Abstract
Many studies have attempted to explain the evolution of cooperation, yet little attention has been paid to what factors control the amount or kind of cooperation performed. Kin selection theory suggests that more cooperation, or help, should be given by relatives. However, recent theory suggests that under specific ecological and demographic conditions, unrelated individuals must ‘pay to stay’ in the group and therefore may help more. We tested these contrasting predictions using the cooperatively breeding fish, Neolamprologus pulcher, and found that the degree of work effort by helpers depended on which helping behaviours were considered and on their level of relatedness to the breeding male or female. In the field, helpers unrelated to the breeding male performed more territory defence, while helpers unrelated to the breeding female contributed less to territory defence. In the laboratory, unrelated group members helped more. Our work demonstrates that a number of factors in addition to kinship shape cooperative investment patterns.
Keywords: cooperative breeding, helping behaviour, microsatellites, cichlid fishes, Neolamprologus pulcher, Lake Tanganyika
1. Introduction
For nearly 40 years, the evolution of helping behaviour has largely been attributed to indirect fitness benefits accrued from assisting kin (Hamilton 1964; Griffin & West 2003). Kin selection theory predicts that helpers will be related to the breeders that they are assisting and increased helping effort should be directed towards more closely related individuals. Therefore, altruism that has evolved via kin selection should result in a positive correlation between relatedness and helping effort (due to proportional investment in relatives), or in an ‘all or nothing’ pattern where investment is preferentially directed towards the most closely related relative (Altmann 1979; Weigel 1981; Schulman & Rubenstein 1983). Although some studies on cooperative breeders support these predictions (Clarke 1984; Emlen & Wrege 1988; Mumme 1992; Conrad et al. 1998), several recent investigations have cast doubt on the relative importance and ubiquitous nature of kin selection by showing that helpers are often not relatives (Whittingham et al. 1997; Queller et al. 2000) and that the degree of relatedness is not necessarily correlated with the amount of assistance provided (Wright et al. 1999; Legge 2000; Clutton-Brock et al. 2001). In general, it now appears that many observations of cooperation cannot be explained by kin selection alone.
Recent theoretical work by Kokko et al. (2002) suggests that direct benefits may provide an alternative explanation to kinship for the degree to which individuals help. To be allowed access to a group and enjoy the protection afforded by the breeders, helpers may work or care for young as a kind of payment of ‘rent’ to the breeders (Gaston 1978), and unrelated individuals may in fact be required to help more (Kokko et al. 2002).
We tested the predictions from kin selection (relatives help more) and ‘pay-to-stay’ theory (non-relatives help more) using a small cooperatively breeding cichlid fish, Neolamprologus pulcher, found in Lake Tanganyika in Africa. This species lives in small colonies consisting of several distinct groups, each group defending a small territory at depths of three to 45 m along the rocky edges of the lake. Groups usually contain a single breeding pair and 1–14 helpers of both sexes (Taborsky & Limberger 1981; Balshine et al. 2001). Helpers and breeders share in (i) brood care (cleaning and fanning eggs and larvae); (ii) territory defence (against predators as well as conspecific and heterospecific space competitors) and (iii) territory maintenance (removing snails, clearing sand or debris from the breeding shelter), all of which are collectively termed work effort (Taborsky & Limberger 1981; Taborsky 1984; Balshine et al. 2001; Buchner et al. 2004). N. pulcher helpers have different life-history trajectories; they may inherit a breeding position in their natal group, or disperse to another group to help or breed (see figure 1; Stiver et al. 2004).
Figure 1.
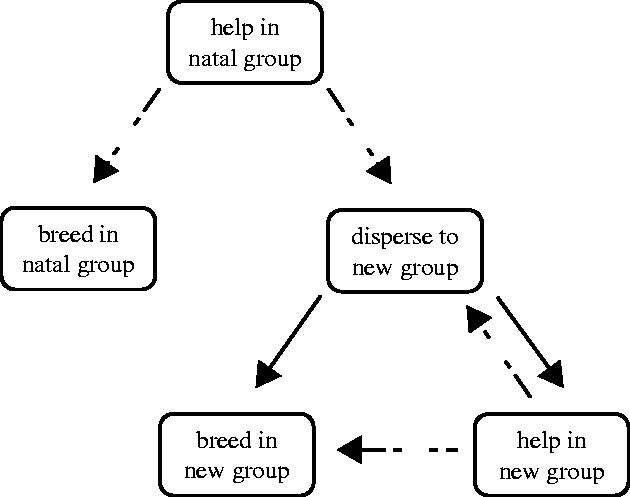
Alternative life-history strategies of N. pulcher. Dashed lines indicate that individuals are not obliged to move between strategies.
A number of life-history traits (sex, size and social rank) appear to affect the amount of help (work effort) provided by individual N. pulcher helpers. Two previous field studies have shown that female breeders perform more brood care and defence (Taborsky & Limberger 1981; Balshine et al. 2001) suggesting that in helpers, too, sex may influence the frequency of help provided. One of these previous field studies also reported that larger helpers perform more defence than smaller helpers (Taborsky & Limberger 1981). Body size and social rank are often correlated within a social group (Abbott & Dill 1989), but in the N. pulcher groups in this study, the most dominant helpers in each group were not necessarily large fish and no direct relationship between dominance and body size was found across groups (see results). Furthermore, Cant & Field (2001) argued that dominant helpers, those close to inheritance of a breeding spot, should perform less work (help) in order to maintain energy reserves for future breeding. Based on their theoretical model, we predicted that dominant N. pulcher helpers (irrespective of body size) would help less. Hence, to fully explore the selective factors affecting the degree of cooperation in social groups of N. pucher, we examined the influence of a constellation of three life-history traits: helper sex, size and social rank on the degree of help (or work effort).
Initial work on N. pulcher suggested that helpers were likely to be offspring of the breeders of their group (Taborsky & Limberger 1981; Taborsky 1984, Taborsky 1985). When individuals live for an extended period with their close relatives, there is increased opportunity for them to engage in altruistic acts to gain the indirect benefits of aiding the survival and reproduction of their kin (Hamilton 1964). However, whether or not individuals live with kin does not rule out the possibility that cooperation may have evolved via pay to stay, as has been suggested by recent studies in N. pulcher (Taborsky 1984; Balshine-Earn et al. 1998; Bergmüller & Taborsky 2005). Hence, we tested the predictions of both kin selection and ‘pay-to-stay’ theory to determine the factors selecting for evolution and maintenance of cooperation in the N. pulcher.
2. Material and Methods
We studied helping (or work effort) of N. pulcher helpers in both the field and in the laboratory. Fieldwork was conducted on the Zambian shores of Lake Tanganyika in Kasakalawe Bay. For details of the study area and field methodologies, including fishes' capture, measurement, sexing, marking and observations, see Balshine-Earn et al. (1998) and Balshine et al. (2001). In the field, the frequency of helping behaviour was recorded in a total of 99 helpers from 45 groups (54 of these individuals were also genetically sampled; see below). For each fish observed, we knew its body length, sex and social rank within the dominance hierarchy. Behaviours recorded included feeding, defence, brood chamber visits, territory maintenance (digging and carrying) and intragroup interactions (aggression and submission). Observations on focal helpers (mean=3 observations per fish, range=1–6) were recorded on polyvinyl chloride slates for 10–15 min per watch while scuba diving at 7–11 m depth. In the field, it was impossible to record the frequency of specific brood chamber activities as these were performed under rocks; thus, number of visits was used as a proxy measure of brood chamber activity. Hence, it is possible that for some recorded ‘helping acts in the brood chamber’ the helper may have been hiding or resting and not helping.
To assess the relatedness between helpers and breeders, we collected fin tissue from 141 helpers and at least one breeder in each of 46 groups (101 could be compared with their breeding male, 110 to their breeding female). For 70 of the 141 helpers (in 26 groups), we obtained fin tissue from both the breeding male and female, enabling us to estimate a coefficient of relatedness of helpers (r-values; Queller & Goodnight 1989) to both their breeding male and breeding female. This large genetic dataset was used to examine overall degrees of relatedness between helpers and breeders. In addition, 54 of these genetically sampled helpers (from 34 groups) were also observed behaviourally (see above).
To gather tissue for genetic analysis, a small piece of fin tissue was cut from the dorsal or anal fin of each fish and preserved in 95% ethanol for transport and storage. The tissue samples were extracted in the laboratory and analysed using five microsatellite loci: LOC101 (Brandtmann et al. 1999), ML007 (Kohler 1997), Pzeb3 (Van Oppen et al. 1997), US-758/773 (Schliewen et al. 2001) and US-780/783 (Schliewen et al. 2001; see Stiver et al. 2004 for further details). PCR products were generated using these primer pairs and then run on an automated capillary sequencer (ABI Prism 310 Genetic Analyser, Perkin Elmer). Genescan and Genotyper software were used for the genetic profiles.
We excluded an adult as the genetic parent of a particular offspring when the alleles in the breeder's genotype failed to match those found in offspring at one or more loci. In two cases where two possible breeders were potential parents of the helper, but both could not be parents (e.g. they matched at the same allele in an offspring), parentage was assigned to the breeder with the higher estimate of pairwise relatedness to the helper. Relatedness values of breeder–helper pairs were estimated using the program Kinship 1.3.1 (Goodnight & Queller 1999). Group relatedness was calculated by averaging all estimates of pairwise relatedness between helpers in a group (see Queller et al. 2000).
Although relatedness is typically viewed as a continuous variable, we also examined how helpers categorized into broad related versus unrelated helper classes differed in terms of their helping effort. The broad categories have been used in other studies (Alderson et al. 1999; Woolfenden et al. 2003) and probably better reflect how individuals perceived changes in group composition when a parent or step-parent is removed through common event such as a take-over or predation (Stiver et al. 2004). Dichotomous categories of helpers as relatives or non-relatives (of the breeding male or female) were generated based on a previously hypothesized relatedness distribution (between helpers and breeders, r=0.5; Taborsky 1984; Taborsky 1985) using the simulation function in Kinship (Goodnight & Queller 1999; Queller et al. 2000). Individuals with r-values falling below a 95% confidence interval of the mean were considered to be unrelated (Alderson et al. 1999). Further details of the genetic analyses can be found in Stiver et al. (2004) and Dierkes (2004).
In order to test the whether related or unrelated helpers help more under simpler conditions, we manipulated groups in the laboratory so that one of two extreme conditions held: all helpers were either related to both breeders (parents and their offspring, r=0.5, n=11) or unrelated to both breeders (r=0.0, n=11). The fishes for the laboratory experiments were either wild caught or first generation laboratory stock. Fourteen groups were housed in 80×40× 40 cm3 aquaria; eight groups were housed in 100×40×40 cm3 aquaria. Each aquarium contained two sponge filters, three ceramic flower pot shelters, one mirror, an electric water heater and 5 cm (depth) of sand as substrate. The light–dark regime was kept at 13 h light to 11 h dark throughout the study. Water temperature ranged between 25.5–28.6 °C. Fishes were fed twice daily (once with commercial dry fish flakes and once with fresh Artemia, frozen Daphnia and chironomid larvae).
In the laboratory, each group member was measured, sexed and marked and each focal helper was observed on three separate occasions for ten minutes. All helping was recorded, including visits to the brood chamber, digging, carrying and defence against conspecific neighbours. Behavioural watches were averaged to provide a mean for each individual's helping effort and then helping effort was averaged for each group.
All 22 laboratory groups (11 related and 11 unrelated) had been set up approximately two years previously for another study (see Dierkes et al. 1999); fishes in these groups were familiar with one another and helpers in the unrelated groups had in fact spent more time in these ‘new’ groups than they had in their natal group. Groups differed in group size (3–10 individuals), sex ratio and the size distribution of individuals within groups; these factors could not be controlled without influencing group dynamics. However, the related and unrelated groups were matched as far as was possible and these variables did not influence differences in helper work effort between the two relatedness conditions in the laboratory (see table 1). Additionally, Balshine et al. (2001) showed that these factors do not influence work effort by helpers.
Table 1.
Average group and helper sizes as well as sex ratios in the 11 related and the 11 unrelated groups in the laboratory.
| factors | related groups | unrelated groups | test statistics | p |
|---|---|---|---|---|
| group size | 6.5 | 6.5 | 60.5 | >0.99 |
| (max–min) | (3–10) | (3–10) | ||
| body length (in mm) | 47.0 | 47.0 | 57.0 | 0.81 |
| (min–max) | (34–54) | (39–57) | ||
| sex ratio | ||||
| groups with male bias | 7 | 5 | 1.01 | >0.50 |
| groups with female bias | 2 | 2 | ||
| groups with equal sex ratios | 2 | 4 |
Mann–Whitney U-statistics were conducted on the group size and helper size data and a log–linear analysis was performed on the sex ratio data. None of these factors differed between related and unrelated groups.
We used Statview 5.0 for statistical analyses. Because sex of helper significantly influenced workload (see results), we controlled for it by comparing males and females separately (results presented are with sexes combined, as their helping patterns only differed in terms of the amount performed). Whenever possible, we normalized using the transformation. When transformations failed to normalize the data, non-parametric tests were employed. All p-values are two tailed and corrected for ties.
As relatedness values were calculated as a pairwise measure of the degree of genetic similarity between a helper and a breeder, relatedness values for two helpers from the same group were not independent. To control for such cases, we randomly selected a single helper from each group to assess the relationship between helping effort and relatedness to breeders. We present only results calculated using one helper per group. (In general, calculations using all available helper–breeder dyads did not change the results and are summarized in the Electronic Appendix).
3. Results
(a) General patterns of helping in the field
In the field, helpers performed an average (±s.e.) of 0.47±0.03 helping acts every minute (n=99). Visiting the brood chamber was the most common behaviour observed (0.24±0.02, n=99) followed by territory defence (0.18±0.02, n=99) and maintenance (0.04±0.02, n=99). Female helpers provided more help than male helpers (unpaired t-test, t=2.30, p=0.02; figure 2a) and this sex difference was driven by the number of brood chamber visits (t=2.22, p=0.03). No relationship was found between helper size and amount of help provided (Z=−1.23, R2=0.016, p=0.21, n=99). As Cant and Field's (2001) model predicts, we found that dominant helpers (ranks 1–3) tended to do less work than subordinate helpers (ranks 4 and lower; t=−1.71, p=0.09; figure 2b). We also performed a forward stepwise regression to determine which of the three above factors (helper size, sex, and social status) most strongly influenced the observed variation in helping effort. Only sex explained a significant proportion of the variance (R2=0.07, p=0.02; size partial R=−0.16, dominance partial R=0.06, sex partial R=0.26).
Figure 2.
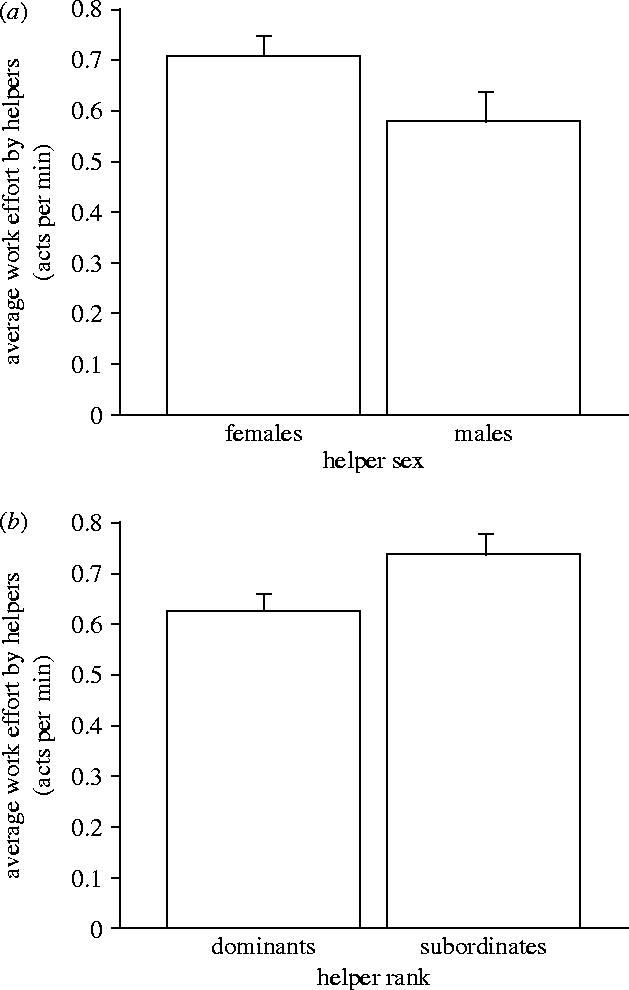
Factors influencing amount of help given by helpers (mean work effort ±s.e.; work effort is defined as mean number of helping acts per minute; data transformed as in §2). (a) Female helpers (n=48) help more than male helpers (n=28). Fishes can be sexed externally by examination of the genital papilla. Males have pointed cone shaped genitalia, female genitalia are larger, rounder with a distinctive slit. (b) Dominant helpers (ranks 1–3, n=78) helped less than subordinate (ranks 4 and lower, n=21) helpers. Ranks were determined behaviourally from the focal watches and were based on the number of aggressive and submissive acts given and received.
(b) General field relatedness patterns between helpers and breeders.
Previously, helpers were assumed to be the offspring of the breeders (Taborsky 1984; Taborsky 1985). Of the 141 helpers that were genotyped, 118 (84%) were excluded as the offspring of the breeders they were helping. The exclusion probability for each locus ranged from 0.273–0.749 and the combined exclusion probability across the five loci was 0.98 (see DeWoody et al. 2000 for this calculation). In general, helpers were more related to their breeding female than to their breeding male (Paired t-test, t=−2.26, p=0.03, n=26; figure 3). By testing the distribution of relatedness of helper–breeder pairs against a simulated distribution of r=0.5 (n=1000), we could determine whether helpers were, on average, first-order relatives (offspring or siblings) of the male or female breeders. Helper relatedness to both the breeding male and female was significantly lower than expected if r=0.5 (helper to breeding male: unpaired t-test, t=−11.4, p<0.0001, n=26; helper to breeding female: t=−7.8, p<0.0001, n=26). Although helpers were not closely related to either breeder, this does not exclude the possibility that helpers (within a group) were all close kin. However, we calculated a mean relatedness among group members and found an average relatedness coefficient of 0.16±0.03, significantly lower than expected for full siblings (tested using a simulated r=0.5 distribution; t=−12.9, p<0.0001, n=68).
Figure 3.
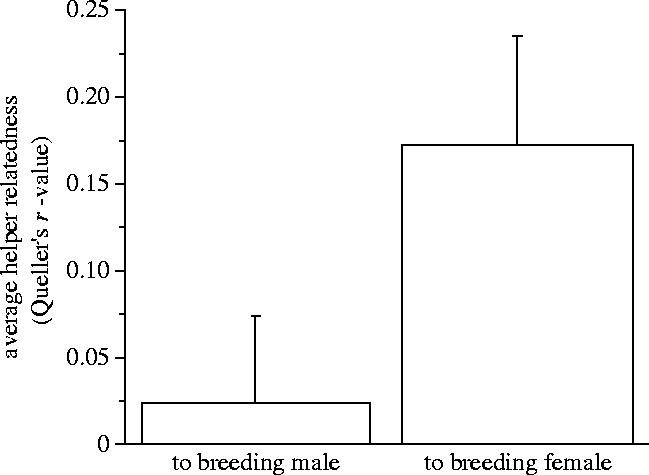
Average relatedness (mean r±s.e.) of helpers to the breeding male versus the breeding female. Relatedness was calculated using r-coefficients developed by Queller & Goodnight (1989). Data comes from 26 groups of N. pulcher found in Kasakalawe Bay.
(c) Testing kin selection and pay-to-stay theory
Helping effort of a helper was not associated with its overall average relatedness to the two breeders in its group (Spearman rank correlation, ρ=0.002, n=17, p=0.99). However, recent empirical work suggests that relatedness of helpers to each breeder must be considered separately (Magrath & Whittingham 1997; Richardson et al. 2003). While overall helping effort was not correlated with relatedness to the breeder male (ρ=0.002, p=0.99, n=17) or breeder female (ρ=0.002, p=0.99, n=17), helpers performed more defensive acts as their relatedness to the breeding male decreased (ρ=−0.49, p=0.03, n=22). Additionally, helpers performed more defensive acts as their relatedness to the breeding female increased (ρ=0.47, p=0.01, n=29). This variation in defence behaviour was not driven by a negative correlation between helper's relatedness to their breeding male versus relatedness to their breeding female, as these relatedness variables tended to be positively correlated (Pearson correlation, R2=0.12, Z=1.76, p=0.08, n=26).
Some authors have argued that individual helpers may help in an ‘all or none’ fashion, directing help only towards individuals they are closely related to (Altmann 1979; Weigel 1981; Schulman & Rubenstein 1983). Helpers could be categorized as either ‘closely related’ or ‘unrelated’ to the male or female breeder. Helpers were classified as related if their calculated pairwise relatedness to the male or female breeder fell within the 95% confidence interval of the mean of a simulated relatedness distribution for first-order relatives [r=0.5], and were classified as unrelated if they fell below this 95% confidence interval see Methods). Again, helpers unrelated to the breeding male performed more defence (U=25.0, p=0.02; figure 4a). In contrast, helpers unrelated to the breeding female performed less defence (U=46.5, p=0.01; figure 4b).
Figure 4.
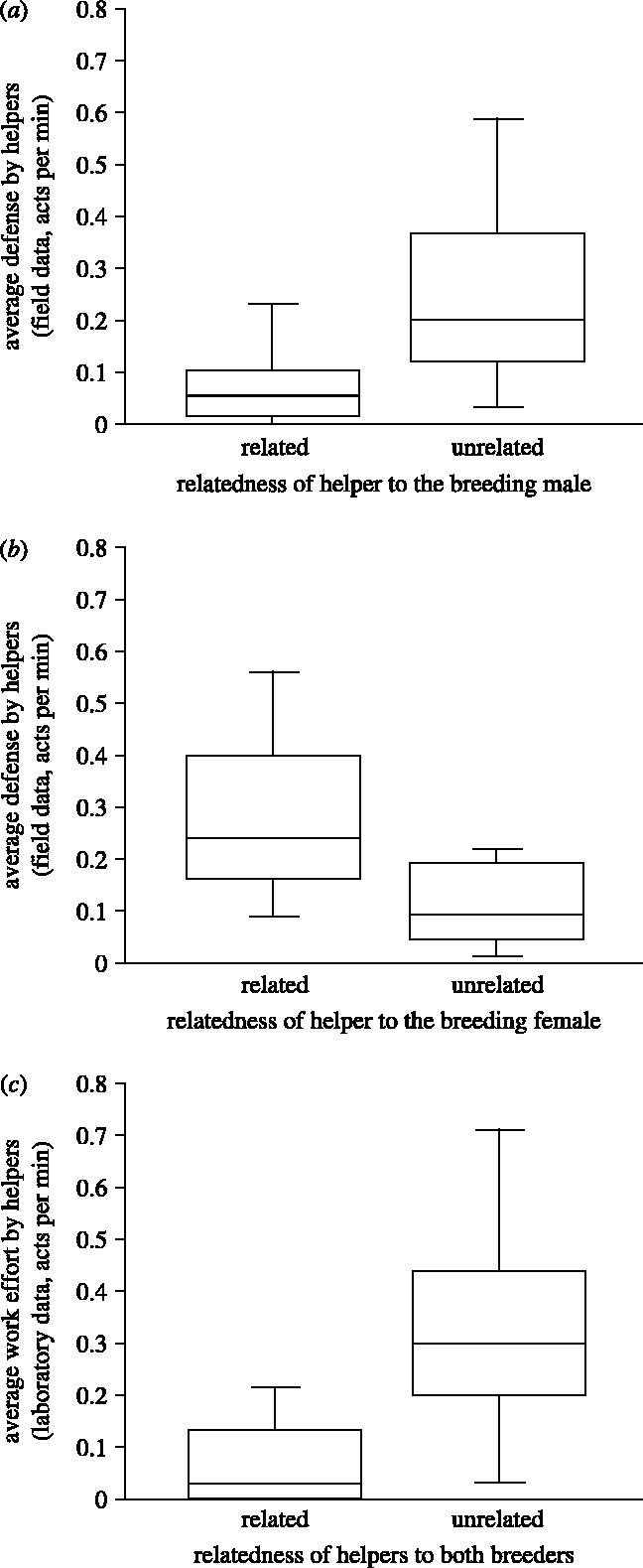
(a) In the field, helpers that were unrelated to the breeding male (n=12) performed more territory defence than did those related to the breeding male (n=10). (b) In the field, helpers that were unrelated to the breeding female (n=15) performed less territory defence than did those related to the breeding female (n=14). (c) In the laboratory, helping was more frequent in groups where helpers were unrelated to both breeders (nR =11, nUR =11).
In the laboratory, defence (against neighbours) was the most common behaviour observed (measured in mean acts by helpers/group/minute ±s.e.=0.10±0.03, n=22), followed by brood chamber visits (0.06±0.02, n=22) and territory maintenance (0.04±0.02, n=22); overall rates of helping (group average) were lower (0.2±0.05, n=22) than those observed in the field (0.46 -±0.04, n=45; U=196.5, p<0.0001). Helping was more frequent in unrelated groups in the laboratory (U=20.5, p=0.008; figure 4c).
4. Discussion
(a) In the field, why were helpers typically unrelated to breeders?
Helpers were on average, related to breeding females at the level equivalent to first cousins and they were typically unrelated to the breeding male. Two factors probably contribute to the overall low degrees of relatedness between helpers and breeders (mean r±s.e.=0.10±0.05). (i) Helpers can disperse to other groups and (ii) breeder turnover rates are rapid (Stiver et al. 2004). Helpers in the field were more closely related to breeding females, consistent with observations that male breeding tenure is typically shorter than that of female breeders (Stiver et al. 2004).
(b) Why do unrelated helpers help?
Several other recent studies have shown that unrelated helpers exist and as a consequence, there has been a shift in the relative importance placed on direct benefits (Wright et al. 1999; Legge 2000; Clutton-Brock et al. 2001; Clutton-Brock 2002; West et al. 2002). Increased performance of certain helping behaviours by unrelated individuals provides support for the hypothesis that helpers may ‘pay rent’ to stay in the group. Helping as payment to stay is theoretically predicted when relatedness between helpers and breeders is low and ecological constraints on independent breeding are high (Kokko et al. 2002). These conditions are met in our study population of N. pulcher (Heg et al. 2004).
(c) Why did helpers unrelated to the breeding male perform more territory defence, while helpers related to the breeding female performed more territory defence?
Predators can injure or kill defending helpers (Balshine et al. 2001); hence, defence is typically considered a more risky form of help than either territory maintenance or brood care. Those helpers related to the breeding female as well as those unrelated to the breeding male appeared more willing to take on this risky behaviour, supporting both the predictions of kin selection and of pay to stay.
Breeders themselves may influence the type of help performed by related versus unrelated helpers, by preventing helpers from entering the brood chamber (Werner et al. 2003). Breeders may be excluding unrelated helpers from the brood chamber, as these helpers may be more likely to sneak reproduction or cannibalize eggs and fry (von-Siemens 1990; Dierkes et al. 1999; Dierkes 2004). A negative relationship between the amount of brood care and defence provided by helpers (Wilcoxon signed ranks test: Z=−2.5, n=99, p=0.01) suggests a trade-off among helping behaviours perhaps in terms of relatedness.
In the laboratory, helpers helped more when they were unrelated to the breeders. How relatedness is assessed in this species has not yet been determined; however, a number of studies have shown that teleost fishes are capable of kin and familiar fish recognition (Balshine-Earn & Lotem 1998; Griffiths & Magurran 1999; Ward & Hart 2003). It is possible that in the laboratory, the cue that the unrelated helpers were responding was a change in group composition, rather than to a change in their relatedness to the breeders per se. However, breeder replacement occurs frequently in the field; hence, this laboratory experiment mirrored natural replacement events. Familiarity was unlikely in practice to be very different between unrelated and related groups in this study, as unrelated helpers had been with their breeders in excess of two years.
(d) Relatedness, group size and skew
A number of recent theoretical papers make predictions about how relatedness influences group size and the degree of sharing between group members of reproduction (skew) or resources, (Higashi & Yamamura 1993; Reeve et al. 1998; Johnstone & Cant 1999; Hamilton 2000; Johnstone 2000). One might ask how our results relate to these predictions. First, Higashi & Yamamura (1993) argued that as relatedness between group members is increased, group size will increase and may even exceed an optimal size. No correlation between group size and relatedness of helpers to breeders was found in groups from the field (Spearman rank correlation, ρ=−0.16, p=0.42, n=26). Second, Hamilton's (2000) recruiter–joiner model suggests that as relatedness increases, so should the degree of sharing of a communal resource (i.e. food). Although food is not shared in N. pulcher groups (zooplankton is consumed while in communal aggregations in the water column without any overt signs of competition; Balshine et al. 2001), shelter is shared. Future studies will further investigate how shelter use varies with relatedness.
Third, concession or optimal skew models suggest that groups with high relatedness will have little reproductive equality, while incomplete control models argue the opposite, i.e. that groups with high relatedness will more equally distribute reproduction (Reeve et al. 1998; Johnstone & Cant 1999). Our results show that groups of N. pulcher are not highly related but, unfortunately, little is known about the degree of skew in wild populations. Laboratory studies are now underway to examine the degree of male and female skew and its influence on helping.
Our results show that work effort by helpers varies with the degree of relatedness and sex of helper. Defence frequency varied with relatedness to a particular breeder, suggesting that helpers react to more complex cues than their average relatedness to the breeders. Unrelated individuals may need to help more in order to be permitted residence in the territory, while related individuals may help to increase their fitness benefits by aiding their kin. The fact that related and unrelated helpers were observed to perform different amounts of helping behaviours underscores the potential differences in the underlying causes of helping.
Acknowledgments
We thank B. Leach, F. Neat, H. Reid and N. Werner for assisting in collection of the behavioural data and the fin samples from the field; A. Kuntz and M. Maan for collecting fin tissue samples; R. Achmann and J. Quinn for advice with the genetic techniques and data analysis; E. Bressler, J. Desjardins, D. Earn and S. Marsh-Rollo, the journal editor and two anonymous referees for helpful comments on the manuscript. The work was funded by an NSERC research grant and a Royal Society fellowship to S.B. and Fonds zur Förderung der wissenschaftlichen Forschung (P 10916-Bio) to M.T. This research was conducted with the permission and cooperation of Leonard Mwape and the Zambian Ministry of Agriculture, Food and Fisheries.
Supplementary Material
References
- Abbott J.C, Dill L.M. The relative growth of dominant and subordinate juvenile steelhead trout (Salmo gairdneri) fed equal rations. Behaviour. 1989;108:104–113. [Google Scholar]
- Alderson G, Gibbs H.L, Sealy S.G. Determining the reproductive behaviour of individual brown-headed cowbirds using microsatellite DNA markers. Anim. Behav. 1999;58:895–905. doi: 10.1006/anbe.1999.1220. [DOI] [PubMed] [Google Scholar]
- Altmann S.A. Altruistic behaviour: the fallacy of kin deployment. Anim. Behav. 1979;27:958–959. [Google Scholar]
- Balshine-Earn S, Lotem A. Individual recognition in a cooperatively breeding cichlid: evidence from video playback experiments. Behaviour. 1998;135:369–386. [Google Scholar]
- Balshine-Earn S, Neat F.C, Reid H, Taborsky M. Paying to stay or paying to breed? Field evidence for direct benefits of helping behaviour in a cooperatively breeding fish. Behav. Ecol. 1998;9:432–438. [Google Scholar]
- Balshine S, Leach B, Neat F, Reid H, Taborsky M, Werner N. Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher) Behav. Ecol. Sociobiol. 2001;50:134–140. [Google Scholar]
- Bergmüller R, Taborsky M. Experimental manipulation of helping in a cooperative breeder: helpers ‘pay to stay’ by pre-emptive appeasement. Anim. Behav. 2005;69:19–28. [Google Scholar]
- Brandtmann G, Scandura M, Trillmich F. Female–female conflict in the harem of a snail cichlid (Lamprologus ocellatus): behavioural interactions and fitness consequences. Behaviour. 1999;136:1123–1144. [Google Scholar]
- Buchner A.S, Sloman K.A, Balshine S. The physiological effects of social status in the cooperatively breeding cichlid Neolamprologus pulcher. J. Fish Biol. 2004;65:1080–1095. [Google Scholar]
- Cant M.A, Field J. Helping effort and future fitness in cooperative animal societies. Proc. R. Soc. B. 2001;268:1959–1964. doi: 10.1098/rspb.2001.1754. 10.1098/rspb.2001.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.F. Co-operative breeding by the Australian bell miner Manorina melanophrys Latham: a test of kin selection theory. Behav. Ecol. Sociobiol. 1984;14:137–146. [Google Scholar]
- Clutton-Brock T.H. Breeding together: kin selection and mutualism in cooperative vertebrates. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Brotherton P.N.M, O'Riain M.J, Griffin A.S, Gaynor D, Kansky R, Sharpe L, McIlrath G.M. Contributions to cooperative rearing in meerkats. Anim. Behav. 2001;61:705–710. [Google Scholar]
- Conrad K.F, Clarke M.F, Robertson R.J, Boag P.T. Patterns of paternity in the co-operatively breeding bell miner (Manorina malanophrys) Condor. 1998;100:343–349. [Google Scholar]
- DeWoody J.A, Fletcher D.E, Wilkins S.D, Nelson W.S, Avise J.C. Genetic monogamy and biparental care in an externally fertilizing fish, the largemouth bass (Micropterus salmoides) Proc. R. Soc. B. 2000;267:2431–2437. doi: 10.1098/rspb.2000.1302. 10.1098/rspb.2000.1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierkes, P. 2004 Ph.D. thesis, Konrad Lorenz-Institut für Vergleichende Verhaltensforschung (KLIVV), 1160, Vienna, Austria.
- Dierkes P, Taborsky M, Kohler U. Reproductive parasitism of broodcare helpers in a cooperatively breeding fish. Behav. Ecol. 1999;10:510–515. [Google Scholar]
- Emlen S.T, Wrege P.H. The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 1988;23:305–315. [Google Scholar]
- Gaston A.J. The evolution of group territorial behavior and cooperative breeding. Am. Nat. 1978;112:1091–1100. [Google Scholar]
- Goodnight K.F, Queller D.C. Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol. Ecol. 1999;8:1231–1234. doi: 10.1046/j.1365-294x.1999.00664.x. [DOI] [PubMed] [Google Scholar]
- Griffin A.S, West S.A. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302:634–636. doi: 10.1126/science.1089402. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Magurran A. Schooling decisions in guppies (Poecilia reticulata) are based on familiarity rather than kin recognition by phenotype matching. Behav. Ecol. Sociobiol. 1999;45:437–443. [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour, I and II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton I.M. Recruiters and joiners: using optimal skew theory to predict group size and the division of resources within groups of social foragers. Am. Nat. 2000;155:684–695. doi: 10.1086/303349. [DOI] [PubMed] [Google Scholar]
- Heg D, Bachar Z, Brouwer L, Taborsky M. Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. B. 2004;271:2367–2374. doi: 10.1098/rspb.2004.2855. 10.1098/rspb.2004.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi M, Yamamura N. What determines animal group size? Insider–outsider conflict and its resolution. Am. Nat. 1993;142:553–563. [Google Scholar]
- Johnstone R.A. Models of reproductive skew: a review and synthesis. Ethology. 2000;106:5–26. [Google Scholar]
- Johnstone R.A, Cant M.A. Reproductive skew and the threat of eviction: a new perspective. Proc. R. Soc. B. 1999;266:275–279. 10.1098/rspb.1999.0633 [Google Scholar]
- Kohler, U. 1997 Zur Struktur und Evolution des Sozialsystems von Neolamprologus multifasciatus (Cichlidae, Pisces), des kleinsten Schneckenbuntbarsches des Tanganjika-Sees. Ph.D. thesis, Ludwig-Maximilians-Universität, München.
- Kokko H, Johnstone R.A, Wright J. The evolution of parental and alloparental effort in cooperatively breeding groups: when should helpers pay to stay? Behav. Ecol. 2002;13:291–300. [Google Scholar]
- Legge S. Helper contributions in the cooperatively breeding laughing kookaburra: feeding young is no laughing matter. Anim. Behav. 2000;59:1009–1018. doi: 10.1006/anbe.2000.1382. [DOI] [PubMed] [Google Scholar]
- Magrath R.D, Whittingham L.A. Subordinate males are more likely to help if unrelated to the breeding female in cooperatively breeding white-browed scrubwrens. Behav. Ecol. Sociobiol. 1997;41:185–192. [Google Scholar]
- Mumme R.L. Do helpers increase reproductive success? An experimental analysis in the Florida scrub jay. Behav. Ecol. Sociobiol. 1992;31:319–328. [Google Scholar]
- Queller D.C, Goodnight K.F. Estimation of genetic relatedness using allozyme data. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Queller D.C, Zacchi F, Cervo R, Turillazzi S, Henshaw M.T, Santorelli L.A, Strassmann J.E. Unrelated helpers in a social insect. Nature. 2000;405:784–787. doi: 10.1038/35015552. [DOI] [PubMed] [Google Scholar]
- Reeve H.K, Emlen S.T, Keller L. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders. Behav. Ecol. 1998;9:267–278. [Google Scholar]
- Richardson D.S, Komdeur J, Burke T. Altruism and infidelity among warblers. Nature. 2003;422:580. doi: 10.1038/422580a. [DOI] [PubMed] [Google Scholar]
- Schliewen U, Rassman K, Markmann M, Market J, Kocher T, Tautz D. Genetic and ecological divergence of a monophyletic cichlid species pair under fully sympatric conditions in Lake Ejagham, Cameroon. Mol. Ecol. 2001;10:1471–1488. doi: 10.1046/j.1365-294x.2001.01276.x. [DOI] [PubMed] [Google Scholar]
- Schulman S.R, Rubenstein D.I. Kinship, need, and the distribution of altruism. Am. Nat. 1983;121:776–788. [Google Scholar]
- Stiver K.A, Dierkes P, Taborsky M, Balshine S. Dispersal patterns and status change in a cooperatively breeding fish; evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 2004;65:91–105. [Google Scholar]
- Taborsky M. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 1984;32:1236–1252. [Google Scholar]
- Taborsky M. Breeder–helper conflict in a cichlid fish with broodcare helpers: an experimental analysis. Behaviour. 1985;95:45–75. [Google Scholar]
- Taborsky M, Limberger D. Helpers in fish. Behav. Ecol. Sociobiol. 1981;8:143–145. [Google Scholar]
- Van Oppen M.J.H, Rico C, Deutsch J.C, Turner G.F, Hewitt G.M. Isolation and characterization of microsatellite loci in the cichlid fish Pseudotropheus zebra. Mol. Ecol. 1997;6:387–388. doi: 10.1046/j.1365-294x.1997.00188.x. [DOI] [PubMed] [Google Scholar]
- von-Siemens M. Broodcare or egg cannibalism by parents and helpers in Neolamprologus brichardi (Poll 1986) (Pisces: Cichlidae): a study on behavioural mechanisms. Ethology. 1990;84:60–80. [Google Scholar]
- Ward A.J.W, Hart P.J.B. The effects of kin and familiarity on interactions between fish. Fish Fish. 2003;4:348–358. [Google Scholar]
- Weigel R.M. The distribution of altruism among kin: a mathematical model. Am. Nat. 1981;118:191–201. [Google Scholar]
- Werner N.Y, Balshine S, Leach B, Lotem A. Helping opportunities and space segregation among helpers in cooperatively breeding cichlids. Behav. Ecol. 2003;14:749–756. [Google Scholar]
- West S.A, Pen I, Griffin A.S. Cooperation and competition between relatives. Science. 2002;296:72–75. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- Whittingham L.A, Dunn P.O, Magrath R.D. Relatedness, polyandry and extra-group paternity in the cooperatively-breeding white-browed scrubwren (Sericornis frontalis) Behav. Ecol. Sociobiol. 1997;40:261–270. [Google Scholar]
- Woolfenden B.E, Gibbs H.L, Sealy S.G, McMaster D.G. Host use and fecundity of individual female brown-headed cowbirds. Anim. Behav. 2003;66:95–106. [Google Scholar]
- Wright J, Parker P.G, Lundy K.J. Relatedness and chick feeding effort in the cooperatively breeding Arabian babbler. Anim. Behav. 1999;58:779–785. doi: 10.1006/anbe.1999.1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


