Abstract
The impact of rare but positive events on the design of organisms has been largely ignored, probably due to the paucity of recordings of such events and to the difficulty of estimating their impact on lifetime reproductive success. In this respect, we investigated the size of spider webs in relation to rare but large prey catches. First, we collected field data on a short time-scale using the common orb-weaving spider Zygiella x-notata to determine the distribution of the size of prey caught and to quantify the relationship between web size and daily capture success. Second, we explored, with an energetic model, the consequences of an increase in web size on spider fitness. Our results showed that (i) the great majority of prey caught are quite small (body length less than 2 mm) while large prey (length greater than 10 mm) are rare, (ii) spiders cannot survive or produce eggs without catching these large but rare prey and (iii) increasing web size increases the daily number of prey caught and thus long-term survival and fecundity. Spider webs seem, therefore, designed for making the best of the rare but crucial event of catching large prey.
Keywords: orb-web spider, web efficiency, web size, web evolution, rare events, large prey
1. Introduction
Rare but crucial events may shape traits in many organisms. Most studies that have been carried out have examined rare events of negative impact. For example, seashore organisms adapt their size, body shape and their attachment forces to rare waves of unusually large amplitude (Gaines & Denny 1993; Denny 1995). The whole concept of a ‘safety factor’ in plant and animal biomechanics refers to these rare events, which can have devastating effects (Friedland & Denny 1995; Niklas 1997). In contrast, the impact of rare but positive events on the design of organisms has been far less studied, probably because these events are only rarely quantified in the field and because their consequences on organism fitness are less evident and open to exploration than for negative events (e.g. predation).
In this context, we investigated an animal construction, the spider web. Spider webs are fascinating animal constructions due to their complex, often artistic geometry and astonishing material properties (Shear 1986; Foelix 1996; Blackledge et al. 2003; Craig 2003). The remarkable silk properties are the focus of many biomechanical studies whose aim is to detangle the effects of chemical composition and the physical properties of the numerous silk components on the silk properties (for a review see Vollrath & Knight 2001). The architectures of orb webs are products of complex behaviours and are subject to strong selective pressure. Specific architectures seem to be the result of convergent evolution, because species using the same web architectures evolved independently of one another (Blackledge & Gillespie 2004). The prime function of the orb web is to catch prey and, thus, the nature of catches could play an important role in the evolution of web architectures. However, the relationship between web architecture (particularly web size) and web efficiency in the field has been addressed only in few studies, and remains unclear (Uetz et al. 1978; Rypstra 1982; Higgins 1990; Opell 1990; Sherman 1994; Heiling 1999; Blackledge & Wenzel 2001; Watanabe 2001). Different field investigations of orb-weaving spiders show that the great majority of prey caught are small (Gillespie & Caraco 1987; Ridwan 1993; Sherman 1994; Watanabe 2001). However, large prey, even if scarce, could strongly influence the spider's energetic gain, reproductive success and consequently foraging strategy. So, here, we hypothesize that web size is greatly influenced by highly positive though rare events, catches of large prey.
Orb-weaving spiders often completely replace their web every day, thereby modifying web size (Sherman 1994; Venner et al. 2000). Web building is very costly in energy (Peakall & Witt 1976; Venner et al. 2003) and increasing web investment is often considered to increase capture efficiency. The choice of web size should, therefore, strongly influence the spider's lifetime reproductive success. There is, however, no study which simultaneously tackles the link between web architecture and capture success, and which explores the actual variability in web size and its consequences on fitness.
The first aim of this work is therefore (i) to determine, in the field, the distribution of the size of prey caught; (ii) to estimate the relative contribution of each prey size in spider energy intake and (iii) to quantify the relationship between web size and daily capture success.
Assessing the impact of potentially rare and large prey on web size requires us to convert daily, short term, benefits into spider survival and reproductive gains. Our second aim was therefore to test how very large prey, together with an increase in web size, could affect spiders' fitness. To do that, we built an energetic and stochastic model in which both gains and losses were a function of web size. This model was based on numerous biological data resulting from field and laboratory experiments.
2. Methods
(a) Field observations
The field study was conducted in France (Nancy, Meurthe and Moselle) on adult female Zygiella x-notata, an orb-weaving spider which occurs commonly on the outside of buildings. Thus, we focused on a population that naturally settled on a building (2.3 m×52 m). This population was surveyed every day over 2 years, with individual identification by means of tagging. Spiders did entirely develop on our study site, from hatching and emergence from egg sacs, to growth and maturation, mating and egg-laying (Venner 2002).
Over 6 non-consecutive days, we selected spiders that had built their webs on the same morning (table 1). We recorded the geometry of each web in a non-invasive and non-disturbing way to calculate the capture thread length (CTL), the spiral thread coated with glue (Venner et al. 2001). CTL is used as a surrogate for web size (see below) and precisely reflects the spider's energetic investment in building (Venner et al. 2003). We quantified the natural prey capture success (number and size of prey) in relation to CTL without removing spiders from their web. Otherwise, prey capture success could be strongly underestimated as the large prey, usually subdued by the resident spider, could then escape.
Table 1.
Dates of the field study, number of females studied daily and mean capture thread length (CTL).
| day 1 | day 2 | day 3 | day 4 | day 5 | day 6 | |
|---|---|---|---|---|---|---|
| date (1999) | September 8 | September 10 | September 15 | September 21 | October 8 | October 27 |
| number of females | 38 | 46 | 50 | 32 | 33 | 28 |
| CTL (mean±s.e.) | 6.94±0.49 | 7.11±0.34 | 7.13±0.42 | 8.21±0.38 | 7.69±0.50 | 7.05±0.63 |
CTL was homogeneous throughout the six non-consecutive days; ANOVA: F5,221=1.06, p=0.38. We used 73 females to conduct the field study. The observations were carried out throughout the whole period of adult female development implemented in the model.
A previous study in an adjacent population showed that prey were rarely caught at night (Ridwan 1993). Transect sampling was therefore performed every 20 min from 07.00 to 20.00, and prey caught in each web recorded (number, size of prey caught). We never removed captured prey from the webs because this could damage webs, disturb spiders and affect following captures. Prey body length was estimated with a ruler brought as close as possible to the prey. Prey length was recorded to the nearest millimetre for values up to 10 mm. Larger prey were measured to the nearest 5 mm due to the curvature of their bodies.
We determined both prey-size and gain distributions. Gain distribution corresponded to the relative contribution of each prey-size class to energy intake. It was estimated using the prey dry weight calculated from the central value of the prey-size class presented in figure 1 (see table 2 for the correspondence between prey-size and prey weight) and the probability of catching such prey (determined from the observed prey-size distribution). The relationship between the web's CTL and the number of prey caught (NPC) was assessed with generalized linear models for dependent data, a method which takes into account multiple sampling of the same individual (generalized estimation equation models; GEE). The relationship between the web's CTL and prey size was assessed by linear regression. Moreover, we determined the relationship between expected daily gain and CTL by multiplying the mean number of daily prey caught in a given web by the weight of the prey. The former was estimated using a negative binomial distribution and the latter using the observed prey-size distribution and prey weight for each size class. First we tested this relationship considering all prey size. Then we excluded the largest prey (body length greater than 10 mm) from the analysis to estimate their impact on daily gain.
Figure 1.
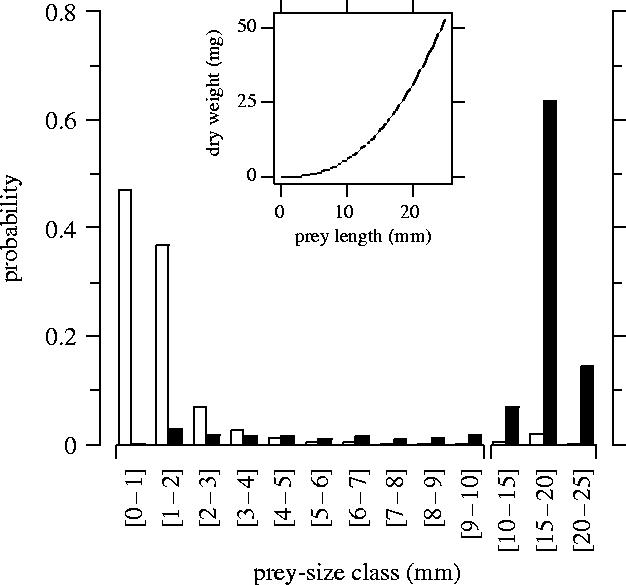
Observed prey-size distribution (n=376, open bars) and gain distribution (filled bars). Gain distribution corresponds to the relative contribution of each prey-size class in energy intake. The relationship between prey length and dry weight is given by Schoener (1979).
Table 2.
Parameters used in the stochastic model.
| Parameter symbol | parameter | value |
|---|---|---|
| SW0 | initial spider weight (weight at last moult) | 33 mg (Venner 2002) |
| SWStarv | spider weight at starvation time | 25 mga |
| SWegg-laying | spider weight just before egg-laying | 80 mga |
| SWD | spider weight on day D | variable |
| αm | daily basal metabolic expenditure | 0.4 mg (Venner et al. 2003) |
| MaxNP | maximum number of prey caught daily | 15 |
| x | number of prey caught daily | random variable |
| 0≤x≤MaxNP | ||
| negative binomial distribution | ||
| Lgn | length of the nth prey caught on a given dayb | random variable |
| 0.5≤Lgn≤22.5 | ||
| observed distributionc | ||
| DWn | dry weight of the nth prey caught on a given day | 0.022×(Lgn2.4 (Schoener 1979) |
| AR | prey assimilation rate | 0.7844a |
| CWG | coefficient of weight gain conversion (dry weight into fresh weight) | 2.487a |
| MaxGain | maximum daily gain | 23.2 mga |
See Electronic Appendix for details.
Lgn varies in 1 mm steps from 0.5 to 9.5 mm and in 5 mm steps from 12.5 to 22.5 mm, in accordance with observed prey-size distribution.
Poisson and negative binomial distributions are inadequate to model the prey-size distribution (χ2-test; p<0.0001 in both cases).
Factors other than CTL were also tested in our analyses. However, web area and CTL were found to be highly and positively correlated (n=227, R2=0.74, p<0.0001). Mesh size was not correlated with CTL (n=227, R2=0.0002, p=0.85). Several studies suggest that prey capture success is dependent on mesh size (Ap Rhisiart & Vollrath 1994; Sandoval 1994; Schneider & Vollrath 1998). However, in our field study, daily capture success was independent of mesh size (NPC: n=227; R2=0.002, p=0.54; prey size: n=376, R2=0.006, p=0.07). Thus, we only present the results obtained with CTL.
(b) General structure of the energetic model
A stochastic model was chosen to account for the random nature of catches and prey weights. This model was based on biological data resulting from laboratory experiments (table 2; see below for details), and published evidence on metabolism related to web construction (Venner et al. 2003). In this model, the weight of an animal fluctuates daily as a function of gains and expenditures. Expenditure is the sum of losses due to basal metabolism (αm) and web building activities. In the model, spiders had to replace their web with a new one of the same size each day, from their last moult until either death or egg-laying, whichever came first. The losses due to building increase with increasing web size and body weight. The daily expenditure (spider weight loss; SWL) was adapted from Venner et al. (2003),
| 2.1 |
where SW and CTL correspond to the spider weight and the capture thread length of the web built on a given day, respectively.
The daily gains (fresh weight intake) are a function of web size and were first expressed as the NPC using a negative binomial distribution. Second, the length and dry weight of each prey were determined from the observed prey-size distribution. Dry weight was multiplied by two coefficients: one converting dry weight into fresh weight, and the other being the assimilation rate. To model the spider's highest digestive ability, we quantified the maximum daily gain in the laboratory. The model ran for 70 days. This duration was based on the observed median date of adult emergence (early September) and the first freezing days observed in the wild, after which the spiders did not renew their web regularly. The initial state of a spider refers to its body weight just after its adult moult. A spider dies of starvation if its body weight attains a critical low level. Spiders stop growing and start laying eggs if their weight attains a critical upper level.
The consequences of the capture of rare but large prey on a spider's fitness were estimated by means of spider survival and reproduction. In a first set of simulations, we used the observed distribution of prey size, taking into account all prey-size classes (‘all prey’ set) while in a second and third set of simulations we excluded, respectively, the largest prey (body length larger than 10 mm; ‘small prey’ set) and the smallest prey (body length smaller than 10 mm; ‘large prey’ set). Moreover, we determined, from the all prey set, the averaged number of large prey caught for spiders that died from starvation and for those that could reach egg-laying.
The all prey set was also used to assess the consequences of increasing web size on fitness. We compared the survival rate, the proportion of egg-laying spiders and the delay before egg-laying for spiders building webs of various sizes (from CTL=1 to 14 m), assuming that the spiders kept a constant web size throughout their growth. Ten thousand simulations were made for a given web size in each set. Estimation of the variables for the model was based on a large number of biological data resulting from laboratory experiments (table 2 and Electronic Appendix).
3. Results
(a) Field data
Adult female Z. x-notata caught on average fewer than two prey per day in the field (mean±s.e.=1.7±0.1 prey; n=227). Most prey were quite small; 84% were smaller than 2 mm (figure 1). Small prey represent a high proportion of a spider's diet, but a minor fraction of a spider's energetic gain. Prey larger than 10 mm were rare, accounting for less than 3% of captures. However, these large but rare prey, mainly crane flies, represented most of a spider's gain, because of the nonlinear relationship between body size and dry weight of prey.
The gain from one large prey is equivalent to that of the smaller prey caught on 60 days (respectively, 23.2 and 0.38 mg per day on average). Large prey must therefore play a crucial role in the spider's development.
Prey size did not vary significantly with web size (linear regression: n=376 prey, F1,375=3.32, R2=0.006, p>0.07). In contrast, the mean and variance of the NPC per day increased with increasing web size (figure 2; n=227 webs; GEE with estimated negative binomial variance: , negative binomial dispersion parameter=1.844, t226=2.739, p<0.01). Web size had a major impact on the expected daily gain, which doubled from small to large webs (figure 3). Expected daily weight gain strongly increased (by a factor 6.5) due to the rare but large prey.
Figure 2.
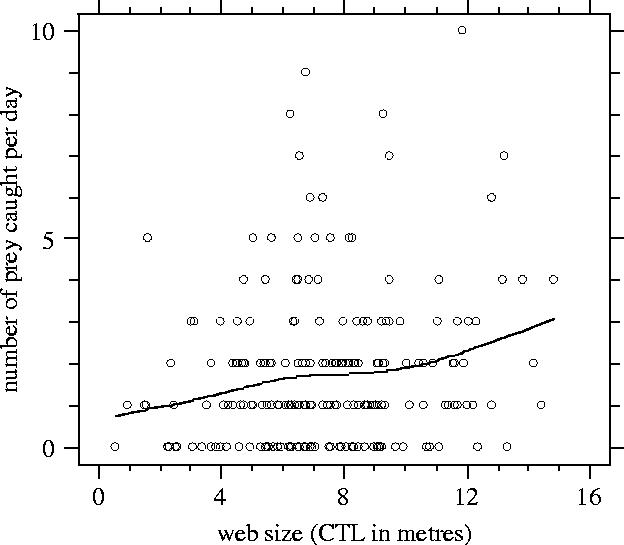
Number of prey caught as a function of web size (capture thread length or CTL). A smoothing spline was applied with d.f.=4.
Figure 3.
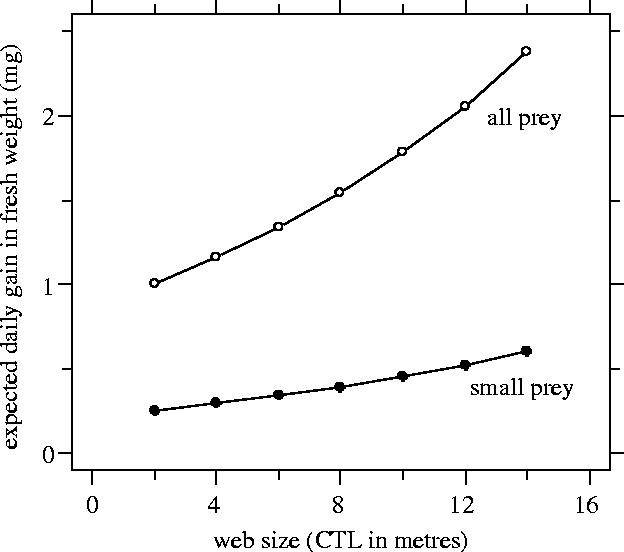
Expected daily gain as a function of web size (capture thread length or CTL). Expected gain was determined considering either all prey (open circles) or only small prey—less than 10 mm (filled circles).
(b) Simulations results
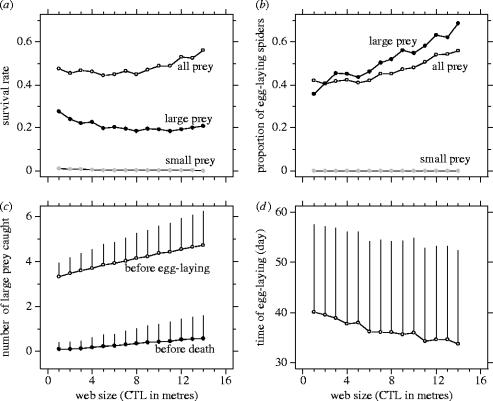
Building webs of a large size is an advantage only if the short-term benefits translate into benefits in terms of survival and reproduction. Simulations revealed that adult female spiders' survival rate, as well as their success of egg-laying, was almost totally dependent on catching large and rare prey (figure 4a,b). Survival rate was insignificant without large prey, while it was greater than 0.5 considering large prey (χ12=109 555, p<0.0001) and no spiders could lay eggs without the large prey (figure 4b). However, survival rate was lower considering only the large prey than considering all prey (χ12=22 875, p<0.0001; figure 4a). Thus, prey smaller than 10 mm allowed the spider to increase their survival rate until they caught large and rare prey.
Figure 4.
Impact of rare and large prey and web size (capture thread length or CTL) on life history traits: results of simulations. (a) Survival rate was determined considering either all prey (open circles) or only small prey—less than or equal to 10 mm (grey circles)—and only large prey—greater than 10 mm (filled circles). (b) The proportion of egg-laying spiders increased with web size due to large prey. (c) The number of large prey caught (mean+s.d.) by egg-laying spiders (open circles) and by spiders that died from starvation (filled circles). (d) Time to the first oviposition (mean+s.d.) decreased with web size considering all prey.
The large prey are crucial for both surviving and producing eggs. A single large prey, supplemented by a normal ration of small prey, enables a spider to live for the entire adult growth period (figure 4c). Between three and four large prey, supplemented by the small ones, enable some females to lay eggs, depending on web size.
Spiders building large webs have to increase the number of large prey caught to lay eggs (ANOVA: F13,31 458=343.4, p<0.0001; figure 4c). However, building large webs, thereby increasing the NPC, offers clear advantages in terms of survival rate, probability of laying eggs and maturation time (figure 4a: χ132=642.8, p<0.0001; figure 4b: χ132=765.4, p<0.0001; figure 4d: ANOVA F13,31 459=33.9, p<0.0001). The increase by 25% of survival rate with increasing web size depended on small as well as large prey (figure 4a). In contrast, the increase by 53% of probability of laying eggs with increasing web size only depended on large prey (figure 4b).
4. Discussion
Adult female spiders Z. x-notata caught on average less than two prey per day in the field. Most prey are quite small and large prey are rarely caught, in accordance with several field studies on orb-weaving spiders (Gillespie & Caraco 1987; Ridwan 1993; Sherman 1994; Watanabe 2001). These large preys play a crucial role in spider development, even if they are only caught on average once every 20 days during female adult growth (i.e. over approximately 70 days). Around 60 days of the usual food portion of small prey are needed to make up the same amount of food contained in a single large prey. Moreover, explorations with the stochastic model show that spiders cannot survive or produce eggs without catching large but rare prey. Consequently, foraging strategy in spiders could be designed for extreme but positive events, the large prey captures. When we added small prey to the large prey sample, the survival rate of spiders more than doubled (see figure 4a): this suggests that more frequent but smaller prey could play a complementary role in keeping the spider alive until it catches these large and rare prey.
Our work suggests that the prime function of webs is to capture rare and large insects. Increasing web size increases energetic expenditure, and thus constrains spiders to increase the number of large prey caught to lay eggs. However, building large webs is worthwhile, even considering the extra costs: the results of simulations show clear advantages in building large webs in terms of increased survival rate, higher probability of egg-laying and shorter maturation time to egg-laying. In such circumstances, why do spiders not always build large webs? First, the availability of particular nutrients may represent a strong constraint on building large webs (Eberhard 1988), but we lacked quantitative data to implement this observation. Second, building activity is increasingly costly with increasing spider body weight (Venner et al. 2003). Considering this constraint, the optimal strategy for bigger spiders could consist of reducing their web size. Third, Sherman (1994) suggests that spiders may switch their resource allocation from foraging towards reproduction before egg-laying, and may reduce their web size accordingly. Finally, predation can play an important role in the evolution of web architecture (Blackledge et al. 2003) and web size could be the result of an energy-predation trade-off: increasing web size increases web-building duration (Venner 2002) and potential exposure to predators. Thus, optimal web size could be smaller than the size predicted considering only losses and gains in energy.
Our work clearly shows not only that large prey are highly valuable at the time of capture, but that they are plainly necessary. Without them, death is likely to occur and no reproduction can be guaranteed. Building larger webs increases the number of prey caught, and thus the likelihood of catching rare but large prey. Spider webs, along with ant-lion pits (Lucas 1986; Griffiths 1996), are one of the most fascinating animal constructions. Such designs are subjected to evolutionary pressure from rare events of utmost importance. Rare and positive events could play a crucial role in the evolution of many organism traits. This phenomenon could be largely underestimated in ecology due to the paucity of recordings of such events in the field and the difficulty of estimating their impact on lifetime reproductive success.
Acknowledgments
We thank O. Dangles, E. Desouhant and F. Menu for their critical reading of the manuscript. We thank members of the laboratory ‘expression et évolution des comportements’ of Nancy University for their support. This work was supported by GDR CNRS 2155 ‘E´cologie Comportementale’.
Supplementary Material
References
- Ap Rhisiart A, Vollrath F. Design features of the orb web of the spider, Araneus diadematus. Behav. Ecol. 1994;5:280–287. [Google Scholar]
- Blackledge T.A, Gillespie R.G. Convergent evolution of behavior in an adaptive radiation of Hawaiian web-building spiders. Proc. Natl Acad. Sci. USA. 2004;101:16 228–16 233. doi: 10.1073/pnas.0407395101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge T.A, Wenzel J.W. State-determinate foraging decisions and web architecture in the spider Dictyna volucripes (Araneae Dictynidae) Ethol. Ecol. Evol. 2001;13:105–113. [Google Scholar]
- Blackledge T.A, Coddington J.A, Gillespie R.G. Are three-dimensional spider webs defensive adaptations? Ecol. Lett. 2003;6:13–18. [Google Scholar]
- Craig C.L. Oxford University Press; New York: 2003. Spider webs and silk. [Google Scholar]
- Denny M.W. Predicting physical disturbance. Mechanistic approaches to the study of survivorship on wave-swept shores. Ecol. Monogr. 1995;65:371–418. [Google Scholar]
- Eberhard W.G. Behavioral flexibiliy in orb web construction: effects of supplies in different silk glands and spider size and weight. J. Arachnol. 1988;16:295–302. [Google Scholar]
- Foelix R. Oxford University Press; New York: 1996. Biology of spiders. [Google Scholar]
- Friedland M.T, Denny M.D. Surviving hydrodynamic forces in a wave-swept environment: Consequences of morphology in the feather boa kelp, Egregia menziesii (Turner) J. Exp. Mar. Biol. Ecol. 1995;190:109–133. [Google Scholar]
- Gaines S.D, Denny M.W. The largest, smallest, highest, lowest, longest, and shortest: extremes in ecology. Ecology. 1993;74:1677–1692. [Google Scholar]
- Gillespie R.G, Caraco T. Risk-sensitive foraging strategies of two spider populations. Ecology. 1987;68:887–899. [Google Scholar]
- Griffiths D. Pit construction by ant-lion larvae: a cost-benefit analysis. J. Anim. Behav. 1996;55:39–57. [Google Scholar]
- Heiling A.M. Why do nocturnal orb-web spiders (Araneidae) search for light? Behav. Ecol. Sociobiol. 1999;46:43–49. [Google Scholar]
- Higgins L.E. Variation in foraging investment during the intermolt interval and before egg-laying in the spider Nephila clavipes (Araneae: Araneidae) J. Insect Behav. 1990;3:773–783. [Google Scholar]
- Lucas J. The biophysics of pit construction by ant-lion larvae (Myrmeleon, Neuroptera) Anim. Behav. 1986;30:651–664. [Google Scholar]
- Niklas K.J. Mechanical properties of Black Locust (Robinia pseudoacacia L.) Wood. size- and age-dependent variations in sap- and heartwood. Ann. Bot. 1997;79:265–272. [Google Scholar]
- Opell B.D. Material investment and prey capture potential of reduced spider webs. Behav. Ecol. Sociobiol. 1990;26:375–381. [Google Scholar]
- Peakall D.B, Witt P.N. The energy budget of an orb-weaving spider. Comp. Biochem. Physiol. A. 1976;54:187–190. doi: 10.1016/s0300-9629(76)80094-1. [DOI] [PubMed] [Google Scholar]
- Ridwan, A. 1993 Réponses comportementales aux variations de facteurs faunistiques du milieu chez une araignée à toile géométrique Zygiella x-notata (Clerck) (Araneae, Araneidae). Doctorate thesis, Nancy (Fr), University of Nancy.
- Rypstra A.L. Building a better insect trap; an experimental investigation of prey capture in a variety of spider webs. Oecologia. 1982;52:31–36. doi: 10.1007/BF00349008. [DOI] [PubMed] [Google Scholar]
- Sandoval C.P. Plasticity in web design in the spider Parawixia bistriata: a response to variable prey type. Funct. Ecol. 1994;8:701–707. [Google Scholar]
- Schneider J.T, Vollrath F. The effect of prey type on the geometry of the capture web of Araneus diadematus. Naturwissenschaften. 1998;85:381–394. [Google Scholar]
- Schoener T.W. Length–weight regressions in tropical and temperature forest-understory insects. Ann. Entomol. Soc. Am. 1979;73:106–109. [Google Scholar]
- Shear W.A. Stanford University Press; Stanford: 1986. Spiders: webs, behavior and evolution. [Google Scholar]
- Sherman P.M. The orb-web: an energetic and behavioural estimator of spider's dynamic foraging and reproductive strategies. Anim. Behav. 1994;48:19–34. [Google Scholar]
- Uetz G.W, Johnson A.D, Schemske D.W. Web placement, web structure, and prey capture in orb-weaving spiders. Bull. Br. Arachnol. Soc. 1978;4:141–148. [Google Scholar]
- Venner, S. 2002 Stratégies comportementales et modèle d'optimisation dynamique à horizon non fini: succession des constructions de toiles chez une araignée orbitèle Zygiella x-notata (Clerck). Doctorate thesis, Nancy (Fr), University of Nancy.
- Venner S, Pasquet A, Leborgne R. Web-building behaviour in an orb-weaving spider Zygiella x-notata: influence of experience. Anim. Behav. 2000;59:603–611. doi: 10.1006/anbe.1999.1327. [DOI] [PubMed] [Google Scholar]
- Venner S, Thevenard L, Pasquet A, Leborgne R. Estimation of the web's capture thread length in orb-weaving spiders: determining the most efficient formula. Ann. Entomol. Soc. Am. 2001;94:490–496. [Google Scholar]
- Venner S, Bel-Venner M.C, Pasquet A, Leborgne R. Body mass-dependent cost of web-building behaviour in an orb-weaving spider Zygiella x-notata. Naturwissenschaften. 2003;90:269–272. doi: 10.1007/s00114-003-0420-9. [DOI] [PubMed] [Google Scholar]
- Vollrath F, Knight D.P. Liquid crystalline spinning of spider silk. Nature. 2001;410:541–548. doi: 10.1038/35069000. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Effects of web design on the prey capture efficiency of the uloborid spider Octonoba sybotides under abundant and limited prey conditions. Zool. Sci. 2001;18:585–590. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.