Abstract
Human social behaviour is influenced by attributing mental states to others. It is debated whether and to what extent such skills might occur in non-human animals. We here test for the possibility of ravens attributing knowledge about the location of food to potential competitors. In our experiments, we capitalize on the mutually antagonistic interactions that occur in these birds between those individuals that store food versus those that try to pilfer these caches. Since ravens' pilfer success depends on memory of observed caches, we manipulated the view of birds at caching, thereby designing competitors who were either knowledgeable or ignorant of cache location and then tested the responses of both storers and pilferers to those competitors at recovery. We show that ravens modify their cache protection and pilfer tactics not simply in response to the immediate behaviour of competitors, but also in relation to whether or not they previously had the opportunity of observing caching. Our results suggest that the birds not only recall whom they had seen during caching, but also know that obstacles can obstruct the view of others and that this affects pilfering.
Keywords: raven, Corvus corax, food caching, knowledge attribution
1. Introduction
Living in a complex social world requires sophisticated knowledge about conspecifics (Jolly 1966; Humphrey 1976) that allows individuals to predict the behaviour of others and to develop tactics to manipulate others (e.g. Byrne & Whiten 1988). With respect to these socio-cognitive skills, the key question concerns the understanding of mental states, i.e. whether individuals know that others have perceptions, intentions or beliefs (Premack & Woodruff 1978). The ability of attributing mental states to others is considered one of the major factors demarcating humans from non-human animals (e.g. Cheney & Seyfarth 1990; Visalberghi & Fragaszy 1990; Tomasello & Call 1997; Heyes 1998; Povinelli et al. 2000).
Recently, the assumption of an all-or-nothing approach with non-human animals lacking any capacities for attending to mental states has been challenged theoretically and empirically (Byrne & Whiten 1992; Whiten 1996; Allen & Bekoff 1997; Tomasello et al. 2003). Primates (e.g. Hostetter et al. 2001; Povinelli et al. 2003; Tsutsumi et al. 2003), dogs Canis familiaris (Gácsi et al. 2004) and even birds, such as bee-eaters Merops orientalis (Watwe et al. 2002), show situation-dependent recognition of human's attention. Great apes discriminate between intentional and accidental actions of humans (Call & Tomasello 1998; Call et al. 2004) and visually signal to conspecifics only when the intended recipient is already looking and thus potentially able to see them (Tomasello et al. 1994; Liebal et al. 2004). Moreover, when chimpanzees Pan troglodytes compete with dominant conspecifics over hidden food, they instantly differentiate between individuals that can and those that cannot see the reward (Hare et al. 2000), respectively, those that have and have not seen its hiding behind an opaque barrier (Hare et al. 2001). However, when chimpanzees interact cooperatively with humans to gain hidden food (e.g. through begging gestures) they merely learn over many trials to discriminate between a knowledgeable and an ignorant person (Povinelli & Eddy 1996; Reaux et al. 1999). It has been suggested that the chimpanzee's capacity to reason about the others' view is expressed specifically in tasks that simulate evolutionary salient problems (Hare et al. 2000, 2001; but see Povinelli & Vonk 2003).
Ravens are scavengers that cache temporary surpluses of food and food that is contested by others. In the wild and in captivity, they are sensitive to conspecifics during caching and selectively place caches at a distance from others (Lorenz 1935; Heinrich & Pepper 1998) and/or behind objects that might obstruct the others' view (Bugnyar & Kotrschal 2002). If potential pilferers come near one of those caches they are chased off and/or the food is re-cached elsewhere (Heinrich 1999; Bugnyar & Kotrschal 2002). However, such cache-guarding is costly. It constrains foraging and reduces the ability to make further caching trips from communal foraging sites (Heinrich 1999). Pilferers differ markedly in their effectiveness of finding caches depending on whether or not they have witnessed caching (Heinrich & Pepper 1998). Like Pinyon jays Gymnorhinus cyanocephalus (Bednekoff & Balda 1996) and western scrub jays Aphelocoma californica (Emery & Clayton 2001), ravens possess observational spatial memory that allows them to exactly remember the locations of caches they have seen others make (Bugnyar & Kotrschal 2002). We here examine whether ravens differentiate between potential competitors who have, and those who have not, had the opportunity to observe the cache making, and thus could assess who would probably pose a high, or low, threat to the caches. By focusing first on storers and then on pilferers, we stepwise control for behavioural alternatives to judge the risk of pilfering.
2. Storer's perspective
(a) Experiment 1
Ravens that had previously stored food in a spacious aviary compartment were faced with the choice of retrieving their caches either (i) in private, (ii) in the presence of a subordinate competitor that had the opportunity to watch the caching from an adjacent compartment (observer) or (iii) in the presence of a subordinate competitor that had not watched the caching (non-observer) because its view was blocked by opaque curtains. We predicted that storers should be more likely to recover caches when confronted with former observers than non-observers or when alone. Differentiation between competitors could result from attributing knowledge (that seeing caching leads to knowing about cache location), from using their own perspective (remembering who was present/absent at caching) or from using behavioural cues such as different approach patterns. In the latter case, we would predict observers to go more directly for the caches than non-observers.
(b) Methods
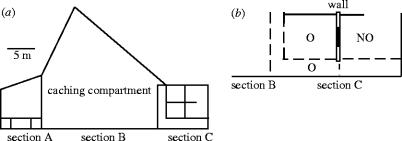
We used seven hand-raised ravens (five males, two females; six birds were in their second year, one male was in his fifth year of age), all of whom were familiar with experimental testing and had participated in a previous study on food caching. Birds were marked with coloured rings for individual identification. They were housed together in an outdoor aviary composed of three sections (section A: 30 m2, section B: ca. 100 m2, section C: 64 m2) separated by wire partitions (figure 1a). Sections A and B contained trees and natural ground cover. Section C consisted of three small compartments (12, 12, 24 m2) and interconnecting pathways (total of 16 m2). Compartments contained horizontal poles for perching and were separated by wooden walls. Walls included windows that could be covered by opaque curtains to manipulate visual access between compartments. Birds were allowed to freely roam in the entire complex during most of the day. For testing, the designated storer was placed into section B, its potential competitors were positioned in compartments of section C and subjects that did not participate in that trial were confined to section A. Birds were fed on their normal diet after the tests. Water was provided ad libitum.
Figure 1.
Sketch of aviary complex, indicating (a) the location of the test compartment (section B) and (b) the position of competitors at caching (section C). Observers (O) had visual access to the storer in section B (broken lines symbolize wire mesh), whereas the view of non-observers (NO) was totally obscured by an opaque wall (white bar) and closed curtains (solid bar). Subjects in the pathway (O below) could have visual access to both potential competitors (O above, NO), as the compartment's side wall was made of wire mesh.
Tests were conducted from February to April 2003 on 3–5 d week−1 and usually started at 09:00 h. Each test consisted of a caching trial and a retrieval trial for focal subjects (n=three trials per treatment). During caching trials, the storer received three pieces of food (meat, 10 g per piece) that it was free to cache in the entire section B. On average, a storer created 3.6 (±0.3 s.e.) caches per trial of which it immediately recovered 0.9 (±0.1), leaving 2.7 (±0.3) caches for the retrieval trial. Caching trials were terminated when all food was cached and/or consumed by the storer and, on average, lasted for 5 (±0.3) min. During every caching trial, two potential competitors were enclosed in section C. The non-observer was in a compartment with the windows covered by a curtain so that its view of the storer was completely obscured. The observer was in a compartment with a transparent window and/or in its adjacent pathway and always had clear view of the storer (figure 1b). Both competitors occasionally gave calls and thus were in acoustical contact with the storer.
After each caching trial, the storer was removed from section B. Five minutes later, the retrieval trial started in which one subject (in-private treatment) or two subjects (competitive treatment) were allowed to recover and/or pilfer the cached food for 10 min. In the in-private treatment for storers, the storer was allowed to return to section B in the absence of competitors. In the in-private treatment for pilferers, an observer and a non-observer were allowed to search for the caches in the absence of the storer. In the competitive treatment, the storer gained access to section B together with an observer or non-observer. The order of treatments per experiment as well as the identity of competitors per treatment changed in a pseudo-randomized order. In each treatment, birds were confronted with the same competitor in the first trial and, in case the birds' position in dominance rank hierarchy allowed for more combinations, with different competitors in the second and third trial. Competitors were always dominant over the focal subject. Dominance status of individuals was known before the onset of the experiments and calculated on the basis of approach–retreat interactions.
Owing to the size of the test compartment (section B), video-taping turned out to be inappropriate to capture the behaviour of both competitors simultaneously. (Focusing on two birds with a single camera produced pictures that were too small to allow identification of individual markings; using two cameras appeared to disturb the birds as it required two people frequently changing positions and exchanging information on the locations and behaviour of the subjects.) Therefore, T.B. recorded all data by direct observation with pre-drawn sketches of the test compartment and a voice recorder (Olympus Pearlcorder S701), standing outside the aviary next to the wire partition of section B. Birds were fully habituated to T.B. taking behavioural protocols from that position, which was a standard procedure executed since fledging for 1–3 h d−1. During caching trials, he recorded the location of caches, the order in which they were made and the number of immediate recoveries by the storer. During retrieval trials, he measured the number of caches recovered by the storer, the number and location of searches by the competitor, as well as the number of caches found. In addition, he measured the time (s) until the first cache was recovered/pilfered. When a bird was digging for the food (mean±s.e. time to find food=7±1 s), he estimated the distance between competitors based on relative size of the enclosure. He also noted all social interactions between subjects during retrieval trials. Videos made in the pilot phase were used to validate the reliability of data collection. T.B. and a second person who was familiar to the ravens but not involved in the present experiment showed a high level of agreement (Cohen's κ=0.87) on their ratings of behavioural categories mentioned above.
We used a Wilcoxon signed-ranks test and a Friedman test on the individuals' mean values across trials to compare the birds' performance between treatments. When applying Wilcoxon test, we calculated the exact p values using Table V in Sokal & Rohlf (1995). As the numbers of caches recovered and pilfered depend on the activity of the storer during caching trials, we calculated those data as a proportion of caches that were present at the start of retrieval trials. Focusing on either the dominant or the subordinate individual reduced our sample size to n=6 subjects. For all analysis, results are given two-tailed and alpha was set at 0.05.
(c) Results and discussion
As predicted, storers retrieved a significantly higher proportion of caches when they were paired with birds that had previously observed them caching than when paired with birds that could not witness the caching event (n=6, T=0 and p=0.032; figure 2a). Furthermore, the storers' response was exhibited only when observers were moving towards the caches (n=6, T=0 and p=0.032; figure 2b). If a conspecific did not come close (less than 2 m) to the caches, recover rates did not differ between observer, non-observer and in-private trials (χ22=1.83 and p=0.4; figure 2b). Although observers found significantly more caches than non-observers when tested in private (n=6, T=0 and p=0.032), their pilfer success did not differ from that of non-observers in the presence of storers (n=6, T=3 and p=0.16), indicating that the selective cache protection of storers paid off.
Figure 2.
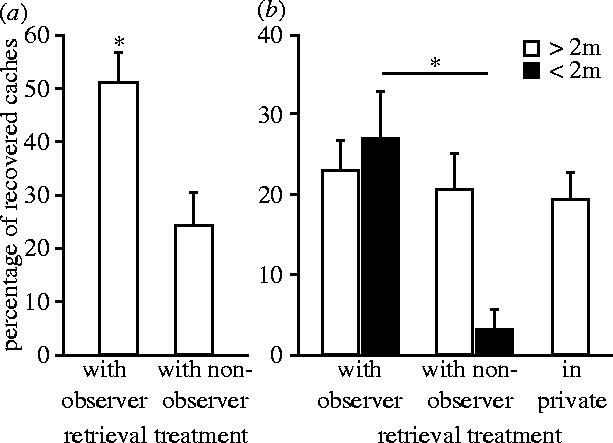
Mean (±s.e.) percentage of caches recovered by storers (a) with previous observers (‘knowers’) and non-observers (‘ignorants’) and (b) when those competitors were moving towards the caches (black bars) or did not come close to the caches (white bars). Exact Wilcoxon signed-ranks test. Asterisk denotes p<0.05.
We also predicted that, if the storers' decision to recover caches is based on the behaviour of pilferers, then there should be differences in the directedness of approach to the caches between observers (knowledgeable pilferers) and non-observers (ignorant pilferers). When tested in private, observers were quicker than non-observers to approach a cache site and they were searching less and at fewer places (table 1). However, when paired with the storer, observers did not differ significantly from non-observers in any of those parameters (table 1). Thus, in contrast to the prediction and despite their knowledge on cache location, observers did not go directly for the caches in the presence of storers. Similar to non-observers, they engaged in apparent searching at various places of the aviary before they approached a cache site. Storers did not wait close by their caches nor did they initiate more agonistic interactions with observers than non-observers before pilfering attempts (table 1). Thus, it is unlikely that overt intimidation by storers prevented observers from straight-forward pilfering.
Table 1.
Behaviour of observers and non-observers when tested in private and with storer.
| behaviour | type of visual experience | exact Wilcoxon test | |||
|---|---|---|---|---|---|
| observer | non-observer | n | T | p | |
| latency to approach (s) | |||||
| in private | 42±8 | 116±20 | 6 | 1 | 0.06 |
| with storer | 82±13 | 131±31 | 6 | 4 | >0.2 |
| time spent searchinga(s) | |||||
| in private | 5±2 | 38±9 | 6 | 0 | 0.03 |
| with storer | 11±4 | 26±9 | 6 | 3 | 0.16 |
| number of places visitedb | |||||
| in private | 0.4±0.1 | 3.8±1 | 6 | 0 | 0.03 |
| with storer | 1.3±0.3 | 2.3±0.8 | 6 | 7 | >0.2 |
| storer's distance (m) at pilfering | 5±0.4 | 6±0.5 | 5 | 1 | 0.13 |
| numbers of agonistic interactionsc | 3.4±0.4 | 2.8±0.5 | 6 | 7 | >0.2 |
A Wilcoxon signed-rank test compared the effect of visual experience for each of the behaviours listed in the solitary and social condition. Exact p-values are given if possible. Data are mean±s.e.
Time spent digging in substrate to find first cache.
Number of places that were searched to find first cache.
Before pilfering.
Taken together, our results show that ravens selectively recover caches in the presence of former observers, confirming previous findings that ravens secure hidden food from conspecifics that could pose a threat to their caches (Heinrich 1999; Bugnyar & Kotrschal 2002). Moreover, the present results provide little support for the hypothesis that approach patterns of potential pilferers allow storers to distinguish knowledgeable from ignorant competitors. Still, storers could have oriented on subtle behavioural cues not measured by the human experimenter. More probably, storers could have remembered the identity of the bird that was watching the caching episode. Responding solely to those individuals that they could see at the time of caching (as opposed to what the competitor has or has not seen) would be a simple but efficient rule to explain the selective cache protection in ravens.
3. Pilferers' perspective
(a) Experiment 2
We designed this experiment to further evaluate the possibility that ravens know what others have and have not seen, and to exclude that the differentiation between competitors is based on the birds' own view at caching. Here we focused solely on the interactions between potential pilferers. We proposed that ravens would apply different pilfer tactics depending on whether or not their competitors could also see the caching event and thus would be knowledgeable or ignorant on cache location. In front of ignorant dominants that would be unlikely to find caches themselves, knowers should delay pilfering until dominants were occupied with searching at some distance from the cache to reduce the risk of attracting the other's attention while digging for the food. However, the same tactic would be disadvantageous with competitors that also had the chance for observational learning during caching. Since observers have little problems in finding a cache, being first at the cache should be the best way for either individual to secure the reward and consume it before being approached. Thus, with knowledgeable competitors, we expected ravens not to delay but go directly for the caches. We tested these opposing predictions by allowing birds to pilfer previously observed caches either (i) in private, (ii) together with a conspecific that had also witnessed caching (co-observer) or (iii) with a conspecific that was present during caching but prevented from watching by an opaque curtain (non-observer). During caching, both co-observer and non-observer were present and visible to the focal subject. We thus controlled for the possibility that observers could base their decision on memory of their own perspective at the time of caching. During pilfering, we measured who started approaching the cache, who was first at the cache, and who received the food, to check for the possibility of behavioural cueing.
In contrast to the previous study, we here had a human experimenter serve as storer in order to hold the cache number constant to one, thereby enabling us to analyse the success of different pilfer tactics in terms of whether or not the food was gained. Furthermore, we tested both subordinate and dominant subjects to address the birds' tactic flexibility. We predicted that ravens should delay pilfering only when ignorant competitors were dominant because those could displace them from caches. However, birds should speed up pilfering with any knowledgeable competitor, independent of its social status.
(b) Methods
Subjects were the same as in experiment 1. Tests were conducted from May to June 2003 and followed the same logic as in experiment 1. This time, a human experimenter cached only one piece of meat (25 g) in a randomly chosen location of section B, while three ravens, one designated as non-observer and two as observers, were enclosed in section C (figure 1b). The non-observer was in a compartment from which its view of the caching room (section B) was totally obscured by a wooden wall and covered windows. Both observers had full visual access to the caching room either from a compartment with an uncovered window or from the adjacent pathway, of which the walls were made of wire mesh. The observer in the pathway became the focal subject in the subsequent retrieval trial because its position allowed a view of the human storer and both potential competitors (figure 1b).
During retrieval trials, the focal observer got access to section B either in private, together with the co-observer or together with the non-observer (n=2 trials per treatment with a dominant and a subordinate competitor). In each treatment, birds were confronted with the same competitor in the first trial and, in case the birds' position in dominance rank hierarchy allowed for more combinations, with a different competitor in the second trial. Data collection and analysis were the same as in experiment 1.
(c) Results and discussion
Compared with in-private trials, observers significantly delayed pilfering with dominant non-observers (i.e. focal subject subordinate), but directly went for the cache with either co-observer (χ42=11.35 and p=0.023; figure 3). The key-result is that the mean time to pilfer was approximately 10 times longer for the focal subject when paired with a dominant non-observer than with a dominant co-observer (n=6, T=0 and p=0.032). Despite their delayed approach, focal subjects were first at the cache in all but one of the cases with non-observers. In contrast, they were first at the cache in only half of the cases with co-observers. In those observer–observer pairs, subordinates were more often the first to start approaching (χ12=4 and p<0.05), although they were passed by the dominant right before the cache in 79% of the cases. Mean distance to competitors at pilfering was 6 m (±0.6 s.e.) with a dominant non-observer compared with 0.6 m (±0.8 s.e.) with a dominant co-observer (n=6, T=0 and p=0.032). Dominant observers got the hidden food in all cases when the competitor was a non-observer and in 79% (±1 s.e.) of the cases when the competitor was a co-observer. As subordinates, birds performed poorly with co-observers (successful in 12±1%), but significantly better with non-observers (successful in 69±1%; n=6, T=0 and p=0.032). Thus, speeding up pilfering with knowledgeable dominants appears to be a best-of-a-bad-job tactic for subordinates, whereas delaying pilfering with ignorant dominants pays off.
Figure 3.
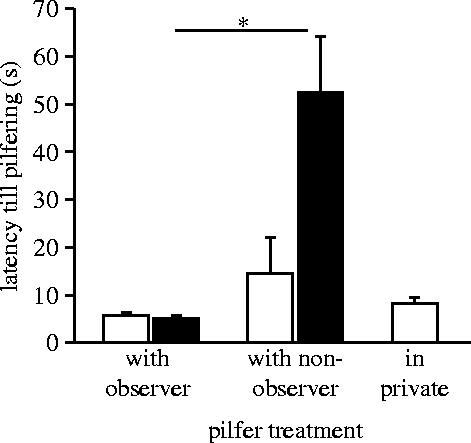
Mean (±s.e.) latency till pilfering of observers when together with knowledgeable and ignorant competitors and in private. White bars represent focal subjects as dominants, black bars as subordinates. Exact Wilcoxon signed-ranks test. Asterisk denotes p<0.05.
The specific use of opposing pilfer tactics suggests that ravens judge competitors on the basis of whether or not the others' sight was obstructed at caching, and flexibly integrate this knowledge into their decision making, together with the relative dominance status and previous role of competitors. Learning throughout the experiment does not account for the results since all subjects showed the responses immediately. Behavioural cueing while approaching the cache would be a possible alternative, but does not seem to be relevant because birds often started approaching the cache ahead of knowledgeable dominants, when they needed to be quick. Since both observers and co-observers always hurried to the cache (figure 3), subordinates could not achieve this head start by first checking whether the opponent was going for the cache or not. Moreover, birds markedly changed their behaviour in the presence of naïve dominants, since they delayed pilfering and searched at other places rather than directly at the cache sites. Apparently, pilferers avoided provoking the counter tactics of those competitors, corroborating the results of experiment 1 and supporting the idea that foraging ravens may engage in functional tactical deception (Bugnyar & Kotrschal 2002).
Again, it is possible that ravens have oriented on behavioural cues people do not readily perceive. For instance, individuals could have indicated their state of knowledge while observing the caching event (e.g. by calling and/or conspicuously following the storer along the wire-partitioning), which then could have been remembered by the competitor. However, detailed video-records on the birds' observing behaviour in a follow-up study revealed that observers hardly drew attention to themselves and, compared with non-observers, were relatively silent (own unpublished data).
4. General discussion
Our studies demonstrate that ravens show fairly sophisticated skills when competing with conspecifics over hidden food. As storers, they selectively retrieved caches with individuals that were visible at the time of caching, and as pilferers, they hurried to the caches only with those competitors that had also had visual access to the caching room and thus were likely to pilfer the caches themselves. These results suggest that ravens not only recall whom they had seen during caching (experiment 1), but also know that the others' view can be obstructed by opaque structures (experiment 2) and that this affects their chances of finding cached food. Hence, ravens show a level of understanding that, similar to chimpanzees (Tomasello et al. 2003), may exceed the recognition of behavioural attributes that are associated with seeing (i.e. presence versus absence of competitors, different approach pattern).
This interpretation stands in line with other recent findings in ravens and other corvids, all indicating an enormous cognitive potential in the social domain (Emery & Clayton 2004). Pinyon jays are able to infer the relative dominance rank of unfamiliar individuals after watching them competing over food with known conspecifics (Paz-y-Miño et al. 2004). Western scrub jays and ravens actively hide from view of potential competitors at caching, whereby scrub jays take into account the properties of shade (Dally et al. 2004) and ravens the exact position of observers for the possibility of using dead angles (Bugnyar & Heinrich 2003). Ravens even follow the direction of a human's gaze to specific locations behind a visual barrier (Bugnyar et al. 2004).
However, showing some understanding of the view of others, i.e. that their line of sight can be interrupted by obstacles, does not allow to infer a human-like understanding of mental states in ravens. On the one hand, individuals could have learned about the relationship of an observer's viewpoint to its later competitive behaviour prior to the experiments during daily foraging (e.g. Heyes 1998). Thereby, they could have even come to understand how to deal with competitors in front and behind obstacles without the need of taking their visual perspective (Call 2001; Hare et al. 2001; Povinelli & Vonk 2003). On the other hand, the label ‘theory of mind’ (Premack & Woodruff 1978) appears to cover a wide range of socio-cognitive processes (Tomasello et al. 2003), some of which may occur in some non-human animals whereas others do not. Ravens appear to be one of the candidates for evaluating the concept ‘see’.
Acknowledgments
We thank M. Stöwe for assistance and U. Aust, R. Biegler, M. Bouton, G. Gaydon, C. M. Heyes, L. Huber, K. Kotrschal, I. M. Pepperberg, and two anonymous referees for valuable comments. T.B. has been funded by Erwin-Schrödinger grants J2064, J2225 and R31-B03 of the Austrian Science Fund. The experiments here described were approved by the Institutional Animal Care and Use Committee at the University of Vermont (on 1 November 2002, Protocol No. 01-054). Permits for ravens include US Federal Fish and Wildlife Permit Number MB689376-0, State of Maine Department of Inland Fisheries and Wildlife Permit 22077, and Vermont Fish and Wildlife Department Scientific Collecting Permit.
Footnotes
Present address: Konrad Lorenz Research Station Grünau and Department of Behaviour, Neurobiology, and Cognition, University of Vienna, Althanstrasse 14, 1090 Wien, Vienna, Austria.
References
- Allen C, Bekoff M, editors. Species of mind. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- Bednekoff P.A, Balda R.P. Social caching and observational spatial memory in pinyon jays. Behaviour. 1996;133:807–826. [Google Scholar]
- Bugnyar T, Heinrich B. Hiding in food-caching ravens, Corvus corax. Rev. Ethol. Suppl. 2003;5:57. [Google Scholar]
- Bugnyar T, Kotrschal K. Observational learning and the raiding of food caches in ravens, Corvus corax: is it “tactical deception”? Anim. Behav. 2002;64:185–195. [Google Scholar]
- Bugnyar T, Stöwe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc. R. Soc. B. 2004;271:1331–1336. doi: 10.1098/rspb.2004.2738. 10.1098/rspb.2004.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R.W, Whiten A, editors. Machiavellian intelligence. Social expertise and the evolution of intellect in monkeys, apes and humans. Clarendon Press; Oxford: 1988. [Google Scholar]
- Byrne R.W, Whiten A. Cognitive evolution in primates: evidence from tactical deception. Man. 1992;27:609–627. [Google Scholar]
- Call J. Chimpanzee social cognition. Trends Cogn. Sci. 2001;5:388–393. doi: 10.1016/s1364-6613(00)01728-9. [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. Distinguishing intentional from accidental actions in orangutans (Pongo pygmaeus), chimpanzees (Pan troglodytes) and human children (Homo sapiens) J. Comp. Psychol. 1998;112:192–206. doi: 10.1037/0735-7036.112.2.192. [DOI] [PubMed] [Google Scholar]
- Call J, Hare B, Carpenter M, Tomasello M. Unwilling or unable: Chimpanzees's understanding of human intentional action. Dev. Sci. 2004;7:488–498. doi: 10.1111/j.1467-7687.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- Cheney D.L, Seyfarth R.M. Attending to behaviour versus attending to knowledge: examining monkeys' attribution of mental states. Anim. Behav. 1990;40:742–753. [Google Scholar]
- Dally J.M, Emery N.J, Clayton N.S. Cache protection strategies by western scrub-jays (Aphelocoma californica): hiding food in the shade. Proc. R. Soc. B. 2004;271(Suppl. 6):S387–S390. doi: 10.1098/rsbl.2004.0190. 10.1098/rsbl.2004.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery N.J, Clayton N.S. Effects of experience and social context on prospective caching strategies by scrub jays. Nature. 2001;414:443–446. doi: 10.1038/35106560. [DOI] [PubMed] [Google Scholar]
- Emery N.J, Clayton N.S. Comparing the complex cognition of birds and primates. In: Rogers L.J, Kaplan G, editors. Comparative vertebrate cognition: are primates superior to non-primates? Kluwer Academic Press; The Hague: 2004. pp. 3–55. [Google Scholar]
- Gácsi M, Miklósi A, Varga O, Topál J, Csányi V. Are readers of our face readers of our minds? Dogs (Canis familiaris) show situation-dependent recognition of human's attention. Anim. Cogn. 2004;7:144–153. doi: 10.1007/s10071-003-0205-8. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Agnetta B, Tomasello M. Chimpanzees know what conspecifics do and do not see. Anim. Behav. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Anim. Behav. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Heinrich B, editor. Mind of the raven. Harper Collins; New York: 1999. [Google Scholar]
- Heinrich B, Pepper J.W. Influence of competitors on caching behavior in the common raven, Corvus corax. Anim. Behav. 1998;56:1083–1090. doi: 10.1006/anbe.1998.0906. [DOI] [PubMed] [Google Scholar]
- Heyes C.M. Theory of mind in nonhuman primates. Behav. Brain Sci. 1998;21:101–148. doi: 10.1017/s0140525x98000703. [DOI] [PubMed] [Google Scholar]
- Hostetter A.B, Cantero M, Hopkins W.D. Differential use of vocal and gestural communication by chimpanzees in response to the attentional status of a human. J. Comp. Psychol. 2001;115:337–343. doi: 10.1037//0735-7036.115.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey N.K. The social function of intellect. In: Bateson P, Hinde R, editors. Growing points in ethology. Cambridge University Press; Cambridge: 1976. pp. 303–321. [Google Scholar]
- Jolly A. Lemur social behavior and primate intelligence. Science. 1966;153:501–507. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- Liebal K, Pika S, Tomasello M. Social communication in siamangs (Symphalangus syndactylus): use of gestures and facial expressions. Primates. 2004;45:41–57. doi: 10.1007/s10329-003-0063-7. [DOI] [PubMed] [Google Scholar]
- Lorenz K.Z. Der Kumpan in der Umwelt des Vogels. J. Ornithol. 1935;83:137–215. (see also pages 289–413). [Google Scholar]
- Paz-y-Miño G.C, Bond A.B, Kamil A.C, Balda R.P. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Povinelli D.J, Eddy T.J. What young chimpanzees know about seeing. Monogr. Soc. Res. Child Dev. 1996;61 [PubMed] [Google Scholar]
- Povinelli D.J, Vonk J. Chimpanzee minds: suspiciously human? Trends Cogn. Sci. 2003;7:157–160. doi: 10.1016/s1364-6613(03)00053-6. [DOI] [PubMed] [Google Scholar]
- Povinelli D.J, Bering J, Giambrone S. Toward a science of other minds: escaping the argument by analogy. Cogn. Sci. 2000;24:167–201. [Google Scholar]
- Povinelli D.J, Theall L.A, Reaux J.E, Dunphy-Lelii S. Chimpanzees spontaneously alter the location of their gestures to match the attentional orientation of others. Anim. Behav. 2003;66:71–79. [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978;1:515–526. [Google Scholar]
- Reaux J.E, Theall L.A, Povinelli D.J. A longitudinal investigation of chimpanzees' understanding of visual perception. Child Dev. 1999;70:275–290. [Google Scholar]
- Sokal R.R, Rohlf F.J, editors. Biometry. 3rd edn. Freeman; New York: 1995. [Google Scholar]
- Tomasello M, Call J, editors. Primate cognition. Oxford University Press; New York: 1997. [Google Scholar]
- Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and the use of gestural signals by young chimpanzees: a trans-generational study. Primates. 1994;35:137–154. [Google Scholar]
- Tomasello M, Call J, Hare B. Chimpanzees understand psychological states—the question is which ones and to what extent. Trends Cogn. Sci. 2003;7:153–156. doi: 10.1016/s1364-6613(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Fujita K, Ushitani T. When wild vervets commit theft: are they reading the owner's attention? Rev. Ethologia Suppl. 2003;5:60. [Google Scholar]
- Visalberghi E, Fragaszy D. Do monkeys ape? In: Parker S, Gibson K, editors. “Language” and intelligence in monkeys and apes. Cambridge University Press; Cambridge: 1990. pp. 247–273. [Google Scholar]
- Watwe M, Thakar J, Kale A, Puntambekar S, Shaikh I, Vaze K, Jog M, Paranjape S. Bee-eaters (Merops orientalis) respond to what a predator can see. Anim. Cogn. 2002;5:253–259. doi: 10.1007/s10071-002-0155-6. [DOI] [PubMed] [Google Scholar]
- Whiten A. When does smart behaviour-reading become mind-reading? In: Carruthers P, Smith P.K, editors. Theories of theories of mind. Cambridge University Press; Cambridge: 1996. pp. 277–292. [Google Scholar]