Abstract
When isolated from predators, costly and no longer functional anti-predator behaviour should be selected against. Predator naiveté is often pronounced on islands, where species are found with few or no predators. However, isolation on islands involves other processes, such as founder effects, that might be responsible for naiveté or reduced anti-predator behaviour. We report the first comparative evidence that, in macropodid marsupials, isolation on islands may lead to a systematic loss of ‘group size effects’—a behaviour whereby individuals reduce anti-predator vigilance and allocate more time to foraging as group size increases. Moreover, insular animals forage more, and are less vigilant, than mainland ones. However, we found no evidence that animals on the mainland are ‘flightier’ than those on islands. Remarkably, we also found no evidence that isolation from all predators per se is responsible for these effects. Together, these results demonstrate that anti-predator behaviour may indeed be lost or modified when animals are isolated on islands, but it is premature to assume that all such behaviour is affected.
Keywords: isolation on islands, persistence of anti-predator behaviour, relaxed selection
1. Introduction
When isolated from predators, costly and no longer functional anti-predator behaviour should be eliminated by selection (Kavaliers 1990; Magurran et al. 1995; van Damme & Castilla 1996; Magurran 1999). Indeed, species on isolated islands, such as the Galápagos, are often described as being predator-naive (Darwin 1839; Lack 1947; Curio 1966). The mechanism underlying this loss is hypothesized to result from a reduction of predation risk on islands either because islands are able to support fewer top predators than adjacent mainland sites, or because islands lost predators following Pleistocene sea-level changes (Blumstein 2002). Because maintaining anti-predator behaviour in the absence of predators is assumed to be costly (Magurran 1999), we would expect that it would be eliminated by selection if there were no benefit. Alternatively, loss of anti-predator behaviour in insular populations could be due to founder effects. If generally true, and regardless of the mechanism, this evolutionary effect has important consequences for conservation biology. For instance, insular birds and mammals are reported to be more vulnerable to human exploitation as well as the introduction of exotic predators than mainland species (Berger et al. 2001; Gittleman & Gomper 2001).
Currently, there is only one systematic, quantitative study demonstrating that isolation on islands with negligible predation risk is responsible for the loss of any anti-predator behaviour (Beauchamp 2004). Species often have more than one predator (Lima 2002; Stoks et al. 2003) and many islands may retain a subset of predators. Thus, it is important to isolate the effects of insularity from isolation from predators.
Species have a variety of anti-predator behaviours (Van Buskirk 2001; Stoks et al. 2003), and some anti-predator traits may be more phenotypically plastic than others (Blumstein 2002). Thus, we might expect different types of behaviour to vary in the degree to which different anti-predator adaptations respond to isolation (Blumstein & Daniel 2002).
In this paper, we focus on macropodid marsupials-kangaroos, wallabies and their relatives—and ask whether insularity affects anti-predator behaviour, and if so, whether this effect is specifically due to the loss of predators. Macropodid marsupials have been isolated on islands since Pleistocene sea-level changes, as well as from more recent anthropogenic translocations (King 1990; Strahan 1995). Some islands are predator-free, some have a reduced set of predators, and mainland Australia has a variety of native and non-native predators (Strahan 1995).
We combined data we collected using identical methods from 23 populations of macropodids representing 14 species, with those collected using different methods by other researchers, to develop a dataset containing 32 populations representing 14 species. These data were then used to study the effects of isolation on islands on three anti-predator behaviours: group size effects, time allocated to foraging and vigilance and flight-initiation distance.
Modifying time allocation as a function of group size is a commonly reported anti-predator behaviour that, for macropodids, is probably most effective against terrestrial mammalian predators (e.g. Blumstein et al. 2004). Two models of predation hazard assessment (detection and dilution; Krause & Ruxton 2002) predict that animals should forage more and allocate less time to anti-predator vigilance as group size increases, because predation risk declines with the addition of alternative prey, and collective vigilance. Animals living with predators commonly trade off foraging with anti-predator vigilance (Bednekoff & Lima 1998; Beauchamp 2003) so we should expect differences when predators are present or not. And, the distance at which animals flee an approaching human—flight initiation distance—is a standardized metric by which to quantify perceived predation risk (Bonefant & Kramer 1996; Blumstein et al. 2003a). We expected that predator-free populations would tolerate closer approach.
2. Material and Methods
(a) Developing the comparative dataset
Most of our original data and methods on time allocation and group size effects have been published (Blumstein et al. 1999, 2001a,b, 2002, 2003b, 2004; Blumstein & Daniel 2002, 2003a,b,c), but we added additional unpublished data on Thylogale thetis (at Lamington National Park, Queensland), and Macropus robustus (at Fowlers Gap, NSW). To summarize our methods, we video-recorded 5 min focal samples of foraging animals. We stood or sat in the open at distances where we did not obviously influence our focal subject's behaviour. Videotapes were scored using an event recorder (e.g. Blumstein et al. 2000). At the beginning of each focal sample we counted the number of conspecifics within 10 and 50 m of the focal subject. For some analyses, we also included three studies of captive animals—Macropus eugenii, T. thetis and Petrogale xanthopus. Macropus eugenii were either wild caught from Kangaroo Island or were first generation offspring from this population. Thylogale thetis had been in captivity for at least one generation and were originally from wild New South Wales populations. Petrogale xanthopus were captive-reared from a population in captivity for many generations. For the first two species, we systematically manipulated group size in captivity. The third study relied on naturalistic observations in a large enclosure. We averaged time allocation for each group size and then fitted linear and logarithmic regressions to these aggregated group size data. By aggregating data, we reduced variation within a group size category and thus increased our ability to detect group size effects if they were present. We then scored a species as having group size effects if there was a significant regression between group size and time allocation or, for less social species, only found alone or in groups of two, whether there was a significant difference in time allocation when alone or in a group. In addition to these systematically collected data (see electronic appendix 1), we included data on the presence or absence of group size effects reported in the literature (Heathcote 1987; Jarman 1987; Johnson 1987; Colagross & Cockburn 1993; Coulson 1999; Payne & Jarman 1999 Wahungu et al. 2001). Because time allocated to foraging may be influenced by body size, we compiled the mean female body size for each species from Strahan (1995).
Populations were scored as being exposed to predators in two ways. Captive populations were scored as predator-free; some subsequent analyses included or excluded these captive populations. Direct observations and published sources (King 1990; Watts 1993; Strahan 1995) were used to identify predator-free insular populations. While many of the free-living studies were conducted in protected areas, we do not exclude human predation as a potential source of occasional mortality. Thus, our analyses focus on the presence of contemporary non-human predators. Moreover, we included anthropogenic isolation (i.e. animals were moved to offshore island) with ‘natural’ Pleistocene isolation. We justify including these species by noting that costly traits may respond rapidly to relaxed selection (e.g. Endler 1980, 1986).
Field data on flight-initiation distance were collected using identical protocols (Blumstein 2002) in 18 free-living populations. Individuals were approached at a constant pace of 0.5 m s−1 to measure the flight initiation distance for each species and at each site. With calibrated paces, we measured the distance from the subject at which we began walking (‘starting distance’), and the distance at which an individual hopped off. This starting distance was used as a covariate in subsequent analyses because of its potentially confounding effect on flight initiation distance (Blumstein 2003).
(b) Developing the phylogeny
A consensus phylogeny was developed that included species of interest. The overall relationship between macropodid ‘groups’ came from Kirsch et al. (1997; fig. 13). This was a DNA–DNA hybridization phylogeny that was broadly in agreement with Flannery's morphological hypothesis (Flannery 1989; fig. 12). For our set of species, we used Setonix brachyurus as an out-group. Petrogale relationships were resolved using Campeau-Peloquin et al.'s (2001) DNA–DNA hybridization phylogeny. The position of Thylogale was supported by Kirsch et al. (1997). The ‘Macropus group’ was less well resolved. Flannery (1989), Sharman (1989) and Burk & Springer (2000) have M. rufogresis, M. eugenii and/or M. agilis grouped in various ways. We follow Flannery and resolve them as a polytomy. In placing M. robustus and M. rufus together, we follow Flannery (1989) and Kirsch et al. (1997) and we follow Flannery in placing M. giganteus and M. fuliginosus together.
Within a species, M. eugenii come from three genetically distinctive populations: the mainland western Australian population (Strahan 1995), the now extinct mainland south Australian population, which exists in New Zealand (Taylor & Cooper 1999), and the Kangaroo Island population. A subspecies of M. rufogriseus is found in Tasmania (Strahan 1995). We assumed nothing about genetic substructure of M. fuliginosus, nor did we assume anything about the substructure of mainland populations of M. giganteus. We did assume that Tasmanian populations were distinct from mainland populations, which were all studied in eastern Australia. Polytomies were then resolved randomly.
(c) Testing the comparative hypotheses
To study group size effects, we used the concentrated changes test (Maddison 1990) to determine whether the evolutionary loss of group size effects was concentrated in populations on islands or in those populations living without non-human predators. For this, analyses used data from the 32 studies that reported the presence or absence of group size effects. Additional analyses were restricted to data collected from free-living populations and those data only collected by us. The concentrated changes test requires a fully resolved phylogeny, hence the random resolve option in Macclade v. 4.03 (Maddison & Maddison 2001) was used to resolve remaining polytomies. The dichotomous traits were optimized on to the resolved tree to reconstruct the ancestor states for each. In all cases, strict parsimony fully resolved the reconstructions.
The ‘actual changes’ simulation option was used for 10 000 replicates to estimate p-values for each reconstruction. Also, to account for incorrect resolutions of the ancestor state, simulations were run with the ‘either ancestral’ option selected. To minimize the possibility of falsely interpreting results as significant, we employed a conservative approach by selecting fewer and as many gains in the distinguished character, and fewer than, as many as, or more losses in the distinguished character than actually counted when calculating the p-value in MacClade. We calculated these tests on the full set of 32 observations, a set of 29 observations that excluded the three captive populations, and a set of 20 free-living populations for which we systematically collected all data.
While the concentrated changes test allowed comparisons between the distributions of two traits on a phylogenetic tree, the contingent states test (Sillén-Tullberg 1993) allowed the use of the phylogenetic reconstruction of characters to ask whether the transition in one character from 0 to 1 or 1 to 0, or the lack of a transition, is equally likely to occur under either state of another character. The main assumption involved is that each branch has an equal probability of state transition. To understand the directionality of the evolution of the three traits, a series of pair-wise contingent states tests using Costa v. 1.03 (Lindenfors 1999) were performed. Specifically, we asked, whether isolation on islands or the loss of all predators was likely to be associated with evolutionary changes in group size effects.
For time allocation and flight initiation distance, we calculated phylogenetically independent contrasts (Felsenstein 2004) using Compare 4.5 (Martins 2003). This comparative method avoids the problems of similarities among closely related species by estimating, and then using in subsequent analyses, the phylogenetically independent evolutionary divergence between traits. These analyses were restricted to data we systematically collected on free-living subjects. We set branch lengths as equal and thus assume a punctuational model of evolution. Multiple regressions were used to control for the effects of body mass while examining the relationship between insularity and isolation from all predators on time allocation, and to control for the effects of starting distance on the relationship between insularity and isolation from all predators on flight initiation distance. Contrast values were regressed through the origin (Felsenstein 2004).
3. Results
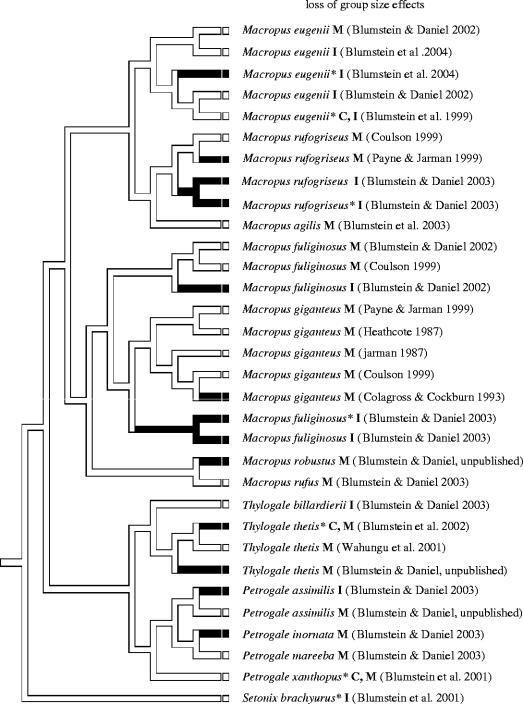
Assuming parsimony, and using the concentrated changes test, we found that the loss of group size effects was significantly more likely to occur in animals living on islands (figure 1; all 32 populations p<0.001; 29 free-living populations p=0.003; 20 free-living populations we studied p=0.003; electronic appendix 2). The contingent states test provided equivocal support for this finding: following isolation on islands, species were significantly likely not to evolve group size effects when data from all 32 populations (table 1) were included (p=0.016), but they were not more likely to lose group size effects (p=0.507). When analyses were restricted to our data only, we found no effect of isolation on the evolution or loss of group size effects (p>0.9). It is not obvious whether this effect can be attributed to the loss of all predators on islands. While the concentrated changes test suggested that animals living without predators were significantly likely not to have group size effects (p<0.001 for all three subsets of data), the contingent states tests did not support this interpretation (table 2). Following the loss of predators, populations were not significantly more likely to gain group size effects (p=0.221), nor were they more likely to lose group size effects (p=0.146) when data from all 32 populations were included. When analyses were restricted to our data only, we found no effect of isolation from predators on the evolution or loss of group size effects (p>0.9).
Figure 1.
Partial phylogeny illustrating the distribution of populations and species in this study. Populations without group size effects are illustrated in black, and those with group size effects are white. Abbreviations: asterisk=predator free, M=mainland population, I=island population, C=captive population. Source of group size effects results are specified.
Table 1.
Effect of isolation on islands on group size effects (GSE).
| independent variable state | dependent variable state | p-value | |
|---|---|---|---|
| all 32 populations | gained GSE | remained without GSE | |
| mainland | 8 | 3 | 0.016 |
| island | 2 | 9 | |
| kept GSE | lost GSE | ||
| mainland | 18 | 6 | 0.507 |
| island | 11 | 6 | |
| 23 populations only | gained GSE | remained without GSE | |
| mainland | 0 | 2 | >0.9 |
| island | 0 | 4 | |
| kept GSE | lost GSE | ||
| mainland | 14 | 3 | >0.9 |
| island | 17 | 5 | |
p-values from the contingent states test that evaluates the hypothesis that evolutionary change in the presence of group size effects is more or less likely when a population is in a particular independent variable state (i.e. found on the mainland or on an island).
Table 2.
Effect of isolation from predators on group size effects (GSE).
| independent variable state | dependent variable state | p-value | |
|---|---|---|---|
| all 32 populations | gained GSE | remained without GSE | |
| no predators | 10 | 9 | 0.221 |
| predators | 0 | 3 | |
| kept GSE | lost GSE | ||
| no predators | 22 | 6 | 0.146 |
| predators | 7 | 6 | |
| twenty-three populations only | gained GSE | remained without GSE | |
| no predators | 0 | 3 | >0.9 |
| predators | 0 | 3 | |
| kept GSE | lost GSE | ||
| no predators | 26 | 7 | >0.9 |
| predators | 5 | 1 | |
p-values from the contingent states test that evaluates the hypothesis that evolutionary change in the presence of group size effects is more or less likely when a population is in a particular independent variable state (i.e. living with or without predators).
Independent contrast analyses on our data collected using identical protocols demonstrated that insularity itself, rather than the loss of all predators, may eliminate some, but not all, anti-predator behaviour. After accounting for non-significant variation explained by body mass (p-values>0.36), there was a significant effect of insularity on time allocated to foraging (p=0.009) and vigilance (p=0.009). Animals on islands were less wary, allocating 20.8% (±7.69 s.d.) of their time to vigilance compared with those on those on the mainland, which allocated 43.6% (±21.8 s.d.). Consequently, insular animals allocated a greater percentage time to foraging, 73.3% (±7.57 s.d.), than did mainland ones, 49.3 (±23.0 s.d.). There was no effect of isolation from all predators on time allocation (p-values>0.85). After significant variation (p<0.001) was explained by the distance we initiated our experimental approaches (Blumstein 2003), and after non-significant variation was explained by body mass (p>0.93), neither isolation on islands (p=0.221), nor from all predators (p=0.790), explained significant variation in flight initiation distance.
4. Discussion
Taken together, these results suggest that some, but not all, anti-predator behaviour is lost following isolation on islands. These results may reflect variation in developmental mechanisms underlying anti-predator behaviour. While some anti-predator behaviour may be heritable (Breden et al. 1987; Riechert & Hedrick 1990; Cousyn et al. 2001), other behaviour may be much more phenotypically plastic. Indeed, both time allocation (Hunter & Skinner 1998; Laundré et al. 2001) and flight initiation distance (Ikuta & Blumstein 2003) may be highly experience-dependent and thus vary for reasons other than variation in the number or types of predators per se.
More importantly, the loss of some anti-predator behaviour types does not necessarily result from the loss of all predators. This result was unexpected and is novel. While our sample size of predator-free populations is small (only 6/32 populations in the analyses of group size effects, and 4/18 populations in the analyses of continuous data were predator-free), our results suggest that although living without predators was associated with a lack of group size effects, the loss of all predators did not necessarily lead, over evolutionary time, to a subsequent loss of group size effects. Similarly, time allocation was influenced specifically by isolation on islands, rather than the presence or absence of predators: insular animals allocated less time to vigilance while foraging. Flightiness is apparently insensitive to either form of isolation because we found no effect of either insular living or the loss of all predators on flight initiation distance.
Thus, other factors, such as founder effects, may play an important role in determining which anti-predator behaviours persist in insular populations. This conclusion is consistent with the pattern of predator naiveté found on the Galápagos; an archipelago where native birds have some risk of predation from a much reduced community of predators, and where founder effects are likely to have been important in the past. It is conceivable that certain behavioural phenotypes (e.g. Sih et al. 2004b) have higher fitness in insular populations. If so, there may be correlated selection against certain anti-predator behaviours. For instance, island living may select against highly ‘reactive’ (Sih et al. 2004a) individuals and this selection will have wide-ranging effects on anti-predator behaviour. Future experimental research in model systems could isolate the effects of founder effects from various degrees of predator isolation.
Acknowledgments
We thank Erin Shelley for help with the comparative analyses, and Terry Ord and two anonymous reviewers for comments on previous versions of the manuscript. The Australian Research Council, Macquarie University, the Marsupial CRC and the UCLA Division of Life Sciences generously supported D.T.B.
Supplementary Material
References
- Beauchamp G. Group-size effects on vigilance: a search for mechanisms. Behav. Processess. 2003;63:111–121. doi: 10.1016/s0376-6357(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp G. Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. B. 2004;271:1039–1042. doi: 10.1098/rspb.2004.2703. 10.1098/rspb.2004.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednekoff P.A, Lima S.L. Randomness, chaos and confusion in the study of antipredator vigilance. Trends Ecol. Evol. 1998;13:284–287. doi: 10.1016/s0169-5347(98)01327-5. [DOI] [PubMed] [Google Scholar]
- Berger J, Swenson J.E, Persson I.-L. Recolonizing carnivores and naive prey: conservation lessons from Pleistocene extinctions. Science. 2001;291:1036–1039. doi: 10.1126/science.1056466. [DOI] [PubMed] [Google Scholar]
- Blumstein D.T. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 2002;29:685–692. [Google Scholar]
- Blumstein D.T. Flight initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manage. 2003;67:852–857. [Google Scholar]
- Blumstein D.T, Daniel J.C. Isolation from mammalian predators differentially affects two congeners. Behav. Ecol. 2002;13:657–663. [Google Scholar]
- Blumstein D.T, Daniel J.C. Developing predictive models of behaviour: do rock-wallabies receive an antipredator benefit from aggregation? Aust. Mammal. 2003a;25:147–154. [Google Scholar]
- Blumstein D.T, Daniel J.C. Foraging behavior of three Tasmanian macropodid marsupials in response to present and historical predation threat. Ecography. 2003b;26:585–594. [Google Scholar]
- Blumstein D.T, Daniel J.C. Red kangaroos (Macropus rufus) receive an antipredator benefit from aggregation. Acta Ethol. 2003c;5:95–99. [Google Scholar]
- Blumstein D.T, Evans C.S, Daniel J.C. An experimental study of behavioural group size effects in tammar wallabies (Macropus eugenii) Anim. Behav. 1999;58:351–360. doi: 10.1006/anbe.1999.1156. [DOI] [PubMed] [Google Scholar]
- Blumstein D.T, Evans C.S, Daniel J.C. 2000. JWatcher 0.9. An introductory user's guide.http://www.jwatcher.ucla.edu [Google Scholar]
- Blumstein D.T, Daniel J.C, Evans C.S. Yellow-footed rock-wallaby (Petrogale xanthopus) group size effects reflect a trade-off. Ethology. 2001a;107:655–664. [Google Scholar]
- Blumstein D.T, Daniel J.C, McLean I.G. Group size effects in quokkas. Aust. J. Zool. 2001b;49:641–649. [Google Scholar]
- Blumstein D.T, Daniel J.C, Schnell M.R, Ardron J.G, Evans C.S. Anipredator behaviour of red-necked pademelons: a factor contributing to species survival? Anim. Conserv. 2002;5:325–331. [Google Scholar]
- Blumstein D.T, Anthony L.L, Harcourt R.G, Ross G. Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol. Conserv. 2003a;110:97–100. [Google Scholar]
- Blumstein D.T, Daniel J.C, Sims R.A. Group size but not distance to cover influences agile wallaby (Macropus agilis) time allocation. J. Mammal. 2003b;84:197–204. [Google Scholar]
- Blumstein D.T, Daniel J.C, Springett B.P. A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology. 2004;100:919–934. [Google Scholar]
- Bonenfant M, Kramer D.L. The influence of distance to burrow on flight initiation distance in the woodchuck, Marmota monax. Behav. Ecol. 1996;7:299–303. [Google Scholar]
- Breden F, Scott M.A, Michel E. Genetic differentiation for anti-predator behaviour in the Trinidad guppy, Poecilia reticulata. Anim. Behav. 1987;35:618–620. [Google Scholar]
- Burk A, Springer M.S. Intergeneric relationships among Macropodoidea (Metatheria: Diprotodontia) and the chronicle of kangaroo evolution. J. Mamm. Evol. 2000;7:213–237. [Google Scholar]
- Campeau-Peloquin A, Kirsch J.A.W, Eldridge M.D.B, Lapointe F.-J. Phylogeny of the rock-wallabies, Petrogale (Marsupialia: Macropodidae) based on DNA/DNA hybridisation. Aust. J. Zool. 2001;49:463–486. [Google Scholar]
- Colagross A.M.L, Cockburn A. Vigilance and grouping in the eastern grey kangaroo, Macropus giganteus. Aust. J. Zool. 1993;41:325–334. [Google Scholar]
- Coulson G. Monospecific and heterospecific grouping and feeding behavior in grey kangaroos and red-necked wallabies. J. Mammal. 1999;80:270–282. [Google Scholar]
- Cousyn C, De Meester L, Colbourne J.K, Brendonck L, Verschuren D, Volckaert F. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA. 2001;98:6256–6260. doi: 10.1073/pnas.111606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio E. How finches react to predators. Animals. 1966;9:142–143. [Google Scholar]
- Darwin C. Henry Colburn; London: 1839. Journal of researches into the geology and natural history of the various countries visited by H. M. S. Beagle, under the command of Captain Fitzroy, R. N. from 1832 to 1836. [Google Scholar]
- Endler J.A. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Endler J.A. Princeton University Press; 1986. Natural selection in the wild. [Google Scholar]
- Felsenstein J. Sinauer; Sunderland, MA: 2004. Inferring phylogenies. [Google Scholar]
- Flannery T.F. Phylogeny of the Macropodidea; a study in convergence. In: Grigg G, Jarman P, Hume I, editors. Kangaroos, wallabies and rat-kangaroos. vol. 1. Surrey Beatty & Sons; Chipping Norton, New South Wales: 1989. pp. 1–46. [Google Scholar]
- Gittleman J.L, Gomper M.E. The risk of extinction—what you don't know will hurt you. Science. 2001;291:997–999. doi: 10.1126/science.291.5506.997. [DOI] [PubMed] [Google Scholar]
- Heathcote C.F. Grouping of eastern grey kangaroos in open habitat. Aust. Wildl. Res. 1987;14:343–348. [Google Scholar]
- Hunter L.T.B, Skinner J.D. Vigilance behaviour in African ungulates: the role of predation pressure. Behaviour. 1998;135:195–211. [Google Scholar]
- Ikuta L.A, Blumstein D.T. Do fences protect birds from human disturbance? Biol. Conserv. 2003;112:447–452. [Google Scholar]
- Jarman P.J. Group size and activity in eastern grey kangaroos. Anim. Behav. 1987;35:1044–1050. [Google Scholar]
- Johnson C.N. Macropod studies at Wallaby Creek. IV. Home range and movements of the red-necked wallaby. Aust. Wildl. Res. 1987;14:125–132. [Google Scholar]
- Kavaliers M. Responsiveness of deer mice to a predator, the short-tailed weasel: population differences and neuromodulatory mechanisms. Physiol. Zool. 1990;63:388–407. [Google Scholar]
- King C.M, editor. The handbook of New Zealand mammals. Oxford University Press; Auckland: 1990. [Google Scholar]
- Kirsch J.A.W, Lapointe F.-J, Springer M.S. DNA-hybridisation studies of marsupials and their implications for metatherian classification. Aust. J. Zool. 1997;45:211–280. [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; 2002. Living in groups. [Google Scholar]
- Lack D. Cambridge University Press; 1947. Darwin's finches. [Google Scholar]
- Laundré J.W, Hernández L, Altendorf K.B. Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Can. J. Zool. 2001;79:1401–1409. [Google Scholar]
- Lima S.L. Putting predators back into behavioral predator–prey interactions. Trends Ecol. Evol. 2002;17:70–75. [Google Scholar]
- Lindenfors P.U.M. Stockholm University; 1999. CoSta. [Google Scholar]
- Maddison W.P. A method for testing the correlated evolution of two binary characters: are gains or losses concentrated on certain branches of a phylogenetic tree? Evolution. 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Maddison W.P, Maddison D.R. Sinauer Associates; Sunderland, MA: 2001. MacClade: analysis of phylogeny and character evolution. Version 4.03. [Google Scholar]
- Magurran A.E. The causes and consequences of geographic variation in antipredator behavior: perspectives from fish populations. In: Foster S.A, Endler J.A, editors. Geographic variation in behavior: perspectives on evolutionary mechanisms. Oxford University Press; New York: 1999. pp. 139–163. [Google Scholar]
- Magurran A.E, Seghers B.H, Shaw P.W, Carvalho G.R. The behavioral diversity and evolution of guppy, Poecilia reticulata, populations in Trinidad. Adv. Study Behav. 1995;24:155–202. [Google Scholar]
- Martins E.P. Department of Biology, Indiana University; Bloomington, IN: 2003. COMPARE, version 4.5. [Google Scholar]
- Payne A.L, Jarman P.J. Macropod studies at Wallaby Creek. X. Responses of eastern grey kangaroos to cattle. Wildl. Res. 1999;26:215–225. [Google Scholar]
- Riechert S.E, Hedrick A.V. Levels of predation and genetically based anti-predator behaviour in the spider, Agelenopsis aperta. Anim. Behav. 1990;40:679–687. [Google Scholar]
- Sharman G.B. Opening address—a chromosome phylogeny of kangaroos. In: Grigg G, Jarman P, Hume I, editors. Kangaroos, wallabies and rat-kangaroos. vol. 1. Surrey Beatty & Sons; Chipping Norton, New South Wales: 1989. pp. v–vii. [Google Scholar]
- Sih A, Bell A.M, Johnson J.C. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004a;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A.M, Johnson J.C, Ziemba R.E. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 2004b;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Sillén-Tullberg B. The effect of biased inclusion of taxa on the correlation between discrete characters in phylogenetic trees. Evolution. 1993;47:1182–1191. doi: 10.1111/j.1558-5646.1993.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Stoks R, McPeek M.A, Mitchell J.L. Evolution of prey behavior in response to changes in predation regime: damselflies in fish and dragonfly lakes. Evolution. 2003;57:574–585. doi: 10.1554/0014-3820(2003)057[0574:EOPBIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Strahan R. Reed Books; Chatswood, New South Wales: 1995. The mammals of Australia. [Google Scholar]
- Taylor A.C, Cooper D.W. Microsatellites identify introduced New Zealand tammar wallabies (Macropus eugenii) as an “extinct” taxon. Anim. Conserv. 1999;2:41–49. [Google Scholar]
- Van Buskirk J. Specific induced response to different predator species in anuran larvae. J. Evol. Biol. 2001;14:482–489. [Google Scholar]
- van Damme R, Castilla A.M. Chemosensory predator recognition in the lizard Podarcis hispanica: effects of predation pressure relaxation. J. Chem. Ecol. 1996;22:13–22. doi: 10.1007/BF02040196. [DOI] [PubMed] [Google Scholar]
- Wahungu G.M, Catterall C.P, Olsen M.F. Predator avoidance, feeding and habitat use in the red-necked pademelon, Thylogale thetis, at rainforest edges. Aust. J. Zool. 2001;49:45–58. [Google Scholar]
- Watts D. Peregrine Press; Kettering, Tasmania: 1993. Tasmanian mammals: a field guide, Revized Edition. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.