora, Sweden), S. Svensson (Municipality of Gotland, Sweden), C.-A. Hæggström (Department of Ecology, University of Helsinki, Finland), G. Weimarck (Göteborg, Sweden), P.H. Salvesen (The Norwegian Arboretum, University of Bergen, Norway), A. Skogen (University of Bergen, Norway), J. Kollmann & J.M. Bañuelos (Department of Ecology, Royal Veterinary and Agricultural University of Denmark, Copenhagen, Denmark), J. Lawesson (Institute of Biological Sciences, University of Aarhus, Denmark), G. Stachowiak (Salzwedel, Germany), D. Frank (Halle, Germany), R. May (German Federal Agency for Nature Conservation, Bonn, Germany), W. Härdtle (Institute of Ecology and Environmental Chemistry, University of Lüneburg, Germany), M. Diekmann (Vegetation Ecology and Conservation Biology, University of Bremen, Germany); and climatic data by K. Boqvist (Swedish Meteorological and Hydrological Institute, Norrköping, Sweden), W. Ernst (Deutscher Wetterdienst, Offenbach, Germany), L. Ågren (Ecological Research Station of Uppsala University, Färjestaden, Sweden), S. Kristiansen (Meteorological Institute, Oslo, Norway), K.M. Erhardi (Danish Meteorological Institute, Copenhagen, Denmark), T. Mitchell (Tyndall Centre for Climate Research, Norwich, United Kingdom), T. Carter & S. Fronzek (Finnish Environment Institute, Helsinki, Finland). We also thank G. Marion and S. Bierman (BIOSS, Edinburgh, United Kingdom) for statistical advice and two anonymous referees for helpful comments on earlier versions of the manuscript. Funding by the following agencies is kindly acknowledged: German Research Foundation (Project WA 1523/5-1), and the EC (within the FP 6 Integrated Project ‘ALARM’; GOCE-CT-2003-506675).
Supplementary Material
References
- Addo-Bediako A, Chown S.L, Gaston K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. 10.1098/rspb.2000.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andergassen S, Bauer H. Frost hardiness in the juvenile and adult life phase of ivy (Hedera helix L.) Plant Ecol. 2002;161:207–213. 10.1023/A:1020365422879 [Google Scholar]
- Banuelos M.J, Kollmann J, Hartvig P, Quevedo M. Modelling the distribution of Ilex aquifolium at the north-eastern edge of its geographical range. Nord. J. Bot. 2004;23:129–142. [Google Scholar]
- Begon M, Harper J.L, Townsend C.R. 3rd edn. Blackwell; Oxford: 1996. Ecology—individuals, populations and communities. [Google Scholar]
- Berger, S. 2003 Ilex aquifolium—Bioindikator für Klimaveränderung? MSc thesis, Institute of Geobotany, University of Hannover.
- Brommer J.E. The range margins of northern birds shift polewards. Ann. Zool. Fenn. 2004;41:391–397. [Google Scholar]
- Callauch, R. 1983 Untersuchungen zur Biologie und Vergesellschaftung der Stechpalme (Ilex aquifolium). Dissertation, University of Kassel.
- Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960;20:37–46. [Google Scholar]
- Crozier L. Winter warming facilitates range expansion: cold tolerance of the butterfly Atalopedes campestris. Oecologia. 2003;135:648–656. doi: 10.1007/s00442-003-1219-2. 10.1007/s00442-003-1219-2 [DOI] [PubMed] [Google Scholar]
- Enquist F. Sambandet mellan klimat och växtgränser. Geol. Fören. Förhandl. 1924;46:202–213. [Google Scholar]
- Fielding A.H, Bell J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997;24:38–49. [Google Scholar]
- Fischer, W. 1965 Über Wassergehalt und Standortsverhältnisse bei einigen wintergrünen atlantischen Pflanzenarten an der Ostgrenze ihrer Verbreitung in NW-Brandenburg. Diss. Dtsch. Akad. Landwirtschaftswiss., Berlin.
- Folland C.K, Karl T.R. Observed climate variability and change. In: Houghton J.T, Ding Y, Griggs D.J, Noguer M, van der Linden P.J, Dai X, Maskell K, Johnson C.A, editors. Climate change 2001: the scientific basis. Cambridge University Press; Cambridge: 2001. pp. 99–181. [Google Scholar]
- Forsman J.T, Monkkonen M. The role of climate in limiting European resident bird populations. J. Biogeogr. 2003;30:55–70. 10.1046/j.1365-2699.2003.00812.x [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. Climate effects on mountain plants. Nature. 1994;369:448. doi: 10.1038/369448a0. 10.1038/369448a0 [DOI] [PubMed] [Google Scholar]
- Graves J, Reavey D. Longman; Essex: 1996. Global environmental change—plants, animals and communities. [Google Scholar]
- Hill J.K, Thomas C.D, Huntley B. Climate and habitat availability determine twentieth century changes in a butterfly's range margins. Proc. R. Soc. B. 1999;266:1197–1206. 10.1098/rspb.1999.0763 [Google Scholar]
- Hill J.K, Thomas C.D, Fox R, Telfer M.G, Willis S.G, Asher J, Huntley B. Responses of butterflies to twentieth century climate warming: implications for future ranges. Proc. R. Soc. B. 2002;269:2163–2171. doi: 10.1098/rspb.2002.2134. 10.1098/rspb.2002.2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmboe J. Kristtornen i Norge. Bergens Museums Aarbok. 1913;7:1–91. [Google Scholar]
- Hughes L. Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 2000;15:56–61. doi: 10.1016/s0169-5347(99)01764-4. 10.1016/S0169-5347(99)01764-4 [DOI] [PubMed] [Google Scholar]
- Humphries M.M, Thomas D.W, Speakman J.R. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature. 2002;418:313–316. doi: 10.1038/nature00828. 10.1038/nature00828 [DOI] [PubMed] [Google Scholar]
- Iversen J. Viscum, Hedera and Ilex as climatic indicators. A contribution to the study of past-glacial temperature climate. Geol. Fören. Förhandl. 1944;66:463–483. [Google Scholar]
- Jensen M.N. Consensus on ecological impacts remains elusive. Science. 2003;299:38. doi: 10.1126/science.299.5603.38. 10.1126/science.299.5603.38 [DOI] [PubMed] [Google Scholar]
- Landis J.R, Koch G.C. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Larcher W. 4th edn. Springer; Berlin, Heidelberg: 2003. Physiological plant ecology. [Google Scholar]
- Leemans R. Biodiversity and global change. In: Gaston K.J, editor. Biodiversity—a biology of numbers and difference. Blackwell; Oxford: 1996. pp. 367–387. [Google Scholar]
- Loesener T. Über die Aquifoliaceen, besonders über Ilex. Mitt. Dtsch. Dendrol. Ges. 1919;28:1–66. [Google Scholar]
- Meusel H, Jäger E, Weinert E. Fischer; Jena: 1965. Vergleichende Chorologie der zentraleuropäischen Flora. [Google Scholar]
- Mitchell T.D, Carter T.R, Jones P.D, Hulme M, New M. Tyndall Centre Working Papers 55, July 2004. 2004. A comprehensive set of high-resolution grids of monthly climate for Europe and the globe: the observed record (1901–2000) and 16 scenarios (2001–2100) (Available at: http://www.tyndall.ac.uk/publications/working_papers/wp55.pdf) [Google Scholar]
- New M, Hulme M, Jones P.D. Representing twentieth century space–time variability. Part I. Development of a 1961–90 mean monthly terrestrial climatology. J. Clim. 1999;12:829–856. [Google Scholar]
- New M, Hulme M, Jones P.D. Representing twentieth century space–time variability. Part 2. Development of 1901–96 monthly grids of terrestrial surface climate. J. Clim. 2000;13:2217–2238. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. 10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. 10.1038/21181 [Google Scholar]
- Peterken G.F, Lloyd P.S. Biological flora of the British Isles. J. Ecol. 1967;55:841–858. [Google Scholar]
- Pott R. Die nacheiszeitliche Ausbreitung und heutige pflanzensoziologische Stellung von Ilex aquifolium L. Tuexenia. 1990;10:497–512. [Google Scholar]
- Prentice I.C, Cramer W, Harrison S, Leemans R, Monserud R.A, Solomon A.M. A global biome model based on plant physiology and dominance, soil properties and climate. J. Biogeogr. 1992;19:117–134. [Google Scholar]
- Root T.L, Price J.T, Hall K.R, Schneider S.H, Rosenzweig C, Pounds J.A. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. 10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Runge F. Die Standorte der Hülse (Ilex aquifolium L.) in der Umgebung des Naturschutzgebietes “Heiliges Meer” bei Hopsten (Westf.) Natur und Heimat. 1950;10:65–77. [Google Scholar]
- Sakai A. Freezing resistance of ornamental trees and shrubs. J. Am. Soc. Hortic. Sci. 1982;107:572–581. [Google Scholar]
- Sitte P, Weiler E.W, Kadereit J.W, Bresinsky A, Körner C. 35th edn. Spektrum Akademischer Verlag; Heidelberg, Berlin: 2002. Strasburger—Lehrbuch der Botanik. [Google Scholar]
- Skov F, Svenning J.C. Potential impact of climatic change on the distribution of forest herbs in Europe. Ecography. 2004;27:366–380. 10.1111/j.0906-7590.2004.03823.x [Google Scholar]
- Svenning J.-C, Skov F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004;7:565–573. 10.1111/j.1461-0248.2004.00614.x [Google Scholar]
- Sykes M.T, Prentice I.C, Cramer W. A bioclimatic model for the potential distributions of north European tree species under present and future climates. J. Biogeogr. 1996;23:203–233. [Google Scholar]
- Thomas C.D, Lennon J.J. Birds extend their ranges northwards. Nature. 1999;399:213. 10.1038/20335 [Google Scholar]
- Thomas C.D, Bodsworth E.J, Wilson R.J, Simmons A.D, Davies Z.G, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. 10.1038/35079066 [DOI] [PubMed] [Google Scholar]
- Walter H, Straka H. 2nd edn. Ulmer; Stuttgart: 1970. Arealkunde—Floristisch-historische Geobotanik. [Google Scholar]
- Walther G.-R. Plants in a warmer world. Perspect. Plant Ecol. Evol. Syst. 2004;6:169–185. 10.1078/1433-8319-00076 [Google Scholar]
- Walther G.-R, Burga C.A, Edwards P.J, editors. “Fingerprints” of climate change—adapted behaviour and shifting species ranges. Kluwer Academic/Plenum Publishers; New York: 2001. [Google Scholar]
- Walther G.-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. 10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Woodward F.I. Cambridge University Press; Cambridge: 1987. Climate and plant distribution. [Google Scholar]
- Woodward F.I, Lomas M.R, Kelly C.K. Global climate and the distribution of plant biomes. Phil. Trans. R. Soc. B. 2004;359:1465–1476. doi: 10.1098/rstb.2004.1525. 10.1098/rstb.2004.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.