Abstract
The occurrence of functional left–right cerebral asymmetries has been documented in a wide range of animals, suggesting that the lateralization of cognitive functions enjoys some kind of selective advantage over the bilateral control of the same functions. Here, we compared schooling performance of fishes with high or low degree of lateralization, which were obtained through selective breeding. Schools of lateralized fishes moving in a novel environment showed significantly more cohesion and coordination than schools of non-lateralized (NL) fishes. Pairs of fishes lateralized in opposite directions were as efficient as pairs of same laterality, suggesting that the performance of lateralized fishes derives from a computational advantage rather than being the consequence of a behavioural similarity among schoolmates. In schools composed of both lateralized and NL fishes, the latter were more often at the periphery of the school while lateralized fishes occupied the core, a position normally safer and energetically less expensive.
Keywords: lateralization, schooling, asymmetry, fishes
1. Introduction
Cerebral lateralization is not a feature unique to humans and three decades of studies have reported behavioural and anatomical asymmetries in a wide range of vertebrate species, from fishes to mammals and birds (reviewed in Rogers & Andrew 2002). Remarkable similarities have been reported even among distantly related species (Sovrano et al. 1999; Bisazza et al. 2002) and it is still debated whether cerebral lateralization arose very early in a common ancestor of all extant vertebrates or evolved independently in different lineages under similar ecological conditions (Andrew 2002; Vallortigara & Bisazza 2002). There are recent reports of anatomical or behavioural asymmetries in invertebrates, for example, spiders (Ades & Ramires 2002), insects (Kells & Goulson 2001; Pascual et al. 2004) and decapods (Byrne et al. 2002), which suggests that lateralization might be even more common than presently thought.
The prevalence of lateralization in the animal kingdom implies that individuals showing lateralization of the cognitive functions enjoy some kind of selective advantage over individuals with bilateral control of the same functions (Rogers 2000, 2002). To date, few studies have investigated this issue. Among free-living chimpanzees, individuals specialized in using one hand were more efficient in termite fishing than individuals not showing such specialization (McGrew & Marchant 1999). Rogers and co-workers observed chicks that had to discriminate food from non-food in the presence of a model predator; normally, lateralized chicks learnt this discrimination more rapidly than chicks that were incubated in the dark and were poorly lateralized (Rogers 2000; Rogers et al. 2004). Studying pigeons, Güntürkün and collaborators (2000) confirmed a better visual discrimination in strongly lateralized individuals. Apparently, lateralization confers some advantages in humans too. Crow et al. (1998) reported that in verbal tests conducted in a large sample of 11 year old children, both left- and right-handed individuals did slightly better than ambidextral individuals did.
Schooling is a very basic feature of aquatic species (Shaw 1978; Wassersug et al. 1981; Motta & Wilga 2001) and may have appeared very early in vertebrate evolution. Over 50% of bony fish species school (Shaw 1978) and true schooling has been reported in a number of cartilaginous fish species and in many other aquatic vertebrates (Klimley 1985; Economakis & Lobel 1998; Benoit-Bird & Au 2003). The ability to swim with others in a coordinated fashion has been demonstrated to have a large impact on individual fitness, as it allows for reduced energetic costs of locomotion, increased safety from predators, improved foraging efficiency and bears a number of other potential benefits (Pitcher et al. 1982a; Pitcher & Parrish 1993; Krause et al. 2000). Being so crucial for survival, schooling behaviour appears an ideal target for studies focusing on the evolution of functional brain asymmetries.
This study compares the performance of schooling in fishes with high or low degree of lateralization, which were obtained through selective breeding. In one experiment, we compared measures of cohesion and alignment in groups made of lateralized individuals and in groups made of non-lateralized (NL) fishes. In a second experiment, we recorded the position occupied within a small school by individuals with different degree of lateralization.
2. Methods
We used three groups of fishes obtained through selective breeding for different degree and direction of lateralization (Bisazza et al. 2001; Vallortigara & Bisazza 2002). A stock population of Girardinus falcatus was maintained in our laboratory since 1992 and bred in population tanks. Selection lines were started in 1997 and 1998. Selection was based on scores in the ‘detour test’. In this test, a subject swims along a runway until it faces a barrier behind which a model predator is visible. The percentage of right and left turns taken by the fishes when leaving the runway is computed for 10 successive trials. The direction taken by a fish is strictly dependent on its eye preference when scrutinizing the stimulus and it is highly heritable in this species (Facchin et al. 1999; Bisazza et al. 2000). Four populations were selected for the direction of laterality (two replicate populations for left turning (LD) and two for right turning (RD)) and one population was selected for low laterality (equal preference to turn left or right). All five populations showed significant response to selection when compared with a control line (Vallortigara & Bisazza 2002). Fishes of RD and LD lines differ in many other lateralized tasks and it was suggested that they have a completely mirror-reversed organization of cerebral functions, while NL fishes have a bilateral representation of most cognitive functions (Bisazza et al. 2001, 2004).
We used adult females (ca. six to seven months old) after they had produced their second litter in the selection experiment (mostly from generations six and seven). They were subdivided into three experimental groups: fishes that turned 80% or more to the left (LD group) fishes that turned 80% or more to the right (RD group) and fishes that turned 50% of times in each direction (NL group). Individuals were maintained in small heterosexual groups (12–15 fishes, ca. 1 : 1 sex ratio) of the same laterality, kept in 70 l glass aquaria with abundant vegetation (Ceratophillum sp.) and artificial lighting 16 h a day; water temperature was maintained at 25±2 °C and all fishes were fed dry fish food and Artemia salina nauplii twice a day.
(a) School cohesion and alignment
We observed schools made of two unfamiliar fishes (either two lateralized or two NL fishes) in a large unknown environment and scored two measures of schooling efficiency, the inter-individual distance and the degree of alignment of the school. We observed a total of 94 schooling pairs belonging to four experimental groups: LL (two LD fishes, n=17) RR (two RD fishes, n=18) MixL (one RD and one LD fish, n=22), NN (two NL fishes, n=37). Fishes were used only once and all individuals were fed to satiation prior to experimentation.
Two fishes matched for size were drawn from two different tanks and moved into a hollow glass cylinder at the centre of the apparatus, a circular opaque plastic tank (100 cm diameter) filled with 15 cm of water. A semispherical ceiling covered the apparatus except for a small central portion to allow video recording of the test. After 5 min, the cylinder was raised from a separate room. Recording started 60 s after the fishes were released and lasted for 4 minutes. Recording was digitized into a computer at two frames per second (a total of 480 images for each school). Frames in which one of the fishes was less than two body lengths from the wall were not included in the analysis. This left an average of 394.15±14.75 frames per school. For each frame, a computer program calculated the distance between the two fishes (snout to snout distance) and the degree of alignment of the school (angle in degrees formed by prolongation of the major body axis of the two fishes).
The computer program then calculated for each school the average distance between the pairs of fishes and the degree of alignment. A ‘schooling index’ was also computed as the geometric mean of these two measures (). The tests were performed in different periods of the year, from spring 2002 to summer 2003. As far as possible, the same number of lateralized and NL fishes were tested each day. In all ANOVA analyses, we considered as factors the month of the year and the average length of the fishes in the school (as a covariate). Statistics for these two factors are reported only when significant. Independent variables were transformed to obtain homogeneity of the variances when necessary.
(b) Position within the school
We observed schools made of three unfamiliar individuals, one LD, one RD and one NL fish in the same conditions of the previous experiment. Thirty adult females (10 RD, 10 LD and 10 NL) were used for this experiment. One hour before the experiment, fishes were given individual identity marks, by dying dorsally one small dot (ca. 1 mm) with 1% toluidine blue solution using a 0.35 mm needle. Three different mark positions were used, randomly assigning the position for each triplet of fishes. Subjects were matched for size with maximum difference of 1 mm within a triplet. The three fishes were released in the experimental tank from a central cylinder after a 5 min settlement period. They were recorded for 20 min after the release. As in the previous experiment, the first 4 min of the test were digitized into a computer, at two frames per second. Frames were analyzed by an expert observer, who was unaware of individuals' identity. For each frame, the common axis of the school was computed as the average direction of the three fishes. We considered only frames in which the angle between each fish and the other two was less than 25° and in which there was a partial overlap of the three bodies along the common axis. The position (left side, right side or central) of each fish inside the school was recorded.
From the video recording, we noted down the number of times a fish lost tight contact with the school, defined by a distance from the nearest fish larger than two body lengths. This was done for the whole 20 min period by an expert, blind observer.
3. Results
(a) School cohesion and alignment
The two measures of schooling efficiency, cohesion (inter-individual distance) and alignment (angle between body axes) were largely independent (Pearson correlation r=0.105; n=94; p=0.314). No significant difference was found between fishes of the two lateralized lines in either measure of schooling efficiency, cohesion (ANOVA: F1,26=0.24, p=0.879; figure 1a) and alignment (F1,26=1.17, p=0.289; month of the year: F3,26=6.24, p<0.01; figure 1b) or in the geometric combination of these two measures, the schooling index (F1,26=0.64, p=0.431; figure 1c). For the subsequent analysis, data of LL and RR fishes were pooled together. Lateralized fishes (LL and RR) performed better than NN fishes in both cohesion (F1,60=4.69; p<0.05; month of the year: F6,60=2.99, p<0.05) and alignment (F1,60=4.29, p<0.05). The different efficiency between lateralized and NL fishes is clearly depicted by the schooling index (F1,60=9.79, p<0.01; month of the year: F6,60=3.12, p<0.05).
Figure 1.
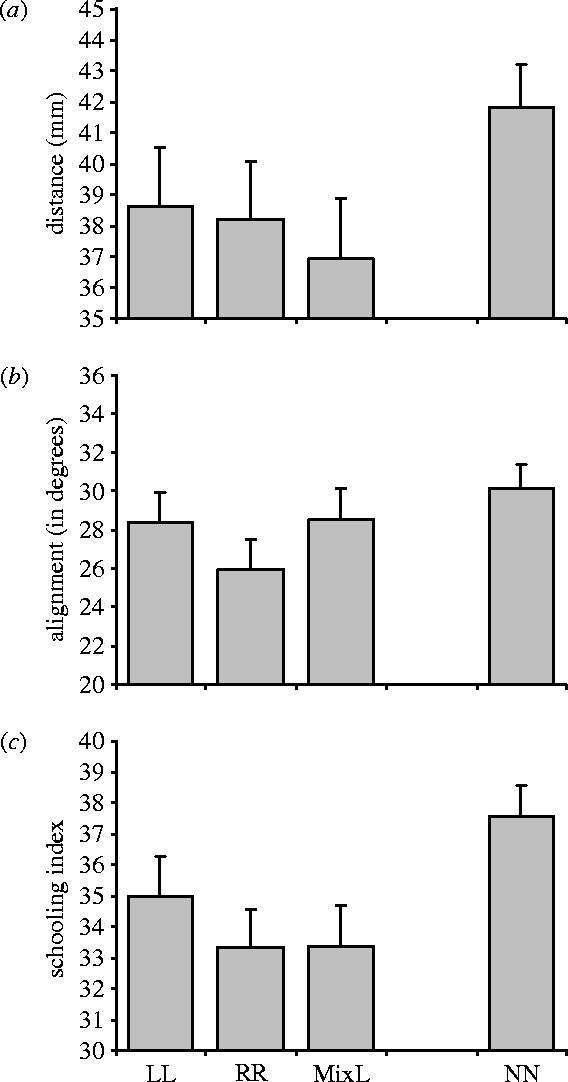
Measures of schooling efficiency (average and s.e.m.) in pairs of lateralized or NL fishes. (a) Inter-individual distance (snout to snout); (b) the degree of alignment of the school (angle between the two body axes); (c) schooling index, computed as the geometric combination of the two previous measures. Fishes were obtained from selective breeding experiments. Pairs of fishes selected to turn left (LL, n=17) or right (RR, n=18) in front of a model predator were compared with pairs of fishes with opposite lateralization (MixL, n=22) and with pairs of NL fishes (NN, n=37).
The performance of schools made of fishes with opposite laterality (MixL) was contrasted with the two former groups excluding the covariate (body length) from the model to allow multiple post-hoc comparison. No significant length difference was however present among these groups (ANOVA: F2,91=2.61, p=0.079).
MixL fishes showed a significantly higher cohesion than NN fishes and did not differ from pairs of fishes with the same laterality, LL+RR (F2,79=3.48, p<0.01; post hoc Sheffé method p<0.05; month of the year: F2,79=2.94, p<0.05). The difference among the three groups in their alignment was not significant (F2,79=2.85, p=0.064) although a strong trend was evident. In the schooling index, MixL fishes, performed significantly better than NN and not differently from the other lateralized fishes, LL+RR (F2,79=6.05, p<0.01; post hoc Sheffé method p<0.05; month of the year: F2,79=3.19, p<0.01).
In many frames (45.1%±15.9), one fish was visibly leading and the other following. This latter individual saw the mate's flank with one eye and the surrounding environment with the other. In a subsample we recorded the position of the follower in schools of LL (n=10), RR (n=10) and NL (n=10) fishes. In RR pairs, the follower tended to keep the leading fishes on the left side more often (61.5%±14.7 of the time; one sample t-test, t9=2.25; p=0.051) so that they saw the surrounding environment with their right eye. In LL pairs, fishes showed a tendency to kept the leading fishes on their right side (56%±10.5; t9=1.81, p=0.1). The difference between RR and LL fishes is significant (Student t-test t18=2.88; p<0.01). NL fishes showed no preference (48.9%±19.2 leading fishes on the right; t9=0.17; p=0.869).
As a reference, we tested a small sample (n=15 pairs) of fishes randomly selected from the stock population. The procedure is the same used with fishes from selected lines. The average efficiency of these 15 pairs (schooling index 38.7±12.3) was lower than that of lateralized pairs (n=57; 33.8±5.6) and very close to that of NN pairs (n=37; 37.1±6.2). The former difference was marginally non-significant (ANOVA F2,106=4.08, p<0.05; post hoc Sheffé method p=0.065)
(b) Position within the school
An average of 131.8 (±69.5) frames per school fit the criteria for the analysis. The central position was occupied most of the time by one of the two lateralized fishes (LD fishes: 43.2%±37.9; RD fishes: 50.1%±37.1) while NL fishes were in the centre of the school in less than 10% of the observations (6.8%±12.1). LD and RD fishes did not differ in the time spent in the central position and both were in the central position significantly more often than NL fishes were (ANOVA F2,27=6.28; p<0.01; post hoc Sheffé method p<0.05).
We recorded 130 cases (average per test: 13±2.7) in which one fish lost tight contact with the school. In 56.9% of the cases the fish was NL, in 21.5% it was LD, and in 21.5% it was RD. These values differ from random expectation (χ22=32.6; p<0.001).
4. Discussion
Lateralized fishes showed more cohesion and were more closely aligned with shoalmates while swimming in a novel environment compared with fishes with a low degree of lateralization. In addition, in schools composed of both lateralized and NL fishes, the former were located in the central position more often than chance and were less likely than NL fishes to lose contact with the rest of the group.
A ‘school’ is commonly defined as a group of fishes moving in the same direction, at the same speed and maintaining a given distance from the neighbours (Pitcher 1983). Theoretical and empirical studies suggest that both the cohesion and the polarity of a school increases the fitness of its components, promoting school integrity and allowing for faster swimming, reduced costs of locomotion and enhanced manoeuvrability (Pitcher & Parrish 1993; Gueron et al. 1996; Viscido et al. 2004). An individual's position inside a school is also important for its fitness. Intra-school positional preferences have been documented in a number of species (Pitcher et al. 1982b; Krause et al. 1998a) and there is good evidence that fishes in a central position suffer reduced predation (Krause et al. 1998b; Bumann et al. 1997) and save energy by enjoying a hydrodynamic advantage (van der Lingen 1995; Svendsen et al. 2003). Both hypoxia and starvation can strongly affect schooling behaviour. Under hypoxia, fishes tend to increase inter-individual distance and food-deprived fishes may prefer the periphery of their school or even reduce their propensity to associate with schoolmates (Krause et al. 1992; Domenici et al. 2002; Hoare et al. 2004). However, we can exclude these latter factors from operating during our experiments.
The observation that lateralized fishes are more aligned, swim in a closer formation and occupy more favourable positions inside the school thus implies that in their natural environment, they probably enjoy advantages such as energy saving or greater protection from predators. More direct evidence of a social advantage comes from heterogeneous schools in the second experiment where the risk of losing contact with schoolmates was twice as large in NL fishes compared with LD or RD fishes.
Interestingly, Rogers & Workman (1989) reported that social behaviour is affected by lateralization in chicks too. They scored the social hierarchy in groups of normally lateralized chicks hatched from eggs exposed to light and chicks from eggs incubated in the dark and hence weakly lateralized. They found that hierarchies were more stable in groups of light-exposed than in groups of dark-incubated chicks.
How can lateralization provide an advantage in group swimming? One may argue that while NL fishes show a random response to environmental stimuli, two similarly lateralized fishes always make the same choice in the same situation (Bisazza et al. 2001, 2004). In a school of the latter, fishes will tend to move more often in the same direction and the integrity and cohesion of the group will be preserved (Rogers 2002; Ghirlanda & Vallortigara 2004). Our experiments indicate that pairs of fishes with opposite direction of lateralization school as efficiently as pairs of fishes with the same laterality direction, although this is more obvious for inter-individual distance than for alignment.
Lateralized fishes must therefore enjoy some other sort of advantage, possibly of a computational nature. The literature provides some theorization of the mechanisms through which this could be achieved. When behavioural alternatives are available, cerebral asymmetries, for example, may reduce the chance of conflicting responses from the two sides of the brain (Andrew et al. 1982). Lateralization may speed up neural processes by avoiding slow inter-hemispheric interactions or by allowing for a more effective parallel processing (Levy 1969; Deacon 1997).
Rogers (2000, 2002) has proposed a different explanation. In many situations, animals face the need to simultaneously process and store different types of information, for example, while searching for food and maintaining vigilance for predators. She suggested that cerebral asymmetries initially evolved to allow individuals coping with divided attention. In fishes, as in most vertebrates, most sensory systems (including the visual system) consist of two laterally placed organs that send most fibres to one side of the brain. Having a lateralized brain may allow individuals to carry out simultaneous processing in an efficient way by channelling the different types of input or output so that each side of the brain carries out a different kind of information processing. Our lateralized fishes could be showing a more efficient schooling since they were able gather information about the position and movements of the shoal mate with one eye (and process it in the contralateral side of the brain, specialized for this task) while scanning the surrounding environment with the opposite eye. Data on sidedness of the followers in experiment 2 support this view.
Overall, our results indicate that in small schools, lateralized individuals enjoy an advantage by associating with fishes of the same type. Whether this effect also holds for larger schools remains to be investigated. Another important direction for future research is to determine if individuals are assorted in schools according to their laterality. Although wide inter-populational differences have been evidenced, most populations are probably composed of individuals with different degree of lateralization (De Santi et al. 2001; Brown et al. 2004). Random association of fishes is expected leading to the formation of schools consisting of both lateralized and NL fishes. Indeed, our data on pairs of fishes randomly selected from the stock population indicate that their schooling efficiency is closer to that of NN fishes. In this context, it would be beneficial for a lateralized fishes to associate with the other lateralized individuals of its population. The same mechanisms underlying spatial assortment by size and familiarity (Krause & Godin 1994; Griffith & Magurran 1999; Couzin & Krause 2003) might work to sort fishes by their laterality. For example, lateralized fishes might evaluate schooling efficiency and, on this basis, decide whether to stay or move to another school, or use past experience to associate more often with those individuals of their population that, in previous encounters, proved to be more effective schoolmates.
Acknowledgments
We wish to thank Culum Brown and Thomas Hope for useful comments on the manuscript; Paola Pezzin and Simone Dalla Chiara for their help with husbandry and analysis of videorecordings; and Massimiliano Martinelli for making the software. This research was financially supported by a grant from MIUR to A.B.
References
- Ades C, Ramires E.N. Asymmetry of leg use during prey handling in the spider Scytodes globula (Scytodidae) J. Insect Behav. 2002;15:563–570. [Google Scholar]
- Andrew R.J. The earliest origin and subsequent evolution of lateralization. In: Rogers L.J, Andrew R.J, editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge: 2002. pp. 70–93. [Google Scholar]
- Andrew R.J, Mench J, Rainey C. Right–left asymmetry of response to visual stimuli in the domestic chick. In: Ingle D.J, Goodale M.A, Mansfield R.J.W, editors. Advances in the analysis of visual behavior. MIT Press; Cambridge: 1982. pp. 197–209. [Google Scholar]
- Benoit-Bird K.J, Au W.W.L. Prey dynamics affect foraging by a pelagic predator (Stenella longirostris) over a range of spatial and temporal scales. Behav. Ecol. Sociobiol. 2003;53:364–373. [Google Scholar]
- Bisazza A, Facchin L, Vallortigara G. Heritability of lateralization in fish: concordance of right–left asymmetry of detour responses between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Sovrano V.A, Vallortigara G. Consistency among different tasks of left–right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia. 2001;39:1077–1085. doi: 10.1016/s0028-3932(01)00034-3. [DOI] [PubMed] [Google Scholar]
- Bisazza A, De Santi A, Bonso S, Sovrano V.A. Frogs and toads in front of a mirror: lateralisation of response to social stimuli in tadpoles of five anuran species. Behav. Brain Res. 2002;134:417–424. doi: 10.1016/s0166-4328(02)00055-4. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Dadda M, Cantalupo C. Further evidence for mirror-reversed laterality in lines of fish selected for leftward or rightward turning when facing a predator model. Behav. Brain Res. 2004;156:165–171. doi: 10.1016/j.bbr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Brown C, Gardner C, Braithwaite V.A. Population variation in lateralized eye use in the poeciliid Brachyraphis episcopi. Proc. R. Soc. B. 2004;271(Suppl. 6):S455–S457. doi: 10.1098/rsbl.2004.0222. 10.1098/rsbl.2004.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumann D, Krause J, Rubenstein D. Mortality risk of spatial positions in animal groups: the danger of being in front. Behaviour. 1997;134:1063–1076. [Google Scholar]
- Byrne R.A, Kuba M, Griebel U. Lateralized eye use in Octopus vulgaris shows antisymmetrical distribution. Anim. Behav. 2002;68:1107–1114. [Google Scholar]
- Couzin I.D, Krause J. Self-organization and collective behavior of vertebrates. Adv. Study Behav. 2003;32:1–67. [Google Scholar]
- Crow T.J, Crow L.R, Done D.J, Leask S. Relative hand skill predicts academic ability: global deficits at the point of hemispheric indecision. Neuropsychologia. 1998;36:1275–1282. doi: 10.1016/s0028-3932(98)00039-6. [DOI] [PubMed] [Google Scholar]
- De Santi A, Sovrano V.A, Bisazza A, Vallortigara G. Mosquitofish display differential left- and right-eye use during mirror-image scrutiny and predator-inspection responses. Anim. Behav. 2001;61:305–310. [Google Scholar]
- Deacon T. The Penguin Press; Harmondsworth: 1997. The symbolic species. [Google Scholar]
- Domenici P, Ferrari R.S, Steffensen J.F, Batty R.S. The effect of progressive hypoxia on school structure and dynamics in Atlantic herring Clupea harengus. Proc. R. Soc. B. 2002;269:2103–2111. doi: 10.1098/rspb.2002.2107. 10.1098/rspb.2002.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economakis A.E, Lobel P.S. Aggregation behavior of the grey reef shark, Carcharhinus amblyrhychos, at Johnston Atoll, Central Pacific Ocean. Environ. Biol. Fishes. 1998;51:129–139. [Google Scholar]
- Facchin L, Bisazza A, Vallortigara G. What causes lateralization of detour behaviour in fish? Evidence for asymmetries in eye use. Behav. Brain Res. 1999;103:229–234. doi: 10.1016/s0166-4328(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S, Vallortigara G. The evolution of brain lateralization: a game-theoretical analysis of population structure. Proc. R. Soc. B. 2004;271:853–857. doi: 10.1098/rspb.2003.2669. 10.1098/rspb.2003.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S.W, Magurran A.E. Schooling decisions in guppies (Poecilia reticulata) are based on familiarity rather than kin recognition by phenotype matching. Behav. Ecol. Sociobiol. 1999;45:437–443. [Google Scholar]
- Gueron S, Levin S.A, Rubenstein D.I. The dynamics of herds: from individuals to aggregations. J. Theor. Biol. 1996;182:85–98. [Google Scholar]
- Güntürkün O, Diekamp B, Manns M, Nottelmann F, Prior H, Schwarz A, Skiba M. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 2000;10:1079–1081. doi: 10.1016/s0960-9822(00)00671-0. [DOI] [PubMed] [Google Scholar]
- Hoare D.J, Couzin I.D, Godin J.G, Krause J. Context-dependent group size choice in fish. Anim. Behav. 2004;67:155–164. [Google Scholar]
- Kells A.R, Goulson D. Evidence for handedness in bumblebees. J. Insect Behav. 2001;14:47–55. [Google Scholar]
- Klimley A.P. Schooling in Sphyrna lewini, a species with low-risk of predation: a non-egalitarian state. Z. Tierpsychol. 1985;70:297–319. [Google Scholar]
- Krause J, Godin J.G.J. Shoal choice in banded killifish (Fundulus diaphanus, Teleostei, Cyprinnodontidae): The effects of predation risk, fish size, species composition and size of shoals. Ethology. 1994;98:128–136. [Google Scholar]
- Krause J, Bumann D, Todt D. Relationship between the position preference and nutritional state of individuals in shoals of juvenile roach (Rutilus rutilus) Behav. Ecol. Sociobiol. 1992;30:177–180. [Google Scholar]
- Krause J, Reeves P, Hoare D. Positioning behaviour in roach shoals: the role of body length and nutritional state. Behaviour. 1998a;135:1031–1039. [Google Scholar]
- Krause J, Ruxton G.D, Rubenstein D.I. Is there always an influence of shoal size on predator hunting success? J. Fish Biol. 1998b;52:494–501. [Google Scholar]
- Krause J, Butlin R.K, Peuhkuri N, Pritchard V.L. The social organization of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol. Rev. 2000;75:477–501. doi: 10.1111/j.1469-185x.2000.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Levy J. Possible basis for the evolution of lateral specialization in the human brain. Nature. 1969;224:614–615. doi: 10.1038/224614a0. [DOI] [PubMed] [Google Scholar]
- van der Lingen C.D. Respiration rate of adult pilchard Sardinops sagax in relation to temperature, voluntary swimming speed and feeding behaviour. Mar. Ecol. Prog. Ser. 1995;129:41–54. [Google Scholar]
- McGrew W.C, Marchant L.F. Laterality of hand use pays off in foraging success for wild chimpanzees. Primates. 1999;40:509–513. [Google Scholar]
- Motta P.J, Wilga C.D. Advances in the study of feeding behaviors, mechanisms, and mechanics of sharks. Environ. Biol. Fishes. 2001;60:131–156. [Google Scholar]
- Pascual A, Huang K.L, Neveu J, Preat T. Brain asymmetry and long-term memory. Nature. 2004;427:605–606. doi: 10.1038/427605a. [DOI] [PubMed] [Google Scholar]
- Pitcher T.J. Heuristic definitions of schooling behaviour. Anim. Behav. 1983;31:611–613. [Google Scholar]
- Pitcher T.J, Parrish J.K. Functions of shoaling behaviour in teleosts. In: Pitcher T.J, editor. Behaviour of teleost fishes. Chapman & Hall; London: 1993. pp. 363–439. [Google Scholar]
- Pitcher T.J, Magurran A.E, Winfield I.J. Fish in larger shoals find food faster. Behav. Ecol Sociobiol. 1982a;10:149–151. [Google Scholar]
- Pitcher T.J, Wyche C.J, Magurran A.E. Evidence for position preferences in schooling mackerel. Anim. Behav. 1982b;30:932–934. [Google Scholar]
- Rogers L.J. Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 2000;73:236–253. doi: 10.1006/brln.2000.2305. [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Advantages and disadvantages of lateralization. In: Rogers L.J, Andrew R.J, editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge: 2002. pp. 126–154. [Google Scholar]
- Rogers L.J, Andrew R.J. Cambridge University Press; Cambridge: 2002. Comparative vertebrate lateralization. [Google Scholar]
- Rogers L.J, Workman L. Light exposure during incubation affects competitive behaviour in domestic chicks. Appl. Anim. Behav. Sci. 1989;23:187–198. [Google Scholar]
- Rogers L.J, Zucca P, Vallortigara G. Advantages of having a lateralized brain. Proc. R. Soc. B. 2004;(Suppl. 6) doi: 10.1098/rsbl.2004.0200. Biol. Lett. S420–S422. 10.1098/rsbl.2004.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw E. Schooling fishes. Am. Sci. 1978;66:166–175. [Google Scholar]
- Sovrano V.A, Rainoldi C, Bisazza A, Vallortigara G. Roots of brain specializations: preferential left-eye use during mirror-image inspection in six species of teleost fish. Behav. Brain Res. 1999;106:175–180. doi: 10.1016/s0166-4328(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Svendsen J.C.S, Kov J.S, Bildsoe M, Teffensen J.F.S. Intra-school positional preference and reduced tail beat frequency in trailing positions in schooling roach under experimental conditions. J. Fish Biol. 2003;62:834–846. [Google Scholar]
- Vallortigara G, Bisazza A. How ancient is brain lateralization? In: Rogers L.J, Andrew R.J, editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge: 2002. pp. 9–69. [Google Scholar]
- Viscido S.V, Parrish J.K, Grunbaum D. Individual behavior and emergent properties of fish schools: a comparison of observation and theory. Mar. Ecol. Prog. Ser. 2004;273:239–249. [Google Scholar]
- Wassersug R.J, Lum A.M, Potel M.J. An analysis of school structure for tadpoles (Anura, Amphibia) Behav. Ecol. Sociobiol. 1981;9:15–22. [Google Scholar]


