Abstract
Ceratogaulus, a member of the extinct fossorial rodent clade Mylagaulidae, is the only known rodent with horns and the smallest known horned mammal. The function of the large, dorsally projecting nasal horns on this burrowing animal has been the subject of wide speculation among palaeontologists; suggested uses range from sexual combat to burrowing. Mammals have evolved adaptations for digging repeatedly; horns and other cranial appendages have also evolved numerous times. These two adaptations co-occur in mammals extremely rarely: only two fossil genera (Ceratogaulus and the xenarthran Peltephilus) and no extant mammals are both horned and fossorial. Tracing the evolution of fossoriality in aplodontoid rodents (the larger clade to which Ceratogaulus belongs) reveals that Ceratogaulus descended from ancestors who dug by head-lifting. Whereas this suggests an obvious explanation for the horns of this rodent, evidence from functional morphology, anatomy, phylogeny and geologic context indicates that the horns in Ceratogaulus were used for defence, rather than digging, and evolved to offset increased predation costs associated with spending more time foraging above ground as body size increased.
Keywords: cranial appendages, adaptation, defence, predation, grasslands, Ceratogaulus
1. Introduction
The evolution of cranial appendages in extinct animals poses a complex problem for palaeontologists. The morphologically and functionally diverse cranial appendages of modern ungulates have been used as analogues for studying the variety of cranial appendages in fossil vertebrates. However, it has proven difficult to apply our knowledge of cranial appendages in extant animals to the diverse horns and cranial adornment in dinosaurs (Goodwin & Horner 2004), brontotheres (Stanley 1974; Janis 1982) and even other fossil artiodactyls with cranial appendages of independent origins (Joeckel 1990). It is rapidly becoming apparent that studies of the evolution of cranial appendages in fossil animals require not just a comparison with modern animals but also study of functional morphology, phylogeny and evolutionary ecology. Horned mylagaulids, in particular, require more than just analogy to determine the adaptive role of horns because there are no modern analogues for a fossorial (digging) animal with horns.
Numerous terrestrial mammals have evolved fossorial adaptations and rodents in particular have repeatedly diversified into underground habitats. Mylagaulids are among the most speciose clades of fossorial rodents and are common in North American Miocene faunas. Phylogenetic analysis of the Aplodontoidea (see Hopkins submitted) reveals that mylagaulids are the sister group to the much less speciose Aplodontinae, including Aplodontia rufa, the mountain beaver, which is also a fossorial animal. Mylagaulids, like Aplodontia, are large rodents with distinct postcranial adaptations to a fossorial life habit, including a broad, robust skull, long, spatulate digging claws, short forelimbs with broad joint articulations and heavily built limb girdles (the fossorial adaptations of mylagaulid postcrania have been described in detail previously; Fagan 1960). Most of the 29 described species of mylagaulids also have flat skull roofs, a feature used by most fossorial mammals in burrow construction. Although the first mylagaulids were described more than 100 years ago, very little is understood about their ecology. As the Aplodontoidea include one of the only horned, fossorial animals ever to evolve, understanding the course of evolution of fossoriality in this group is essential to explain how horns could evolve in a fossorial animal.
Mammals have evolved several distinct methods of burrow excavation. Modern rodents use one of three methods to break up soil (see Stein 2000 for illustrations and a more complete discussion). As with other mammals, the predominant mode of digging among rodents is scratch digging, in which the claws of the manus are used to break up soil. Many rodents also make use of the ever-growing incisors which diagnose the clade to excavate their burrows. Rodents which excavate using both upper and lower incisors in a chewing motion are referred to as chisel-tooth diggers. A final mode of digging, and the least common among rodents, is head-lift digging. In head-lift diggers, the nose (sometimes assisted by the lower incisors) is used like a spade to excavate the burrow, using powerful neck muscles to elevate the head. This last mode has not been identified in any modern North American mammals, but is known in two fossil groups, palaeanodonts (Rose & Emry 1983) from the Oligocene and proscalopid moles (Barnosky 1981) from the Miocene of North America.
The unusual skull morphology in mylagaulids—thickened nasal bones, an extremely short, broad skull and an anteriorly tilted occipital plate—has been the subject of extensive discussion (Fagan 1960; Korth 2000), but the function responsible for shaping the evolution of this unusual morphology has not been determined. While the anteriorly tilted occipital plate is strongly suggestive of extant head-lift diggers, it is necessary to trace the course of this feature's evolution and associated morphology to determine whether head-lift digging is the best explanation of the feature.
Ceratogaulus, the ‘horned gopher’ of the North American Miocene, is a monophyletic clade nested within the Mylagaulidae (figure 1) that includes four species, Ceratogaulus rhinocerus, Ceratogaulus hatcheri (originally described as Epigaulus hatcheri), Ceratogaulus minor (originally described as Epigaulus minor) and Ceratogaulus anecdotus, which are all found exclusively in the Miocene and early Pliocene deposits of the central Great Plains, in Nebraska and northeastern Colorado, USA. (Korth 2000). The group is identified by their large, paired nasal horns (figure 2), a unique feature among rodents and shared by only one other fossorial mammal, the fossil armadillo Peltephilus of the South American Miocene, in which the function of the horns is unknown. Past studies of Ceratogaulus have offered two hypotheses for the functional role of nasal horns in Ceratogaulus: that they are used either for digging (Gidley 1907; Fagan 1960) or sexual combat (Matthew 1902; Gidley 1907; Korth 2000). Consideration of extant horned mammals suggests two other uses for cranial horns. Many horned mammals use their horns in defence against predators (Packer 1983), although they may also be used in sexual combat or display. Alternatively, the horns of mammals, particularly ruminant artiodactyls, are suggested to allow individuals to recognize conspecifics (Vrba 1984). Thus, four adaptive roles are suggested for the horns of Ceratogaulus, three by analogy with modern horned mammals (sexual combat, species recognition or defence) and one (digging) suggested by the novel combination of fossoriality with nasal horns. Alternatively, the presence of horns may simply be a non-adaptation, a feature lacking any function.
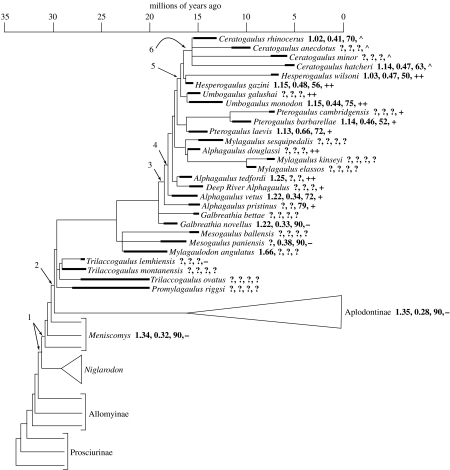
Figure 1.
Phylogeny of the Mylagaulidae. Heavy lines indicate known stratigraphic ranges of taxa; lighter lines indicate ghost lineages or inferred stratigraphic ranges. The relationships with the remainder of the Aplodontoidea are shown schematically without stratigraphic ranges. Numbers following the names of taxa are, in order, the ratio of skull length to skull width (which decreases in head-lift diggers), the ratio of the height of the occipital plate to the length of the skull (which gets larger in head-lift diggers), the angle between the occipital plate and the angle of the palate (‘Angle of occipital’; Korth 2000), which gets smaller in head-lift diggers) and the form of the nasal bones, being (−) thin, (+) thick, (++) with boss or (^) with horn. The arrows indicate the points in aplodontoid evolutionary history at which features putatively relevant to fossoriality evolve. (1: Shortening of skull, trabecular structure appears in wall of bulla; 2: broadening of occipital plate; 3: thickened nasal bones; 4: anteriorly tilted occipital plate, increased lower incisor hypsodonty; 5: nasal bosses; 6: large nasal horns.)
Figure 2.
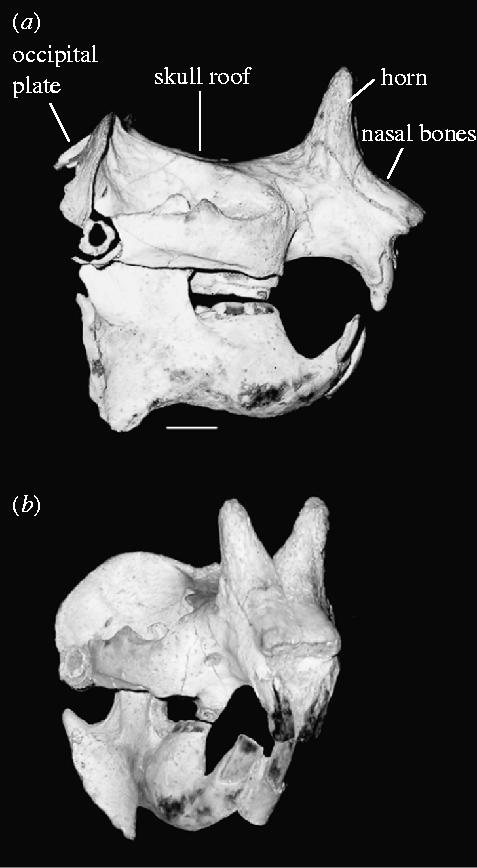
The skull of Ceratogaulus rhinocerus. (a) Lateral view showing the shape and position of the distinctive nasal horns. Important parts of skull morphology are labelled. (b) Anterolateral view showing the morphology of the paired horns. American Museum of Natural History specimen F: AM 65489, Ainsworth Prospecting Locality, Barstovian NALMA Brown County, Nebraska. Scale bar, 1 cm.
2. Material and Methods
Testing a hypothesis of adaptation requires two major components (Greene 1986). First, it must be shown that the feature improves function in the context of the hypothesized selective force or purpose. Second, it must be shown that the trait arose concurrently with the change in the selective regime. Thus, to suggest an adaptive role for the horns of Ceratogaulus, it must first be shown that they were useful for that purpose (adaptation) and second that the hypothesized need for horns arose at the same time as the horns themselves appear in the lineage (adaptation, not exaptation). In looking at a complex adaptation, like the suite of characters indicative of head-lift digging, it is necessary to trace the order and timing of the features' evolution to show that the course of assembly of the adaptation is consistent with the hypothesized explanation (Padian 1987).
Showing the timing of this feature's evolution requires a phylogeny to constrain the point in time when the clade possessing the feature first appeared. The phylogeny used here was generated using 250 morphological characters drawn from the skull and dentition and includes all published species of mylagaulids, as well as an undescribed species of mylagaulid from the Deep River Formation (middle Miocene) of Montana. The matrix was run using 1000 random addition replicates and Tree Bisection and Reconnection in Paup 4.0b10 (Swofford 2002). This analysis is part of a more comprehensive phylogenetic analysis of aplodontoid rodents that will be published elsewhere.
3. Head-lift digging
Head-lift digging is known in only a small number of modern mammals, including members of five to seven genera from three different orders, Marsupialia (Notoryctes, the marsupial mole), Rodentia (Nannospalax; Myospalax, both blind mole-rats; Ellobius, the mole vole; possibly Spalax, also a blind mole-rat and Rhizomys, the bamboo rat) and Afrosoricida (Eremitalpa, the golden mole; Nevo 1999; Stein 2000). All are highly fossorial or subterranean and have extensive skeletal modifications to withstand the immense forces generated by this mode of burrow excavation. Head-lift diggers use the tip of the snout as the primary implement for excavation of burrows. The head and forelimbs are driven forward into the substrate and the animal then extends the forelimbs while simultaneously lifting the head anterodorsally, performing a ‘push-up’ to open the burrow anteriorly (see Gasc et al. 1986 for an illustration). Most of the excavation forces are borne by the greatly enlarged neck muscles, while the forelimbs are mostly used to brace the body. Head-lift diggers can lift many times their body weight with their neck muscles, and frequently must do so while excavating burrows. Some head-lift diggers, in particular spalacids (Zuri et al. 1999), often use the lower incisors simultaneously in the digging motion, as the snout is swept anterodorsally, to aid in breaking up soil. Like most highly fossorial animals, head-lift diggers often also have secondary digging modes that they use when the substrate is ill-suited to head-lift digging; many are also scratch diggers and some are also chisel-tooth diggers.
Several major osteological features identify head-lift diggers. The most important feature that distinguishes this digging mode from others, such as chisel-tooth digging and incisor digging, is the immense force exerted on the muscles that elevate the head. The morphology most affected by these immense forces is that of the occipital region of the skull. The nuchal crest is enlarged and the areas of attachment of the rhomboideus capitus, splenius and rhomboideus cervicalis are expanded (Nevo 1999). The occipital plate is tall, thick and anteriorly tilted to accommodate the increased muscle mass in the neck. The neck vertebrae are often shortened or fused to shorten the output lever for lifting the head (Barnosky 1982a,b; Rose & Emry 1983). The nasal bones are also modified as the primary digging tool. The tip of the nasal bones are thickened to support the calloused nose pad which resists abrasion by the soil (Hildebrand 1985). The skull is short and posteriorly broad across the zygomatic arches and the occipital plate (Nevo 1999). The forelimbs are modified for increased extensor leverage (Stein 2000). This modification of the forelimbs, however, is also expected in a scratch digger. Because many head-lift diggers also use scratch digging when the substrate is hard, these two modes can be very challenging to distinguish from the morphology of postcrania alone.
4. Evolution of fossoriality
Aplodontoid rodents show a pattern of morphological evolution clearly indicative of the evolution of increasing fossoriality and, in mylagauline mylagaulids, head-lift digging. The pattern of evolution of features associated with fossoriality is shown on the phylogeny in figure 1. While most early aplodontoid taxa are preserved exclusively from isolated teeth and fragmentary jaws, enough crania are preserved to say with confidence that basal aplodontoids were fairly generalized in their cranial and postcranial morphology. The morphology of known skulls of Ansomys shanwangensis, Haplomys liolophus, Campestrallomys siouxensis and several Allomys species all show a generalized terrestrial or arboreal morphology. Meniscomys is the first taxon from which well preserved skulls are known that shows any evidence of increasing fossoriality. All three Meniscomys species are known from relatively complete skulls (Hopkins, in press). In contrast with earlier aplodontoid species, Meniscomys has a shortened skull, primarily in the basicranial region between the posterior end of the palate and the anterior extent of the bulla (figure 3). It is not clear whether the skull of Niglarodon, a genus very similar in dental morphology to Meniscomys, was similarly short because the basicranium of Niglarodon is not preserved. Another feature of Meniscomys clearly indicative of a fossorial habit is the structure of the middle ear. The wall of the bulla is cancellous (figure 3) with a complex cellular structure, as noted by Rensberger (1983). This organization of the middle ear is found in several other fossorial rodents, including geomyids (Wilkins et al. 1999), Ctenomys (Schleich & Busch 2004) and Clyomys (Gardner & Emmons 1984). This feature seems to yield improvements in low frequency hearing (Relkin 1988) and may also help reduce transmission of vibrations from the skull to the ear region, improving hearing when the animal's skull is in contact with the ground inside the burrow (Wilkins et al. 1999). The predecessor of this structure can be seen in Allomys, which has several septae dividing the bullae, to a similar degree to that seen in heteromyid rodents or in Octodon (Schleich & Busch 2004); this could be associated with an improvement in low frequency hearing of the sort used by terrestrial animals to avoid predators and may have provided the pathway for the further modification of the bulla in more derived aplodontoids. All Meniscomys, aplodontines and mylagaulids have this cancellous, cellular structure in the wall of the bulla.
Figure 3.
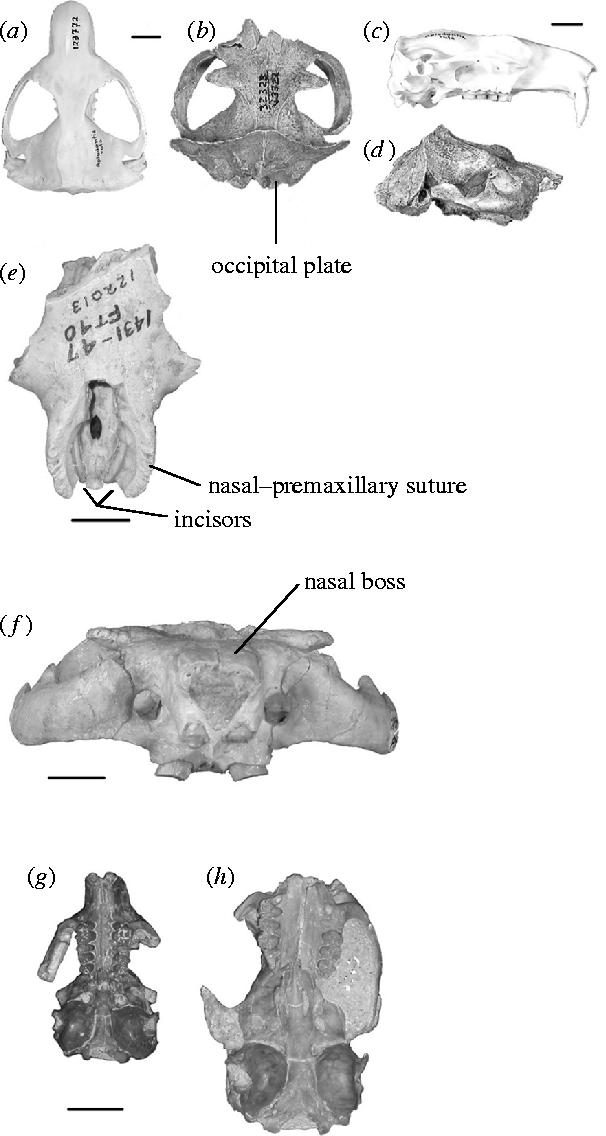
Features relevant to head-lift digging in aplodontoid rodents. (a) Skull of Aplodontia rufa, dorsal view, University of California Museum of Paleontology (UCMP) No. 123772, locality R0, a modern specimen. (b) Skull of Pterogaulus sp., dorsal view, UCMP No. 32323, locality V3322, Big Spring Canyon, Clarendonian NALMA, South Dakota. Same scale bar as (a). (c) Same as (a), lateral view. (d) Same as (b), lateral view. Same scale bar as (c). (e) Rostrum of Pterogaulus cambridgensis, dorsal view, University of Nebraska State Museum (UNSM) No. 122013, locality Ft-40, Cambridge Quarry, Hemphillian NALMA, Nebraska. The nasals are lost, exposing the extremely thick and complex nasal–premaxillary suture. The incisors can be seen at the base of the exposed narial opening. (f) Skull of an undescribed mylagaulid, anterior view, UCMP No. 316994, locality RV7619, Clarendonian NALMA, New Mexico, showing the thick nasal bones and the extremely broad, flat skull. (g) Skull of Meniscomys uhtoffi, ventral view, University of Washington Burke Museum (UWBM) No. 31451, locality UWA4556, Arikareean NALMA, Oregon showing the shortening of the skull between the M3 and the bullae. (h) Skull of Haplomys liolophus, ventral view, UCMP No. 1672, locality 898, Arikareean NALMA, Oregon, showing the much longer skull in this early aplodontoid. Same scale bar as (g). Scale bars are all 1 cm.
Aplodontines have a broader, flatter skull than Meniscomys, indicating a greater degree of fossoriality, and modern Aplodontia is highly fossorial (Carraway & Verts 1993), although not so much so as is inferred for mylagaulids. The major morphological change along the line to aplodontines is the dramatic broadening of the occipital plate, yielding a triangular skull outline with a narrow rostrum and a very broad occipital region. The precise digging mechanics of this extant species have not been studied; however, there is no evidence, either from behavioural observations or from the morphology, that these animals use the head to excavate their burrows.
The thickening of the nasal bones is another feature strongly indicative of head-lift digging. The nasals of all mylagauline mylagaulids are thickened to some degree and those of Umbogaulus and Hesperogaulus have a small boss near the tip (figure 3). The surface of this nasal boss is generally rugose, similar to the surface underlying the nose pad in extant head-lift diggers. This boss is larger and thicker than in any living fossorial animals but mylagaulines are universally larger than any living head-lift diggers. Even in those mylagauline taxa without a nasal boss, the nasal bones are thicker than in earlier mylagaulids and the nasal–premaxillary suture is thickened and strengthened (figure 3).
The anteriorly tilted occipital plate first appears in the paraphyletic Alphagaulus group. This occipital tilt is between 65 and 75 degrees for most mylagaulines, although the tilt is more severe in Hesperogaulus and some derived Ceratogaulus. In all mylagaulines the anterior tilt of the occipital plate seems to accommodate larger neck muscles, as suggested by the increasing thickness of the occipital plate relative to earlier species.
The height of the occipital plate also increases through time in the mylagaulid lineage (figure 1). This increase positions the attachments of the neck muscles higher above the occipital condyles, increasing the lever advantage for the action of the dorsal neck muscles by increasing the input lever arm relative to the output lever (which simultaneously shortens as the skull length decreases). The most significant increase in occipital plate height occurs at the base of the Mylagaulinae but the relative height of the occipital plate continues to increase through time in several mylagaulid lineages.
The incisors are used in digging as a primary or secondary mode of excavation in many subterranean rodents and, particularly, in the sister groups to many modern head-lift digging species. This is generally indicated by long, rapidly growing incisors with their roots set well back in the jaw and skull. In many such rodents, the roots of the incisors ascend all the way to the base of the mandibular condyles. In mylagaulids, the upper incisors are not procumbent and, hence, could not be brought to bear on the substrate for chisel-tooth digging. The lower incisors are more forward-projecting. Neither pair of incisors projects far outside the alveolus and, hence, both are well supported by the bone of the surrounding alveolus. Both upper and lower incisors are very broad and robust with flat anterior faces presenting a chisel-like profile. It has been suggested (Fagan 1960) that these incisors could be used to cut roots encountered during tunnelling; furthermore, they could be used in excavation as they are by Nannospalax, which uses them as part of the ‘spade’ in head-lift digging (Zuri et al. 1999). This possibility is suggested by similarities in the incisor morphology. Mylagaulid lower incisors are strongly curved and, although set within a very short jaw, are rooted more anteriorly than in most rodents. The roots ascend all the way to the dorsal tip of the coronoid process, creating a visible knot in the coronoid process. This feature is derived at the same time as the anterior tilt in the occipital plate first appears (figure 1), suggesting that the use of the incisors in digging arose at the same time as true head-lift digging appeared in mylagaulids.
5. Function of the nasal horns
(a) Digging
Gidley (1907) and Fagan (1960) suggested that the morphology of mylagaulid horns was consistent with their use in excavation. While several mammal species use the head in excavation, none of these species has developed horns. The tip of the snout is used like a spade to scrape at the substrate and, in contrast to the nasal horns of Ceratogaulus, the only modification of the nasal bones is a slight thickening of the anterior tips (Stein 2000). The nasal horns in Ceratogaulus are morphologically inconsistent with use as a digging tool (figure 4). The horns are positioned on the posterior ends of the nasal bones and extend dorsally, perpendicular to the plane of the palate. As a result of their posterior position, using the horns to dig would bring the anterior tip of the nasals against the substrate after a very short sweep of the horns (figure 4a), making digging with the horns extremely inefficient. This motion would be even more inefficient than suggested by this figure, as the anterior surface of a burrow is concave, making it essentially impossible to use the horns without the anterior end of the snout interfering. Furthermore, the occipital plate is tilted anteriorly, so it would be very difficult to exert any force on the substrate anterior to the animal. With the head tilted to direct the horns anteriorly, the muscles that elevate the head would be acting parallel to the lever arm along which the force is exerted, minimizing the lever advantage for the input force (figure 4a). The expectation is that an animal using its horns anteriorly (rather than dorsally) would have the occipital plate positioned vertically or tilted posteriorly. In this configuration, the effective input lever is maximized when the head is lowered, as in the rhinoceros skull in figure 4b. The shape of the horn itself is also very poor for a digging tool. The horns are very thick and broad with large, flat anterior and posterior surfaces (figure 2). Dragging such a broad tool through the soil would create immense resistance, proportional to the large surface area presented to the substrate. Finally, the horn becomes more posteriorly positioned through time (Korth 2000), so that the evolutionary trend is towards a horn which is more poorly suited to digging through time, rather than better suited. Thus, the argument that the horns functioned in digging is not supported by the morphology or the evolutionary progression.
Figure 4.
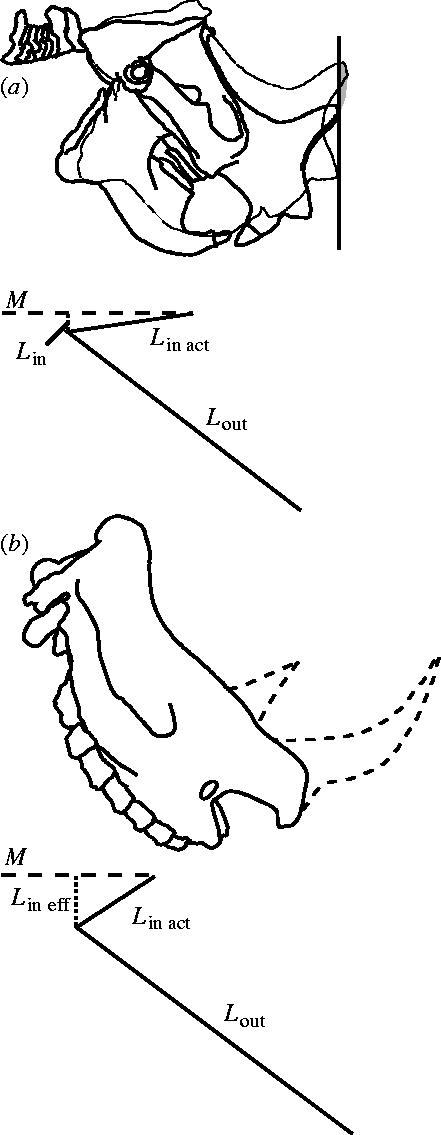
Function of the horns of Ceratogaulus as a potential digging implement. (a) Ceratogaulus skull (a composite drawing from several different specimens as few complete skulls are known). The initial position has the head held low with the occipital plate parallel to the line of the spinal column. The digging stroke ends when the nasal bones contact the substrate. The black line indicates the substrate surface to be excavated. The shaded area is the area excavated by the stroke of the horns. The diagram below the illustration shows the proportions of the lever system (Lin eff=effective input lever; Lin act=actual input lever; Lout=output lever; M=line of muscle action). (b) A rhinoceros skull (not to scale) to illustrate the differing lever system needed to effectively bring horns to bear anteriorly. Same abbreviations as (a).
(b) Sexual combat
Although all authors discussing the function of the horns address the hypothesis of sexual combat (Matthew 1902; Gidley 1907; Fagan 1960; Korth 2000), it is not supported by the evidence at hand. Sexually selected features are generally expected to be sexually dimorphic, which does not seem to be the case for the horns of Ceratogaulus (Korth 2000). Horned and hornless co-occur in only a single locality (Pratt Quarry, Late Miocene of Nebraska) out of 48 occurrences of Ceratogaulus and Pterogaulus (the only mylagaulid genus that occurs in the Great Plains during the same time span), according to MIOMAP (Carrasco et al. 2005). Furthermore, they differ drastically in morphology, particularly in dental features that are never sexually dimorphic in modern mammals. The enlarged P4 (the tooth responsible for most of the chewing function) has narrow, antero-posteriorly elongated enamel lakes in Pterogaulus and broad, rounded lakes in Ceratogaulus. The two groups also differ in the shape and wear pattern of the parafossette, (the large anterolingual enamel lake), a character argued to distinguish two major lineages within the Mylagaulidae (Korth 2000).
Some mammalian species in which horns are not sexually dimorphic still engage in sexual combat, but female horns are secondarily derived in all these species (Kiltie 1985; Geist & Bayer 1988; Roberts 1996). Furthermore, many of the objections that apply to the horns as a digging implement also apply to the use of the horns in sexual combat. Their orientation and position and the morphology of the rest of the skull make it exceedingly difficult to bring them to bear on an opponent of similar size. The cervical vertebrae are shortened antero-posteriorly in all mylagaulids (a feature inherited by Ceratogaulus from ancestral, head-digging mylagaulids), decreasing the flexibility and range of motion of the neck and making it even more difficult for Ceratogaulus to wrestle with their horns.
Many ungulates with horns ill-suited to sexual combat still use them for combat or for sexual display. However, a sexually selected use of the horns is unlikely in Ceratogaulus, as the optic foramen is very small, roughly 0.5–0.66 times the size of that of A. rufa (Wahlert 1974), which itself has very poor vision (Carraway & Verts 1993). The small size of the optic foramen indicates extremely poor visual acuity (Kay & Kirk 2000), meaning that the females would be unlikely to be able to recognize a winner in any sexual displays or sexual combat by the males.
(c) Species recognition and non-adaptation
Horns in ruminant artiodactyls have been hypothesized to have evolved their present morphological diversity to facilitate species recognition (Vrba 1984). This explanation, however, is inconsistent with the horns of a fossorial animal. Large, dorsally projecting cranial appendages are no liability to a cursorial ungulate, but they have a substantial evolutionary cost for a fossorial animal, particularly one which ancestrally used its head extensively in burrowing. The horns would prevent packing the burrow roof with the head, a behaviour common to almost all other fossorial mammals. The protruding cranial appendages would also limit underground mobility, as the horns pose a serious threat of damage to the burrow. The fitness cost of these horns must be offset by some gain because the horns persist in Ceratogaulus for roughly 10 million years (figure 1). More derived Ceratogaulus have taller horns (Korth 2000), suggesting that, rather than being selectively disadvantageous, larger horns are providing some advantage. In an animal with such poor visual acuity, it seems that this feature is unlikely to be so important for species recognition to offset the cost; species recognition is much more likely to be through scent or auditory cues. This argument also applies to rejecting the possibility that horns are non-adaptive.
(d) Defence
Horns are used in defence against predators by almost all horned mammals. Animals will use any weapons at their disposal to fight off predators, and the horns of Ceratogaulus are particularly well suited to defence. The horns are broad and robust (figure 2) and their dorsal orientation and relatively posterior position makes them well suited to protecting the vulnerable eyes and neck. By elevating the head dorsally, the horns would be snapped backward, protecting the areas most commonly attacked by predators. A similar use of postero-dorsal horns has been indicated to decrease predation in horned lizards (Young et al. 2004). As the horns grow taller through evolutionary time, they also become more posteriorly positioned and the height of the occipital plate increases, increasing the leverage for lifting them. By positioning the horns more posteriorly, the output lever is shortened and, because the muscles used to rotate the skull dorsally attach at the top of the occipital plate, the input lever is lengthened. Thus, the dorsal strike with the horns would be more powerful as the ratio of output lever to input lever would be increased. This increased leverage would also be helpful in manoeuvring the horns to deter predators from attacking at the head or neck, as elevating the head dorsally would snap the horns backward, protecting these vulnerable areas. Predation is by far the dominant cause of mortality in most small mammals (Sinclair et al. 2003), so the benefits provided by a mechanism to reduce predation could offset the substantial evolutionary cost of horns in a fossorial mammal.
6. Tests of adaptation
The sequence of acquisition of characters consistent with head-lift digging describes a clear case of progressively increasing fossoriality (figure 1), terminating with the derivation of head-lift digging. The first fossorial aplodontoids seem to be Meniscomys, or possibly the Meniscomyinae as a whole, with characters of the overall skull shape and bulla consistent with a fossorial life habit. Aplodontines and early mylagaulids show increased fossoriality as indicated by the broadening of the occipital region of the skull. Alphagaulus shows another increase in fossoriality with the broadening and flattening of the skull relative to earlier mylagaulids, as well as the acquisition of more robust nasal bones. After the divergence of Alphagaulus pristinus, the anterior tilt in the skull, further thickening of the anterior tips of the nasal bones and the increased lower incisor hypsodonty indicate that mylagaulids have become head-lift diggers. As body size continued to increase in mylagaulids, the relative height of the occipital plate increased to make the elevation of the head more powerful. Finally, in the largest mylagaulids, thick, bony bosses are developed on the anterior ends of the nasals, probably to reinforce the nose pad used in digging. In the largest of mylagaulids, however, this head-lift digging becomes impossible with the evolution of nasal horns.
Distinguishing adaptation from exaptation in the evolution of horns in Ceratogaulus requires knowing the timing of the onset of changes in selective forces and demonstrating that the hypothesized adaptive feature arises at the time that the improved function would become advantageous. The phylogenetic hypothesis for the Mylagaulidae (figure 1) gives a framework for constraining the timing of cranial horn evolution. Ceratogaulus is the largest known mylagaulid, as indicated by cranial dimensions, and is notably larger than its sister clade, Hesperogaulus. Because Ceratogaulus was too large to be purely subterranean (McNab 1966; McNab 1979; Stein 2000), it would have spent a substantial amount of time foraging above ground and its risk of predation was, therefore, elevated relative to its smaller precursors. Rapid escape is impossible for a short-legged, heavy-bodied, long-clawed fossorial animal such as Ceratogaulus, so a formidable defensive mechanism such as horns would substantially decrease predation mortality. The changing selective environment in the mid-Miocene of the Great Plains provides support for horns as a defensive adaptation. The Great Plains became progressively more open and grassy throughout the Miocene (Retallack 2001; Strömberg 2002, 2004; Fox & Koch 2003), providing less cover for small mammals to use in hiding from predators. Predator diversity increases among North American mustelids (Baskin 1998) approximately 17 Myr and among canids (Munthe 1998) approximately 16 Myr, when Ceratogaulus first appears (figure 1).
7. Conclusions
The unusual skull morphology in mylagaulids is easily understood through comparison with modern head-lift diggers. The evolutionary history of the Aplodontoidea clearly shows a process of increasing fossoriality from the late Oligocene through the middle Miocene, during a period of increasing habitat openness in North America (Retallack 2001; Strömberg 2002, 2004; Fox & Koch 2003). Many North American small mammals went underground during this period (Nevo 1999) because more open habitats provided more niche space for fossorial animals (Nevo 1979). By the Mid-Miocene (late Hemingfordian North American Land Mammal Age), mylagaulids had evolved head-lift digging. In modern mammals, this behaviour only occurs in highly fossorial and subterranean mammals that live in desert and grassland habitats. This is consistent with the known environment in much of North America during the Mid-Miocene and with the previously assumed high degree of fossoriality in mylagaulids.
The fact that mylagaulids engaged in head-lift digging may explain why horns arose in this group. The highly enlarged neck muscles in mylagaulids and adaptations for increased leverage in elevating the skull provided a foundation for the development of horns because the power needed to effectively use cranial horns against predators was already in place. The cranial horns may also have arisen by a developmental mechanism similar to the one that yields the nasal bosses in the outgroups of Ceratogaulus. Thus, the unusual mode of digging employed by mylagaulids provides cranial and muscular morphology which is exapted for the use of the horns in defence when horns arise in Ceratogaulus.
Of all the possible explanations for the horns of Ceratogaulus, the only one consistent with morphology and evolutionary history is that they are used in defence against predators. Such a defensive feature is unique among fossorial mammals, most of which retreat into burrows or defend themselves with claws or teeth. The defensive use of horns has been indicated for ungulate horns, particularly those of the female antelope (Packer 1983). The development of defensive horns in mylagaulids may represent an evolutionary trade-off as larger body size mandated increased use of above-ground resources. The persistence of these large-bodied, horned forms for some 10 million years indicates that this was a successful strategy. A similar explanation can be suggested for the horns of Peltephilus, which also arose in a large-bodied fossorial lineage during a period of widespread habitat openness. It appears likely that these two unrelated taxa have convergently evolved defensive horns, a feature unknown in modern fossorial animals.
Acknowledgments
This study is part of a dissertation in Integrative Biology at the University of California, Berkeley. Funding for this study was provided by NSF Doctoral Dissertation Improvement Grant No. DEB-0407873, the University of California Museum of Paleontology (UCMP), the Department of Integrative Biology and a Sigma Xi grant. The author would like to thank A. Barnosky for mentorship and guidance during the project. The study was also greatly helped by discussions with J. Patton, M. Voorhies, A. Bair, K. Gobetz, P. Holroyd, W. Lidicker, W. Clemens and many members of the UCMP and Museum of Vertebrate Zoology (MVZ). C. Janis and an anonymous reviewer provided helpful reviews of the manuscript. Specimens used in this study were provided by the University of Nebraska State Museum, the University of Washington's Burke Museum of Natural History, the American Museum of Natural History, the University of Kansas Museum of Natural History, the MVZ and the UCMP. E. Davis has offered endless help, advice, support and inspiration. This is UCMP contribution No. 1888.
References
- Barnosky A.D. A skeleton of Mesoscalops (Mammalia: Insectivora) from the Miocene Deep River Formation, Montana, and a review of the proscalopid moles: evolutionary, functional, and stratigraphic relationships. J. Vertebr. Paleontol. 1981;1:285–339. [Google Scholar]
- Barnosky A.D. Locomotion in moles (Insectivora, Proscalopidae) from the middle Tertiary of North America. Science. 1982a;216:183–185. doi: 10.1126/science.216.4542.183. [DOI] [PubMed] [Google Scholar]
- Barnosky A.D. A new species of Proscalops (Mammalia, Insectivora) from the Arikareean Deep River Formation, Meagher County, Montana. J. Paleontol. 1982b;56:1103–1111. [Google Scholar]
- Baskin J.A. Mustelidae. In: Janis C.M, Scott K.M, Jacobs L.L, editors. Evolution of tertiary mammals of North America. Terrestrial carnivores, ungulates, and ungulatelike mammals. vol. 1. Cambridge University Press; New York: 1998. pp. 152–173. [Google Scholar]
- Carrasco M.A, Kraatz B.P, Davis E.B, Barnosky A.D. University of California Museum of Paleontology; Berkeley: 2005. Miocene mammal mapping project (MIOMAP) [Google Scholar]
- Carraway L.N, Verts B.J. Aplodontia rufa. Mamm. Species. 1993;431:1–10. [Google Scholar]
- Fagan S.R. Osteology of Mylagaulus laevis, a fossorial rodent from the upper Miocene of Colorado. Univ. Kansas Paleontol. Contrib. Pap. 1960;26:1–29. [Google Scholar]
- Fox D.L, Koch P.L. Tertiary history of C4 biomass in the Great Plains, USA. Geology. 2003;31:809–812. [Google Scholar]
- Gardner A.L, Emmons H.L. Species groups in Proechimys (Rodentia, Echymyidae) as indicated by karyology and bullar morphology. J. Mammal. 1984;65:10–25. [Google Scholar]
- Gasc J.P, Jouffroy F.K, Renous S. Morphofunctional study of the digging system of the Namib Desert Golden Mole (Eremitalpa granti namibensis): cinefluorographical and anatomical analysis. J. Zool. 1986;208:9–35. [Google Scholar]
- Geist V, Bayer M. Sexual dimorphism in the Cervidae and its relation to habitat. J. Zool. 1988;214:45–54. [Google Scholar]
- Gidley J.W. A new horned rodent from the Miocene of Kansas. Proc. US Natl Mus. 1907;32:627–636. [Google Scholar]
- Goodwin M.B, Horner J.R. Cranial histology of pachycephalosaurs (Ornithischia: Marginocephalia) reveals transitory structures inconsistent with head-butting behavior. Paleobiology. 2004;30:253–267. [Google Scholar]
- Greene H.W. Diet and arboreality in the emerald monitor, Varanus prasinus, with comments on the study of adaptation. Fieldiana Zool. 1986;31:1–12. [Google Scholar]
- Hildebrand M. Digging in quadrupeds. In: Hildebrand M, Bramble D.M, Leim K.F, Wake D.B, editors. Functional vertebrate morphology. Harvard University Press; Cambridge, MA: 1985. pp. 89–109. [Google Scholar]
- Hopkins, S. S. B. In press. Morphology of the skull in Meniscomys from the John Day Formation of central Oregon. PaleoBios
- Hopkins, S. S. B. Submitted. Phylogeny and evolutionary history of the Aplodontoidea. Zool. J. Linn. Soc.
- Janis C.M. Evolution of horns in ungulates: ecology and paleoecology. Biol. Rev. 1982;57:261–318. [Google Scholar]
- Joeckel R.M. A functional interpretation of the masticatory system and paleoecology of entelodonts. Paleobiology. 1990;16:459–482. [Google Scholar]
- Kay R.F, Kirk E.C. Osteological evidence for the evolution of activity pattern and visual acuity in primates. Am. J. Phys. Anthropol. 2000;113:235–262. doi: 10.1002/1096-8644(200010)113:2<235::AID-AJPA7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kiltie R.A. Evolution and function of horns and horn-like organs in female ruminants. Biol. J. Linn. Soc. 1985;24:299–320. [Google Scholar]
- Korth W.W. Review of Miocene (Hemingfordian to Clarendonian) mylagaulid rodents (Mammalia) from Nebraska. Ann. Carnegie Mus. 2000;69:227–280. [Google Scholar]
- Matthew W.D. A horned rodent from the Colorado Miocene, with a revision of the Mylagauli, beavers and hares of the American Tertiary. Bull. Am. Mus. Natl Hist. 1902;16:291–310. [Google Scholar]
- McNab B.K. The metabolism of fossorial rodents: a study of convergence. Ecology. 1966;47:712–733. [Google Scholar]
- McNab B.K. The influence of body size on the energetics and distribution of fossorial and burrowing animals. Ecology. 1979;60:1010–1021. [Google Scholar]
- Munthe K. Canidae. In: Janis C.M, Scott K.M, Jacobs L.L, editors. Evolution of tertiary mammals of North America. Terrestrial carnivores, ungulates, and ungulatelike mammals. vol. 1. Cambridge University Press; New York: 1998. pp. 124–143. [Google Scholar]
- Nevo E. Adaptive convergence and divergence of subterranean mammals. Annu. Rev. Ecol. Syst. 1979;10:269–308. [Google Scholar]
- Nevo E. Oxford University Press; New York: 1999. Mosaic evolution of subterranean mammals: regression, progression, and global convergence. [Google Scholar]
- Packer C. Sexual dimorphism: the horns of African antelopes. Science. 1983;221:1191–1193. doi: 10.1126/science.221.4616.1191. [DOI] [PubMed] [Google Scholar]
- Padian K. A comparative phylogenetic and functional approach to the origin of vertebrate flight. In: Fenton M.B, Racey P, Rayner J.M.V, editors. Recent advances in the study of bats. Cambridge University Press; New York: 1987. pp. 3–22. [Google Scholar]
- Relkin E.M. Introduction to the analysis of middle-ear function. In: Jahn A, Santos-Sacchi J, editors. Physiology of the ear. Raven Press; New York: 1988. pp. 103–123. [Google Scholar]
- Rensberger J.M. Successions of meniscomyine and allomyine rodents (Aplodontidae) in the Oligo-Miocene John Day Formation. Univ. Calif. Publ. Geol. Sci. 1983;124:1–157. [Google Scholar]
- Retallack G.J. Cenozoic expansion of grasslands and climatic cooling. J. Geol. 2001;109:407–426. [Google Scholar]
- Roberts S.C. The evolution of hornedness in female ruminants. Behaviour. 1996;133:399–442. [Google Scholar]
- Rose K.D, Emry R.J. Extraordinary fossorial adaptations in the Oligocene palaeanodonts Epoicotherium and Xenocranium (Mammalia) J. Morphol. 1983;175:33–56. doi: 10.1002/jmor.1051750105. [DOI] [PubMed] [Google Scholar]
- Schleich C.E, Busch C. Functional morphology of the middle ear of Ctenomys talarum (Rodentia: Octodontidae) J. Mammal. 2004;85:290–295. [Google Scholar]
- Sinclair A.R.E, Mduma S, Brashares J.S. Patterns of predation in a diverse predator–prey system. Nature. 2003;425:288–290. doi: 10.1038/nature01934. [DOI] [PubMed] [Google Scholar]
- Stanley S.M. Relative growth of the titanothere horn: a new approach to an old problem. Evolution. 1974;28:447–457. doi: 10.1111/j.1558-5646.1974.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Stein B. Morphology of subterranean rodents. In: Lacey E.A, Patton J.L, Cameron G.N, editors. Life underground: the biology of subterranean rodents. University of Chicago Press; 2000. pp. 19–61. [Google Scholar]
- Strömberg C.A.E. The origin and spread of grass-dominated ecosystems in the late Tertiary of North America: preliminary results concerning the evolution of hypsodonty. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002;177:59–75. [Google Scholar]
- Strömberg C.A.E. Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the Great Plains of North America during the late Eocene to early Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;207:239–275. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP: 4.0b10: phylogenetic analysis using parsimony (and other methods) [Google Scholar]
- Vrba E.S. Evolutionary pattern and process in the sister-groups Alcelaphini–Aepycerotini (Mammalia: Bovidae) In: Eldredge N, Stanley S.M, editors. Living fossils. Springer; New York: 1984. pp. 62–79. [Google Scholar]
- Wahlert J.H. The cranial foramina of protrogomorphous rodents; an anatomical and phylogenetic study. Bull. Mus. Comp. Zool. 1974;146:363–410. [Google Scholar]
- Wilkins K.T, Roberts J.C, Roorda C.S, Hawkins J.E. Morphometrics and functional morphology of middle ears of extant pocket gophers (Rodentia: Geomyidae) J. Mammal. 1999;80:180–198. [Google Scholar]
- Young K.V, Brodie E.D, Jr, Brodie E.D., III How the horned lizard got its horns. Science. 2004;304:65. doi: 10.1126/science.1094790. [DOI] [PubMed] [Google Scholar]
- Zuri I, Kaffe I, Dayan D, Terkel J. Incisor adaptation to fossorial life in the blind mole-rat, Spalax ehrenbergi. J. Mammal. 1999;80:734–741. [Google Scholar]