Abstract
Many animals nest or roost colonially. At the start of a potential foraging period, they may set out independently or await information from returning foragers. When should such individuals act independently and when should they wait for information? In a social insect colony, for example, information transfer may greatly increase a recruit's probability of finding food, and it is commonly assumed that this will always increase the colony's net energy gain. We test this assumption with a mathematical model. Energy gain by a colony is a function both of the probability of finding food sources and of the duration of their availability. A key factor is the ratio of pro-active foragers to re-active foragers. When leaving the nest, pro-active foragers search for food independently, whereas re-active foragers rely on information from successful foragers to find food. Under certain conditions, the optimum strategy is totally independent (pro-active) foraging because potentially valuable information that re-active foragers may gain from successful foragers is not worth waiting for. This counter-intuitive outcome is remarkably robust over a wide range of parameters. It occurs because food sources are only available for a limited period. Our study emphasizes the importance of time constraints and the analysis of dynamics, not just steady states, to understand social insect foraging.
Keywords: collective foraging, optimal foraging, information transfer, recruitment, division of labour, social insect
1. Introduction
There are two ways of acquiring information about food sources: by solitary searching (personal information) or by interacting with other foragers (social information). Social information can be based on cues provided inadvertently by individuals exploiting a profitable food source, or signals that actively recruit other foragers (Danchin et al. 2004). Such group foraging based on social information has been described in a number of taxa, including insects, fishes, birds, mammalian carnivores and primates (Giraldeau & Caraco 2000). The benefit of using social information is that it may reduce the cost of individual searching.
Some of the most remarkable cases of information sharing and cooperation among foragers occur in eusocial insects. Members of a colony do not forage purely for their own consumption. The food is stored for later use by the colony. A cooperative strategy allows the colony to focus its foraging efforts rapidly on newly discovered food sources. Several recruitment systems allow workers to acquire information about profitable sources, e.g. recruitment dances of honey bees (von Frisch 1967; Seeley 1995) and tandem runs in ants, in which the successful forager directly leads a nest-mate to the food source (Hölldobler & Wilson 1990). Indirect methods are even more common and often involve pheromone trails or simply pheromones that activate other workers (Hölldobler & Wilson 1990; Dornhaus & Chittka 2004b; Nieh 2004). Furthermore, unemployed foragers can be alerted to the general availability of food sources by the frequency and intensity at which foragers engage them in trophallaxis (Ribbands 1955; De Marco & Farina 2001; Fernández et al. 2003). Scents detected on the bodies of successful foragers may also provide informative signals and cues (Stabentheiner et al. 1995; Biesmeijer & Slaa 2004; Dornhaus & Chittka 2004a; Gil & De Marco 2005).
This information transfer increases the recruits' probabilities of finding a good food source. Thus, it is commonly assumed that recruitment always increases the colony's net energy gain (Lindauer 1961; von Frisch 1967; Ydenberg & Schmid-Hempel 1994). This assumption is questionable (Sherman & Visscher 2002; Dornhaus & Chittka 2004b). Can the costs, in energy or time, of awaiting information outweigh the benefits of communication? If so, when should workers forage independently? By exploring this fundamental issue we can gain new insights about the efficiencies that accrue from information sharing and division of labour in a broader context.
In honey bee colonies for example, worker inactivity is surprisingly common (Lindauer 1952; Kolmes 1985; Seeley 1995). Why? First, such apparently inactive workers may receive information on the needs of the colony while patrolling in the nest (Seeley 1998). Second, they may be able to react quickly to the discovery of profitable food sources. The availability of food sources can be extremely variable. In a dearth it would be counter-productive for all foragers to search in vain and waste energy. On the other hand, if food can be found easily, why should workers stay in the nest to be informed about its location. Thus, the division of labour between pro-active (independent) searchers and re-active (recruitment-guided) searchers is susceptible to optimization as a function of environmental conditions. Using a mathematical model, we study energy gain by a colony as a function both of the probability of finding food sources and the duration of their availability. Contrary to most studies of division of labour, we will not focus on possible mechanisms allowing the workers to distribute themselves appropriately among several tasks or resources. Rather, we address the more general and strategic question of the optimal proportion of workers that should stay inactive in the nest to await information.
2. The model
We advocate the terms ‘pro-active’ workers and ‘re-active’ workers. These terms recognize that some workers (the pro-actives) spontaneously search for food sources. If successful, they forage and may recruit one or more of their nest-mates. Such recruits (the re-actives) search for the food source advertised by the successful nest-mates. In turn, successful re-active searchers become foragers and can recruit additional inactive workers.
The model is designed to determine the optimal proportion of pro-active and re-active searchers, rather than to investigate mechanisms of individual allocation to these roles. In our model, it is unimportant whether the difference between pro-active and re-active workers is ‘hard-wired’ or is influenced by the dynamics of interactions with other nest-mates or their environment. For simplicity and tractability, we assume that the profitability of food sources remains constant, and we do not consider any effect of previous foraging experience (Aron et al. 1993; Raveret-Richter & Waddington 1993; Gil & Farina 2002). Moreover, we assume that successful foragers recruit with a constant probability. We do not consider any variability among workers or any stochasticity at the individual level (Myerscough & Oldroyd 2004).
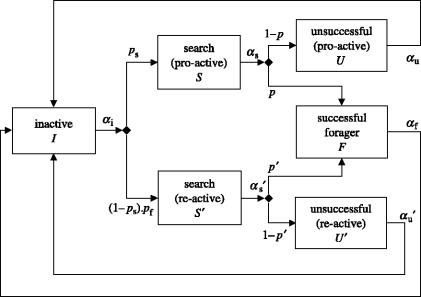
We assume that N workers are distributed among the behaviours described in the boxes in figure 1. At a given time t, I(t) workers are inactive in the nest. Such inactive workers re-evaluate their role at rate αi. When re-evaluation takes place, we assume a constant proportion ps become pro-active searchers S, while the remaining proportion 1−ps stay inactive unless they are recruited by successful foragers, in which case they become re-active searchers. We assume that the probability of recruitment depends on the current number of successful foragers, so on re-evaluation we assume a proportion (1−ps)pf become re-active searchers S′ and the remainder, which did not move to S or S′, stay inactive. Pro-active searchers S change their role at overall rate αs. Of these a proportion p change because they find food and become successful foragers F, while the remainder, a proportion 1−p fail to find food and become unsuccessful pro-active searchers U. These unsuccessful searchers wander around and eventually return to become inactive workers I at rate αu. Similarly, re-active searchers S′ change their role at rate . Of these a proportion p′ become successful foragers F, while a proportion 1−p′ become unsuccessful re-active searchers U′ which eventually return to the nest at rate . The foraging state F includes travel time between the nest and the food source, handling time at the source, unloading time at the nest and time spent recruiting. A small number of successful foragers F change their roles at rate αf and revert to being inactive workers I. At any time, we have . The model is formalized by a system of differential equations which describes the dynamics of the numbers of workers in the different behavioural states, I(t), S(t), S′(t), U(t), U′(t) and F(t)
We assume that the N workers are inactive at the beginning of the day, I(t=0)=N. The numerical values of parameters used in the model are given in table 1. These values have been chosen to represent honeybee foraging but they can be modified to investigate foraging by other species. We have also assessed the effect of modifications of their value on the properties of the system (§2d).
Figure 1.
Flow diagram of the model. The behavioural states are represented by boxes. Each parameter α represents the rate at which the individuals leave a state. The diamonds represent decision points. For example, after the searching state S the workers have a probability p of finding the food source and a probability 1−p of not finding the food source.
Table 1.
| parameter | definition | value |
|---|---|---|
| αs | rate at which workers leave state S (Seeley 1983, 1986; Seeley & Visscher 1988) | 1/50 min−1 |
| rate at which workers leave state S′ (Seeley 1983, 1986; Seeley & Visscher 1988) | 1/50 min−1 | |
| αu | rate at which workers leave state U | 1 min−1 |
| rate at which workers leave state U′ | 1 min−1 | |
| αf | rate at which workers leave the state F (Seeley et al. 1991) | 10−4 min−1 |
| αi | rate at which inactive workers can become pro-active or re-active (Seeley 1995) | 0.1 min−1 |
| σ | proportion of time spent recruiting for a successful forager (Seeley 1986) | 0.1 |
| K | term of proportionality | 50 |
| N | total number of potential foragers (Seeley 1985) | 300 |
| recruit's probability of finding the food due to recruitment only (equation (2.4)) | 0.2 | |
| μ | link between p and p′ (equation (2.3)) | 1 |
| b | energy collected per successful forager per second (Anderson 2001) | 1.9×10−1 W |
| cs | energy cost searching per recruit per second (Anderson 2001) | 3.4×10−2 W |
| ci | metabolic cost per inactive worker per second (Anderson 2001) | 4.2×10−3 W |
| cf | energy cost per successful forager per second (Seeley 1985; Goller & Esch 1990) | 2.7×10−2 W |
| ps | proportion of workers that become pro-active searchers | variable |
| p | probability of finding a food source for pro-active searchers | variable |
| p′ | probability of finding a food source for re-active searchers | variable |
| T | Duration of availability of food | variable |
However, the effect of modifying their values has been assessed with a sensitivity analysis.
The recruitment function pf determines the rate at which inactive workers become re-active searchers. This rate depends on the number of successful foragers. We assume that only a certain proportion of successful foragers recruit. Our default recruitment function assumes that pf is proportional to the number of successful foragers
| 2.1 |
The greater the number of successful foragers, the greater will be the proportion of workers recruited per time unit.
Information may not be transmitted efficiently, however, when there are too many recruiters. For example in a honeybee colony, dances are performed on a limited area of the combs. Follower bees may interfere with each other and the number of recruits per recruiter may decrease with an increase in the absolute number of recruiters. Therefore, we investigated another recruitment function (derived from Deneubourg et al. 1990; Camazine et al. 2001; Sumpter & Pratt 2003) that takes into account the absolute number of recruiters
| 2.2 |
where σ is the proportion of successful foragers recruiting and K is a braking constant that captures the proportionality to the absolute number of successful foragers. When the absolute number of recruiters (σF) increases, pf tends to 1, and the number of recruits per recruiter decreases.
(a) The colony's net energy gain
We compare the foraging performance under different strategies by comparing the colony's net energy intake (Ydenberg & Schmid-Hempel 1994; Denny et al. 2001; Sherman & Visscher 2002; Dornhaus & Chittka 2004b) during a limited time period T. The total net energy gain G(T) during T is a function of the number of successful foragers F, the individual amount of energy b collected by each forager per unit of time, the rate of energy expenditure during travel for successful foragers cf, and the rate of energy expenditure during search cs. We assumed that b is constant over time. We also consider the metabolic cost of inactive workers awaiting information in the nest ci. The colony's net energy gain G(T) is
| 2.3 |
The energy costs of searching and the energy consumed by inactive workers are not trivial. Moreover, food is collected as soon as it is discovered, and the accelerating phase of recruitment can have a substantial effect upon a colony's total energy gain. In such a dynamic system it could be misleading to analyse maximisation of the rate of energy gain at the steady state of the system. Therefore, we investigated fully the dynamics of forager build-up and energy collection.
(b) Optimal proportion of pro-active workers
We assume that the proportion of pro-active workers ps is constant over time t. By resolving the system numerically, we found that, for a given set of parameters, there is a single value of ps that maximizes the colony's net energy gain. This optimal value is dependent upon the duration of food availability T, and the probability of finding the source for a pro-active worker, p. For each pair (T, p), we determined the corresponding value of .
(c) Two models
As a first approximation (model I), we assumed a fixed value for p′ (probability that a recruit finds the food source advertised by successful foragers) whatever p. But if the food source could be easily found by a pro-active worker (p is large), it should also be easily found by a re-active worker that takes advantage of the information collected by successful foragers (so p′ is large). Hence, in model II we linked p and p′
| 2.4 |
where represents the information transmitted through recruitment. Here, if the re-active worker does not find the food source as a direct result of recruitment, it still has a probability of finding it by chance (second part of the equation). If and μ>0, p′ is always larger than p.
(d) Sensitivity analysis
We investigated the effect of the other variables on the predictions of the model by modifying them over a broad range of values. In particular, we assessed the effect of the transition rates (for example the mean searching times were varied from 1 min to several hours), the energetic gain (from 1 to 100 times higher than the physiological or travel costs), and the effect of the number of foragers (from 10 to 10 000). We also investigated other functions than (2.4) to link p and p′ such as a function including a quadratic term. We found that the results of the model were never qualitatively modified.
3. Results
(a) Model I
We used the default recruitment function (equation (2.1)). The optimal proportion of pro-active workers depends upon the duration of food availability (T) and upon p. For each pair (T, p), there is only one optimum proportion that maximizes the colony's energy gain. Such proportions occur in three categories (shown by the three areas delimited by the thick lines in figure 2). First, when the searching costs are greater than the benefits expected over the limited period T, the optimal strategy is to have no pro-active workers . Consequently there is no foraging activity at all. Second, the optimal proportion of pro-active workers could be . Every individual should then start searching on its own. This occurs when the overall costs of inactive workers waiting for information in the nest are higher than the benefits of recruitment. Third, some workers start to search for a profitable food source while some avoid the energy costs of searching by remaining inactive in the nest. Hence, there is an optimum proportion of re-active workers that is less than 1 and more than zero, and it is adaptive for the colony to contain inactive workers while others are foraging.
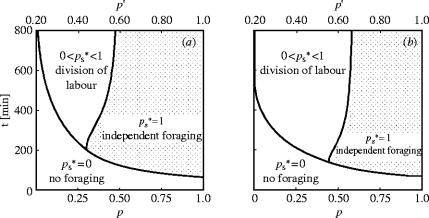
Figure 2.
Model I. Foraging strategies as a function of p, the probability of finding a food source for a pro-active searcher S and Tmax, the duration of availability of food. For these numerical resolutions, we have fixed p′=0.5 and used the default recruitment function (equation (2.1)). is the optimal proportion of pro-active searchers. When the conditions lead to , the best strategy for the foragers is to stay in the nest and not forage. If each worker forages individually. If then the workers specialize in two groups, pro-active or re-active workers. Dashed line, value of p=p′. Dotted area, range of values where despite the fact that the recruitment would increase the probability of finding the source for a re-active individual (p′>p).
The dashed line in figure 2 represents the value p=p′. To the right of this dashed line, p′<p. The re-active searchers have a lower probability of finding the food source after being informed by a successful forager than they would if they had searched unaided as pro-actives. Logically, the optimal proportion of pro-active foragers is . To the left of the dashed line, p′>p, and recruitment increases the probability of finding the food source. Within this range of values of p there is a non-zero proportion of re-active workers that should rely on recruitment to find the source .
Nevertheless, there is a range of values of p<p′ where (dotted area in figure 2). Hence, even though recruitment would increase the re-active's probability of finding food sources, the workers should search for food on their own as pro-actives. The increase of foraging performance due to recruitment is not sufficient to compensate for the costs of inactive workers consuming energy while idly waiting in the nest at the beginning of the foraging period. Numerical analysis reveals that for an extremely long T, the line delimiting the individual foraging and the cooperative foraging areas converges to the dashed line. But surprisingly, this zone of independent foraging remains even with very long periods of food availability (T). Even in the case of T=100 days (which is probably longer than any natural food source), there is a range of values (0.49<p<p′) where all individuals should search for food on their own.
The importance of time constraints can also be illustrated when the probability p of finding the food source equals 1. Even under such circumstances, there is a range of values of T during which the workers should not forage. In the case of an easily discovered but fleeting food source, the cost in time spent searching or travelling between the nest and the feeding site is too high.
(b) Model II
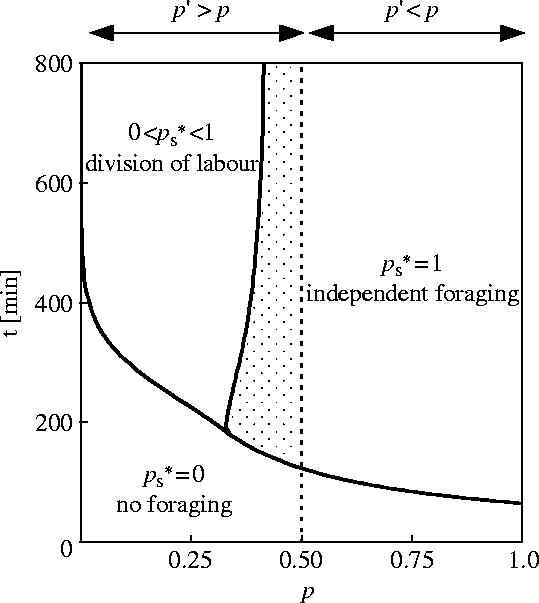
To simplify the analysis of the collective foraging process, we first assumed (in model I) that p and p′ are independent. Nevertheless, if a food source can be easily found by a pro-active searcher (p is high), re-active searchers (which benefit from information gained from successful foragers) should also find it easily (p′ will be high). Thus, the two probabilities p and p′ might be linked (equation (2.4)). Hence, we have computed the optimal proportion of pro-active searchers as a function of T and p with the default recruitment function (equation (2.1)). Nevertheless, the qualitative predictions of the original model are not strongly modified (figure 3a). Recruitment always increases the probability of finding a profitable food source. Yet a range of parameters still remains where the workers should forage individually. Furthermore, this pattern is not modified even when we consider that the number of recruits per recruiter decreases with an increase in the number of recruiters as described in equation (2.2) (figure 3b).
Figure 3.
Model II. Foraging strategies as a function of the probabilities of finding a source (p and p′), and Tmax the duration of availability of food. Probability of finding the source for a pro-active searcher (p, bottom X-axis) and for a recruit (p′, top X-axis) are linked (equation (2.4)). Dotted area, range of values where even though recruitment would increase the probability of finding the source (p′>p). (a) Default recruitment function (equation (2.1)) or (b) second function taking into account a saturation of the recruiters (equation (2.2)).
4. Discussion
(a) The value of information
We have found that under certain environmental conditions, the optimum strategy for a social insect colony is pure independent foraging. This prediction holds even when potential recruits would benefit from information gained from successful foragers by having a higher probability of finding food than they would as independent foragers. This is because of the costs of waiting for information in the recruitment process. Most intriguingly, such hidden costs are sufficiently important to have noticeable effects even when the food source is available for many days. Because information transfer increases the probability of finding the best food sources for individual recruits, it is commonly assumed that recruitment is always advantageous (Lindauer 1961; von Frisch 1967). But our counter-intuitive results raise the question of the value of information in social insect communication. Recruitment is not always adaptive, even when the recruits have a higher probability of finding food. There is not necessarily a direct link between an increase in individual performance after information transfer and collective energy gain over the whole foraging period.
Our study differs from the other theoretical studies that have previously investigated the scout-recruit system in social insects (Johnson et al. 1987; Jaffe & Deneubourg 1992; Anderson 2001). First, we did not assume a strict division of labour between scouts and recruits. Indeed, the notion of scouts should be handled with care. For example, in a model by Jaffe & Deneubourg (1992), the scouts were assumed not to forage at all. As a result, the optimal proportion of scouts was found to decrease as the amount of food increased. But the existence of such single task scouts is highly questionable (Biesmeijer & de Vries 2001).
Second, in contrast to the studies of Johnson et al. (1987) and Anderson (2001), we emphasize the importance of time constraints for the assessment of collective strategies. Under natural conditions, food sources are not present continuously. For example, for honey bees, conditions may change quickly from a nectar famine to a feast. In most plants, optimal nectar availability (maximum volume and sugar concentration) occurs only during a few hours on a restricted number of days (Corbet 1978; Corbet et al. 1979; Cruden et al. 1983; Herrera 1990). Similarly, in the cases of scavenger ants or seed harvester ants, availability of food items can be ephemeral (Wehner 1987; Traniello 1989; Hölldobler & Wilson 1990; Gordon 1991; Traniello et al. 1991). In some rare situations, such as for leaf-cutting ants or ants feeding on aphid dew, the colony could exploit spatially stable and renewable sources for days (Way 1963; Sudd 1987; Sundström 1993; Burd 1996). But in most cases, the colony has to optimize its energy gain within a very limited window of opportunity. Thus, it is potentially hugely misleading to confine the study of the properties of a model only to the steady state of the recruitment process, as is often done in theoretical studies of social insects (Johnson et al. 1987; Bartholdi et al. 1993; Anderson 2001). By investigating recruitment strategies dynamically, we have been able to take into account not only energetic costs, but also time costs and ‘inertia’ at the beginning of the recruitment process. Such time constraints are overlooked in models that only consider such systems at equilibrium. An analysis of the model's properties at the steady state only (T tends to infinity) leads to the erroneous conclusion that some workers should choose to be re-active workers for any p<p′ (i.e. when recruitment increases the probability of finding food sources).
(b) Division of labour
We have studied a simple case of division of labour between pro-active and re-active workers. In the case of a dearth of resources it would be counter-productive for all the individuals to spend large amounts of energy searching. On the other hand, if food sources can be found easily, there is no need for a worker to stay in the nest and wait to be informed about their precise positions. The proportion of pro-active searchers strongly influences the energy spent in searching for food and the amount of energy collected. For any set of environmental conditions, there is an optimal division of labour. In the case of abundant forage, we predict a decrease in the importance of the recruitment (i.e. an increase in the proportion of pro-active searchers). Our theoretical results are consistent with experimental data from the literature. The proportion of pro-active workers is not constant; rather it varies as a function of the environmental conditions (Seeley 1983; Portha et al. 2002). The importance of recruitment is reported to decrease in habitats with abundant sources of food (Waddington et al. 1994; Dornhaus & Chittka 2004b; Dornhaus et al. submitted), or when the sources are more ephemeral (Portha et al. 2002).
Contrary to most studies of division of labour (Bourke & Franks 1995; Gordon 1996; Beshers & Fewell 2001), we have not focused on possible mechanisms that allow workers to distribute themselves among several tasks. We first have to address the fundamental question: are there circumstances in which a division of labour reduces collective performance? One of the great benefits of using foraging as a test case, compared with most other collective tasks, is that the value of many different strategies can be easily quantified by direct comparison of the net amount of energy collected over a limited period. Our study shows that, under particular circumstances, the cost in energy or time incurred by a division of labour and the necessary information transfer can be counter-productive and in this case independent foraging should be favoured.
Acknowledgments
F.X.D.M. was supported by a BBSRC (British Biotechnology and Biological Sciences Research Council) research grant E19832 and A.D. was supported by the DFG (German Science Foundation, Emmy Noether Fellowship).
References
- Anderson C. The adaptive value of inactive foragers and the scout-recruit system in honey bee (Apis mellifera) colonies. Behav. Ecol. 2001;12:111–119. [Google Scholar]
- Aron S, Beckers R, Deneubourg J.L, Pasteels J.M. Memory and chemical communication in the orientation of two mass-recruiting ant species. Insectes Soc. 1993;40:369–380. [Google Scholar]
- Bartholdi J.J, Seeley T.D, Tovey C.A, Vande Vate J.H. The pattern and effectiveness of forager allocation among flower patches by honey bee colonies. J. Theor. Biol. 1993;160:23–40. [Google Scholar]
- Beshers S.N, Fewell J.H. Model of division of labor in insect societies. Annu. Rev. Entomol. 2001;46:413–430. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Biesmeijer J.C, de Vries H. Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav. Ecol. Sociobiol. 2001;49:89–99. [Google Scholar]
- Biesmeijer J.C, Slaa E.J. Information flow and organization of stingless bee foraging. Apidologie. 2004;35:143–158. [Google Scholar]
- Bourke A.F.G, Franks N.R. Princeton University Press; Princeton, NJ: 1995. Social evolution in ants. [Google Scholar]
- Burd M. Server system and queuing models of leaf harvesting by leaf-cutting ants. Am. Nat. 1996;148:613–629. [Google Scholar]
- Camazine S, Deneubourg J.-L, Franks N.R, Sneyd J, Theraulaz G, Bonabeau E. Princeton University Press; Princeton, NJ: 2001. Self-organization in biological systems. [Google Scholar]
- Corbet S.A. Bee visits and the nectar of Echium vulgare L. and Sinapsis alba L. Ecol. Entomol. 1978;3:25–37. [Google Scholar]
- Corbet S.A, Unwin D.M, Prŷs-Jones O.E. Humidity, nectar and insect visits to flowers, with special reference to Crataegus, Tilia and Echium. Ecol. Entomol. 1979;4:9–22. [Google Scholar]
- Cruden R.W, Hermann H.M, Peterson S. Patterns of nectar production and plant-pollinator coevolution. In: Bentley B, Elias T, editors. The biology of nectaries. Columbia University Press; New York: 1983. pp. 80–125. [Google Scholar]
- Danchin E, Giraldeau L.A, Valone T.J, Wagner R.H. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- De Marco R, Farina W. Changes in food source profitability affect the trophallactic and dance behavior of forager honeybees (Apis mellifera L.) Behav. Ecol. Sociobiol. 2001;50:441–449. [Google Scholar]
- Deneubourg J, Aron S, Pasteels J. The self-organizing exploratory pattern of the Argentine ant Iridomyrmex humilis. J. Insect Behav. 1990;3:159–168. [Google Scholar]
- Denny A.J, Wright J, Grief B. Foraging efficiency in the wood ant, Formica rufa: is time of the essence in trail following? Anim. Behav. 2001;62:139–146. [Google Scholar]
- Dornhaus A, Chittka L. Information flow and regulation of foraging activity in bumble bees (Bombus spp.) Apidologie. 2004a;35:183–192. [Google Scholar]
- Dornhaus A, Chittka L. Why do honey bees dance? Behav. Ecol. Sociobiol. 2004b;55:395–401. [Google Scholar]
- Dornhaus, A., Kluegl, F., Oechslein, C., Puppe, F. & Chittka, L. Submitted. Resource distribution and benefits of recruitment in social bees.
- Fernández P.C, Gil M, Farina W.M. Reward rate and forager activation in honeybees: recruiting mechanisms and temporal distribution of arrivals. Behav. Ecol. Sociobiol. 2003;54:80–87. [Google Scholar]
- Gil M, De Marco R.J. Olfactory learning by means of trophallaxis in Apis mellifera. J. Exp. Biol. 2005;208:671–680. doi: 10.1242/jeb.01474. [DOI] [PubMed] [Google Scholar]
- Gil M, Farina W.M. Foraging reactivation in the honeybee Apis mellifera L.: factor affecting the return to known nectar sources. Naturwissenschaften. 2002;89:322–325. doi: 10.1007/s00114-002-0323-1. [DOI] [PubMed] [Google Scholar]
- Giraldeau L.A, Caraco T. Princeton University Press; Princeton, NJ: 2000. Social foraging theory. [Google Scholar]
- Goller F, Esch H.E. Waggle dances of honey bees. Is distance measured through energy expenditure on outward flight. Naturwissenschaften. 1990;77:594–595. [Google Scholar]
- Gordon D.M. Behavioral flexibility and the foraging ecology of seed-eating ants. Am. Nat. 1991;138:379–411. [Google Scholar]
- Gordon D.M. The organization of work in social insect colonies. Nature. 1996;380:121–124. [Google Scholar]
- Herrera C.M. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer-flowering Mediterranean shrub. Oikos. 1990;58:277–288. [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Jaffe K, Deneubourg J.L. On foraging, recruitment systems and optimum number of scouts in eusocial colonies. Insectes Soc. 1992;39:201–213. [Google Scholar]
- Johnson L.K, Hubbell S.P, Feener D. Defense of food supply by eusocial colonies. Am. Zool. 1987;27:347–358. [Google Scholar]
- Kolmes S.A. A quantitative study of the division of labor among worker honey bees. Z. Tierpsychol. 1985;68:287–302. [Google Scholar]
- Lindauer M. Ein Beitrag zur Frage der Arbeitsteilung im Bienenstaat. Z. Vergl. Physiol. 1952;34:299–345. [Google Scholar]
- Lindauer M. Harvard University Press; Cambridge, MA: 1961. Communication among social bees. [Google Scholar]
- Myerscough M.R, Oldroyd B.P. Simulation models of the role of genetic variability in social insect task allocation. Insectes Soc. 2004;51:146–152. [Google Scholar]
- Nieh J.C. Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini) Apidologie. 2004;35:159–182. [Google Scholar]
- Portha S, Deneubourg J.L, Detrain C. Self-organized asymmetries in ant foraging: a functional response to food type and colony needs. Behav. Ecol. 2002;13:776–781. [Google Scholar]
- Raveret-Richter M, Waddington K. Past foraging experience influences honey dance behaviour. Anim. Behav. 1993;46:123–128. [Google Scholar]
- Ribbands C.R. The scent perception of the honeybee. Proc. R. Entomol. Soc. B. 1955;143:367–379. [Google Scholar]
- Seeley T.D. Division of labor between scouts and recruits in honeybee foraging. Behav. Ecol. Sociobiol. 1983;12:253–259. [Google Scholar]
- Seeley T.D. Princeton University Press; Princeton, NJ: 1985. Honeybee ecology, a study of adaptation in social life. [Google Scholar]
- Seeley T.D. Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav. Ecol. Sociobiol. 1986;19:343–354. [Google Scholar]
- Seeley T.D. Harvard University Press; Cambridge, MA: 1995. The wisdom of the hive. [Google Scholar]
- Seeley T.D. Thoughts on information and integration in honeybee colonies. Apidologie. 1998;29:67–80. [Google Scholar]
- Seeley T.D, Visscher P.K. Assessing the benefits of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behav. Ecol. Sociobiol. 1988;22:229–237. [Google Scholar]
- Seeley T.D, Camazine S, Sneyd J. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1991;28:277–290. [Google Scholar]
- Sherman G, Visscher P.K. Honeybee colonies achieve fitness through dancing. Nature. 2002;419:920–922. doi: 10.1038/nature01127. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A, Kovac H, Hagmüller K. Thermal behavior of round and wagtail dancing honey bees. J. Comp. Physiol. B. 1995;165:433–444. [Google Scholar]
- Sudd J.H. Mutualism of ants and aphids. In: Harrewijn P, Minsk A.K, editors. Aphids, their biology, natural enemies and control. Elsevier; Amsterdam: 1987. pp. 355–365. [Google Scholar]
- Sumpter D.J.T, Pratt S.C. A modelling framework for understanding social insect foraging. Behav. Ecol. Sociobiol. 2003;53:131–144. [Google Scholar]
- Sundström L. Foraging responses of Formica truncorum (Hymenoptera; Formicidae) exploiting stable vs. spatially and temporally variable resources. Insectes Soc. 1993;40:147–161. [Google Scholar]
- Traniello J.F.A. Foraging strategies of ants. Annu. Rev. Entomol. 1989;34:191–210. [Google Scholar]
- Traniello J.F.A, Fourcassié V, Graham T.P. Search behavior and foraging ecology of the ant Formica shaufussi: colony-level and individual patterns. Ethol. Ecol. Evol. 1991;3:35–47. [Google Scholar]
- von Frisch K. The Belknap Press of Harvard University Press; Cambridge, MA: 1967. The dance language and orientation of bees. [Google Scholar]
- Waddington K.D, Visscher P.K, Herbert T.J, Richter M.R. Comparisons of forager distributions from matched honey bee colonies in suburban environments. Behav. Ecol. Sociobiol. 1994;35:423–429. [Google Scholar]
- Way M.J. Mutualism between ants and honeydew-producing homoptera. Annu. Rev. Entomol. 1963;8:307–344. [Google Scholar]
- Wehner R. Spatial organization of foraging behavior in individually searching desert ants, Cataglyphis (Sahara desert) and Ocymyrmex (Namib desert) In: Pasteels J.M, Deneubourg J.L, editors. From individual to collective behaviour. Birkhäuser; Basel: 1987. pp. 15–42. [Google Scholar]
- Ydenberg R, Schmid-Hempel P. Modelling social insect foraging. Trends Ecol. Evol. 1994;9:491–493. doi: 10.1016/0169-5347(94)90321-2. [DOI] [PubMed] [Google Scholar]