Abstract
Because of pelagic-larval dispersal, coral-reef fishes are distributed widely with minimal genetic differentiation between populations. Amphiprion akallopisos, a clownfish that uses sound production to defend its anemone territory, has a wide but disjunct distribution in the Indian Ocean. We compared sounds produced by these fishes from populations in Madagascar and Indonesia, a distance of 6500 km. Differentiation of agonistic calls into distinct types indicates a complexity not previously recorded in fishes' acoustic communication. Moreover, various acoustic parameters, including peak frequency, pulse duration, number of peaks per pulse, differed between the two populations. The geographic comparison is the first to demonstrate ‘dialects’ in a marine fish species, and these differences in sound parameters suggest genetic divergence between these two populations. These results highlight the possible approach for investigating the role of sounds in fish behaviour in reproductive divergence and speciation.
Keywords: sound production, Pomacentridae, geographic variation, dialect, speciation
1. Introduction
Changes in behaviour can be a stage leading to speciation (Mayr 1989). A change in communication signals provides a prezygotic isolating system in which receivers are the selective force on the evolution of the signal (Higgins & Waugaman 2004). Geographical variation in acoustic signals occurs in numerous taxa including insects (Simmons et al. 2001; Higgins & Waugaman 2004), frogs (Cocroft & Ryan 1995), birds (Grant & Grant 1996; Slabbekoorn & Smiths 2002) and mammals (Peters & Tonkin-Leyhausen 1999; Bazua-Duran & Au 2004). In birds and mammals, geographic variation in repertoires has been attributed to adaptations to different environmental properties (Bjorgesaeter & Ugland 2004). Local dialects may also lead to reproductive divergence and potentially to speciation. In birds, geographic variation in repertoires has been partly attributed to genetic differences in populations (Baker & Cunningham 1985; Catchpole & Slater 1995).
Little has been published on behavioural and geographic variation in sound production in fishes (Mann & Lobel 1998). Geographic variation in fish sounds were examined in the toadfish Opsanus tau, which has no pelagic dispersal stage; its boatwhistle advertisement call varies seasonally and with location (Fine 1978a,b). In the damselfish Dascyllus albisella, two populations separated by 1000 km (Hawaii–Johnston atoll) had similar call parameters (pulse rate, interpulse interval, dominant frequency and frequency envelope; Mann & Lobel 1998). A borderline difference in pulse duration was likely due to cancellation effects from reflection and not intrinsic to sounds. Similarity is reinforced by the lack of genetic difference between the populations of D. albisella in these regions (McCafferty et al. 2002).
Absence of variation is likely due to the dispersive ability of the coral-reef larvae (Leis 1991, 2002). Larval dispersal is dependent on the duration of larval life, current strength and direction, swimming behaviour and the mortality rates of the larvae (Munro & Williams 1985). Support for widespread, long-range dispersal of Pacific reef fishes is derived from genetic studies. A number of pomacentrid species have no significant allelic variation, even in populations separated by as much as 3000 km (Shaklee 1984; Nelson et al. 2000). However, a significant allelic differentiation occurs in Amphiprion clarkii over a 1500 km range in Japan (Bell et al. 1982), in Amphiprion ocellaris in the Indo-Malayan region (Nelson et al. 2000), but not in Amphiprion melanopus over a 1000 km range on the Great Barrier Reef. In the latter case, genetic homogeneity could be due to the absence of barrier, i.e. along the reef.
Damselfishes are prolific callers that produce a wide variety of sounds (Myrberg 1972; Mann & Lobel 1998), a behaviour pattern also shared by at least 10 species of anemonefish (Takemura 1983; Chen & Mok 1988; Lagardère et al. 2003). These fishes produce aggressive and courtship sounds that differ in the number of pulses, pulse duration and dominant frequency. Additionally, males can produce a grunt sound, which consists of many irregularly, spaced pulses, after a female enters his nest (Myrberg 1972; Kenyon 1994; Mann & Lobel 1998). ‘Pops’ and ‘chirps’ are produced during agonistic interactions between conspecifics or heterospecifics (Luh & Mok 1986; Myrberg et al. 1986).
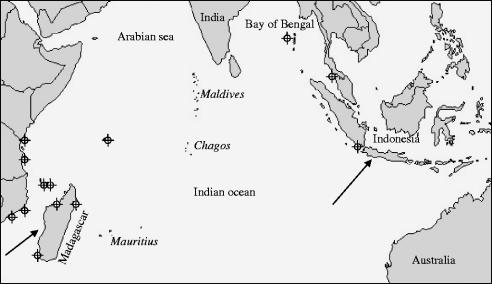
The sounds of Amphiprion akallopisos from Indonesia have been briefly described (Lagardère et al. 2003). The fish lives in groups consisting of a large breeding female, a breeding male and a number of non-breeding males (Fricke 1979). The female develops from a breeding male that changes sex, and she defends a territory and controls potential mates. An opportunity arose to record this species on the west coast of Madagascar, a distance of 6500 km from Indonesia. Because of the large distance of open ocean between the habitats (figure 1), we hypothesized that acoustic signals from the two populations would vary geographically.
Figure 1.
Distribution of Amphiprion akallopisos specimens. Circles are localizations given in Froese & Pauly (2004); arrows correspond to the localizations of the specimens used in this study.
2. Materials and methods
(a) Madagascar
Ten A. akallopisos (male total length (TL): 3.5–4.5 cm and female TL: 6.2–7.4 cm) were collected by scuba diving in the lagoon in front of Tulear (Mozambic canal, west coast of Madagascar). The anemone host (Heterachtis magnifica) was carefully removed from its support and placed in a bucket, and the Amphiprion couple followed its host. Host and fish were stored in a community tank (3.50×0.7×0.2 m) with running seawater. Recordings were made in a smaller tank (1×0.5×0.6 m). The host and the anemonefish pair were placed in the centre of the tank, and a second pair of clownfish was introduced after 15 min. Sounds were recorded for 15 min., after which the intruder couple was removed and replaced in the community tank until the following session.
Sounds were recorded with an Orca hydrophone (sensitivity: −186 dB re 1 V μPa−1) connected via an Orca-made amplifier (ORCA Instrumentation, France) to a mini-disc recorder (JVC, XM-228BK). This system has a flat frequency response range (±3 dB) between 10 Hz and 23.8 kHz. The hydrophone was placed above the sea anemone.
(b) Indonesia
Twelve A. akallopisos from Indonesia were held in an aquarium (3.5×1.75×1.4 m) at La Rochelle, France where they colonized two Heteractis crispa sea anemones (for further information, see Lagardère et al. 2003).
Sounds were recorded with a HTI 16400 hydrophone coupled with a preamplifier and connected to a mini-disc recorder (SONY, MZ-R37SP) or a Sony TCD-D8 digital audio tape-recorder (recording band with: 20–22 000 Hz±1.0 dB). Sounds were recorded when other fishes approached the sea anemone. Although the methodology is not exactly the same, sounds were recorded in both experiments when the fish defends its territory, the sea anemone.
(c) Sound analysis
Sounds were digitized at 44.1 kHz (16 bit resolution) and analysed with AvisSoft-SAS Lab Pro 4.33 software (1024 point Hanning windowed fast fourier transform (FFT)). The resonant frequency of the tank was calculated as 2560 kHz using an equation from Akamatsu et al. (2002), and a low-pass filter of 2.56 kHz was applied for the sounds of Madagascar. Temporal features were measured from the oscillograms and frequency parameters were obtained from power spectra (filter bandwidth 300 Hz, FFT size 256 points, time overlap 96.87% overlap, and a Flat top window). The following parameters were measured: duration in ms; number of pulses in a series; pulse period in ms (measured as the average peak to peak interval between consecutive pulse units in the series); number of peaks in a pulse and dominant frequency (see also figure 2).
Figure 2.
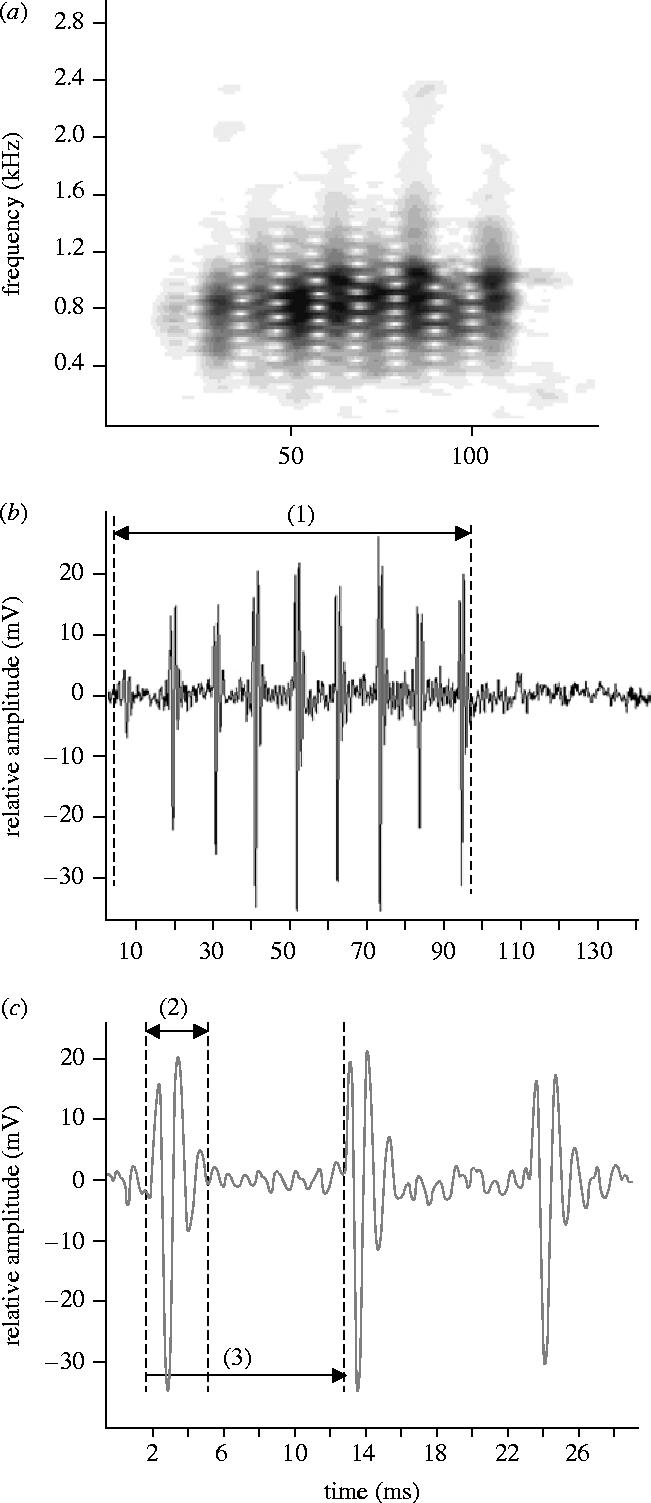
Oscillograms (a) and (b) and sonograms (c) of chirps produced by Amphiprion akallopisos from Madagascar. Characteristics measured in the signal: (1) train duration, (2) pulse length, (3) pulse period.
A t-test was used to compare data between Indonesian and Madagascan fishes, and a one-way ANOVA followed by a Tukey's test compared parameters in Madagascar sounds.
3. Results
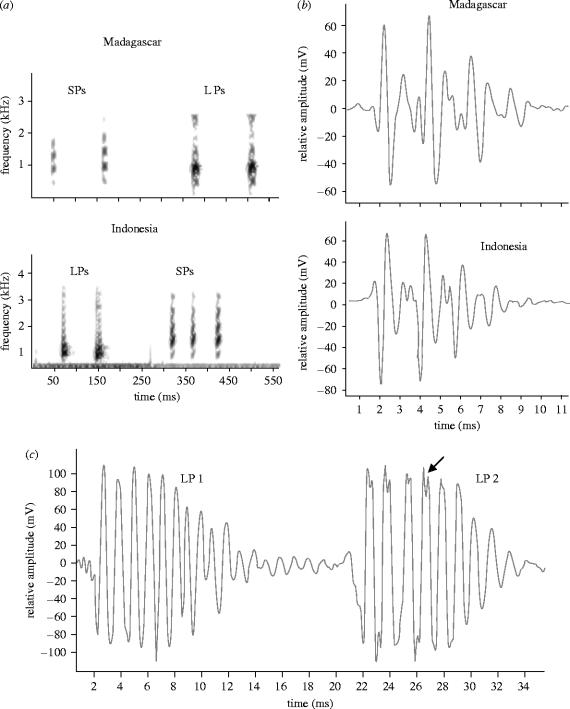
Sound production occurred when other fishes entered the territory (the sea anemone) defended by the pair of clownfish. Since the small male usually remained stationary in the sea anemone tentacles, sounds were likely produced by the female who displayed charge and chase when another specimen approached the host. Three different sound types were identified in the Madagascar fish and two in the Indonesian group. Madagascar fish produced chirps and both groups produced two types of pops, designated as short pops (SPs) and long pops (LPs).
(a) Madagascar sounds
(i) Chirp
Chirps had an average duration of 89 ms and contained 5–12 pulses (table 1; figure 2). Pulse period averaged 11.2 ms and pulse durations ranged from 1.7 to 4.8 ms . Pulses were composed of two or three cycles and damped rapidly (figure 2c). Peak frequency was 665±11 Hz and most sound energy ranged from about 350 to 1600 Hz.
Table 1.
Amphiprion akallopisos.
| Madagascar | Indonesia | p-value | |||
|---|---|---|---|---|---|
| mean±s.e. | n | mean±s.e. | n | ||
| chirp | |||||
| train duration (ms) | 89±4.1 | 47 | |||
| no. of pulses per train | 8.7±0.35 | 46 | |||
| pulse length (ms) | 3±0.07 | 117 | |||
| peak frequency (Hz) | 665±11 | 124 | |||
| no. of peaks per pulse | 2.6±0.05 | 115 | |||
| pulse period | 11.2±0.13 | 257 | |||
| short pop | |||||
| pulse duration (ms) | 8.1±0.1 | 52 | 7.4±0.1 | 37 | 0.0006* |
| no. of peaks per pulse | 7.6±0.1 | 52 | 8.2±0.2 | 37 | 0.0149* |
| peak frequency (Hz) | 1096±26 | 52 | 1088±26 | 37 | 0.8388 |
| long pop | |||||
| pulse duration (ms) | 12.8±0.2 | 146 | 12.1±0.4 | 61 | 0.164 |
| no. of peaks per pulse | 10.8±0.2 | 146 | 8.1±0.2 | 61 | <0.0001* |
| peak frequency (Hz) | 875±11 | 146 | 633±10 | 61 | <0.0001* |
| long pop 1 | |||||
| pulse duration (ms) | 13.3±0.8 | 46 | 11.7±0.7 | 31 | 0.1746 |
| no. of peaks per pulse | 11.7±0.8 | 46 | 8.9±0.7 | 31 | 0.0142* |
| peak frequency (Hz) | 896±28 | 46 | 655±30 | 31 | <0.0001* |
| long pop 2 | |||||
| pulse duration (ms) | 12.8±0.4 | 48 | 12.7±0.5 | 33 | 0.8311 |
| no. of peaks per pulse | 9.1±0.3 | 48 | 7.8±0.3 | 33 | 0.0082* |
| peak frequency (Hz) | 724±9 | 48 | 572±8 | 33 | <0.0001* |
Temporal and frequency parameters of chirps, short pops, long pops combined and separated into type 1 and 2 from Madagascar and Indonesia. p-values are the results of t-test.
(ii) Pops
Both Pop pulses were longer, contained more cycles and presented a lower dominant frequency than chirp pulses (F2,313=588, p<0.0001; figure 3).
Figure 3.
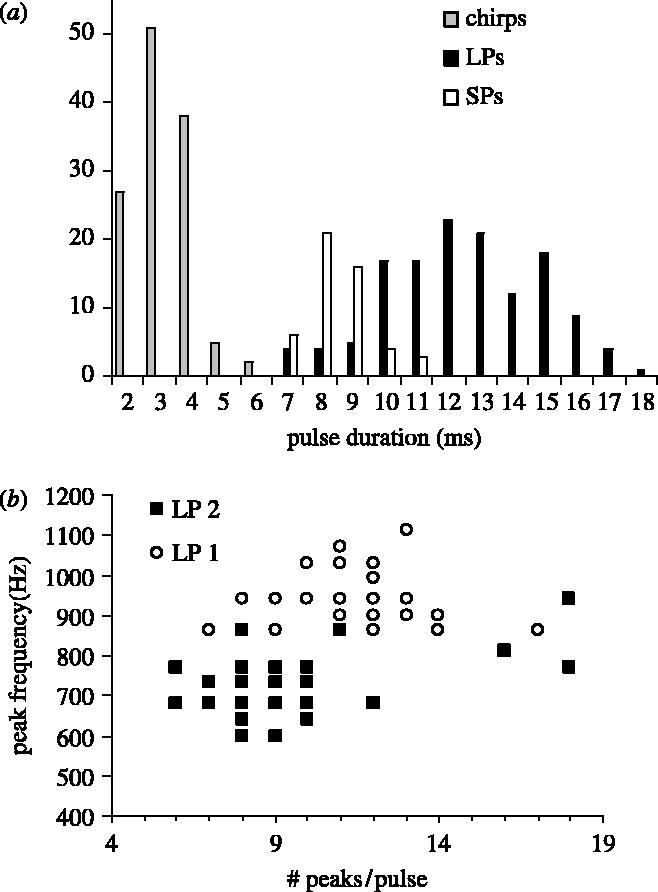
Frequency distribution of the number of peaks in a pulse in the three different sounds in Madagascar (a) and comparison of the number of peaks in LP1s (open circles) and LP2s (filled squares) distribution in relation to the peak frequency (b).
Short pops
Short pops (SP) occurred as single or multiple pulses (2–15) with a period of 57–98 ms (, n=12). Pulses consisted of 5–10 peaks (, n=52; table 1, figures 3 and 4) and varied in duration from 7 to 10 ms (, n=52; table 1, figure 4). Most sound energy ranged from 700 to 2600 Hz with an average peak frequency of 1096 Hz (figure 4).
Figure 4.
Amphiprion akallopisos. (a) Sonograms of the pops produced in Madagascar and Indonesia. (b) Oscillograms of short pops from Madagascar and Indonesia and of (c) the two type of long pops (LP1 and LP2) produced in Madagascar. SPs: short pops, LPs: long pops, arrow indicates the double peaks characteristic of LP2 in Madagascar clownfish.
Long pops
LPs also consisted of a pulse or a train of 2–15 pulses, but they had a greater number of cycles per pulse (, t=7.3, d.f.=196, p<0.0001), a longer duration and a lower peak frequency (, t=8.9, d.f.=196, p<0.0001) than SPs (table 1, figures 3 and 4). Pulse periods were between 20 and 90 ms, and its average (51.3±20 ms, n=65) was lower than in SPs (t=2.7, d.f.=74, p<0.001). Most sound energy ranged from 200 to 2500 Hz with a peak frequency of 875±11 Hz (table 1, figure 4).
LPs were divided into two subgroups: LP1 and LP2 (figure 4; table 2). The position of both types of LP pulses in a train was highly variable with no obvious order. Pulse duration was similar in both sounds. LP1 had a greater number of peaks per pulse (t=3.61, d.f.=89, p<0.001) although there was considerable overlap (figure 3b). Peak frequency exhibited almost a complete separation being higher in LP1 (t=15, d.f.=89, p<0.001) than in LP2 (table 2; figure 4). Oscillograms of LP2 sounds also possessed doubled peaks indicative of high frequency energy (figure 4).
Table 2.
Amphiprion akallopisos.
| LP1s | LP2s | p-value | |||
|---|---|---|---|---|---|
| mean±s.e. | n | mean±s.e. | n | ||
| Madagascar | |||||
| pulse duration (ms) | 12.8±0.5 | 43 | 12.8±0.4 | 48 | 0.9451 |
| no. of peaks per pulse | 11.4±0.4 | 43 | 9.4±0.4 | 48 | 0.0005* |
| peak frequency (Hz) | 934±10 | 43 | 724±9 | 48 | <0.0001* |
| Indonesia | |||||
| pulse duration (ms) | 11.4±0.5 | 28 | 12.7±0.5 | 33 | 0.1268 |
| no. of peaks per pulse | 8.4±0.3 | 28 | 7.8±0.3 | 33 | 0.1666 |
| peak frequency (Hz) | 699±5 | 28 | 572±8 | 33 | <0.0001* |
Comparison of the two types of long pops (LP1s and LP2s) for both Madagascar and Indonesia. p-values are the results of t-test.
(b) Indonesia
Indonesian sounds were compared with those of Madagascar.
(i) Chirp
No chirps were recorded in Indonesian fishes.
(ii) Short pops
Short pops from both localities had similar waveforms, peak frequencies and pulse periods (table 1; figure 4). However, the pulse duration in Madagascar was longer (8.1 versus 7.4 ms, t=3.5, d.f.=87, p=0.0006), but number of peaks per pulse was higher in Indonesia (8.2 versus 7.6, t=2.4, d.f.=87, p=0.015).
(iii) Long pops
LP sounds as a group and, LP1s and LP2s of both localities had similar pulse durations (table 1). The pulse period was higher in Indonesia (t=3.5, d.f.=94, p=0.006). However, the number of peaks and the peak frequencies were lower in Indonesia LPs, LP1 and LP2 (table 1).
4. Discussion
The vocal repertoire of A. akallopisos is larger than previously reported (Lagardère et al. 2003), including three different call types, one of which was further subdivided. Madagascar chirps correspond to chirps described for other damselfish (Amorim 1996). This type of sound is multiply pulsed with shorter pulses and periods, and lower dominant frequencies (respectively, 3 ms, 11.2 ms and 665 Hz in this study) than in pops. The waveforms as well as the longer pulses and higher frequencies of short pops (8.1 ms and 1056 Hz) separate them from long pops (12.8 ms and 875 Hz). Pops are generally made of single pulses, which can be repeated in long encounters (Myrberg 1972). The pulse period of pops is longer than for chirps (ca. 50–70 versus 11.2 ms in this study). Moreover, long pops have been subdivided for the first time into two groups (LP1 and LP2, table 2) on the basis of number of peaks per pulse and peak frequency. Interestingly, SPs, LP1s and LP2s were not produced in a fixed order. The significance of these different calls remains to be established. In addition to identification of species, sex (and maturity) or size, multiple call types may carry information about motivation, dominance status, fitness or be an adaptation to inhibit habituation. Differentiation of agonistic calling into four distinct types indicates a complexity not previously recorded in fish acoustic communication (e.g. Fine et al. 1977).
Much of the biogeography and evolution of Pacific reef fish species have been interpreted on the assumption of wide dispersal via ocean currents, particularly for those with long pelagic-larval stages (Nelson et al. 2000). Regional populations, even when separated by large geographical distances (up to 3000 km), exhibit little or no genetic differentiation (Nelson et al. 2000). Small heterogeneities in allele frequencies were however observed in two populations of A. melanopus separated by a distance of 1000 km of open water (Doherty et al. 1995). This heterogeneity was also reflected in the colour pattern of this species. As a whole, these studies indicate that geographic variation depends on different factors including current strength, larval dispersion abilities, swimming behaviour (Leis 1991, 2002) and the presence of stepping stones (Doherty et al. 1995). However, genetic studies have shown that larval duration does not necessarily affect geographic structure in coral-reef fishes in French Polynesia (Bernardi et al. 2001; Fauvelot & Planes 2002; Fauvelot et al. 2003). According to museum records, A. akallopisos is not found along the coast of India, in Sri Lanka or in the Maldives islands (Froese & Pauly 2004). The large gap (figure 1) between the two populations (East Africa and Indonesia) correlates with the observed difference in the sounds.
This study is the first to report geographic variation in sounds of a coral-reef fish species. In frogs, birds and mammal, geographic variations in sounds are considered prezygotic isolating mechanisms leading to speciation (Cocroft & Ryan 1995; Grant & Grant 1996; Slabbekoorn & Smiths 2002). However, since the two populations in this study appear to be reproductively isolated, differences may result from genetic drift or selection for some other purpose on the sound producing mechanism. Temporal characteristics and pulse grouping patterns are believed to be important in species recognition in fishes, and could then play an important role in speciation.
In the damselfish D. albisella, two populations separated by 1000 km have similar calls except for a possible minor difference in pulse duration attributed to the environment (Mann & Lobel 1998). In A. akallopisos, differences occur in the number of peaks per pulse and peak frequency in all pops as well as in pulse duration in short pops. Pulse duration is the most variable parameter in underwater sound analysis because it can be affected by noise, attenuation and reflection of the signal. Therefore similar pulse durations in the LPs allow us to exclude abiotic parameters as a cause of geographic variation. The only other fish study dealing with a variation of dialect was conducted in the estuarine toadfish O. tau, a polygynous species with male parental care and no pelagic dispersal stage. Variations in call duration and fundamental frequency were recorded in the field, along the Atlantic coast of North America (Fine 1978a) and from sounds evoked by electrical stimulation of the brain (Fine 1978b). The latter study avoids variation due to abiotic characters, and confirms that the dialects can be supported by anatomical structures and output of central pattern generator. In adjacent estuaries, the toadfish populations are likely to exhibit a high degree of reproductive isolation in contrast to the case in coral-reef fishes.
Although this study examined agonistic sounds in defence of an anemone territory, the results suggest a shift in sound parameters that could also reflect a possible role in reproductive divergence and speciation. More studies are needed to determine which factors influence the geographic variation of sounds. Genetic studies provide a baseline for interpreting differences. However, geographic variation could be an adaptation to abiotic conditions (e.g. different acoustic environment with variability in the ambient noise) and/or biotic conditions. Rice & Lobel (2004) proposed the possibility that the pharyngeal jaw apparatus is used in sound production. If this interpretation is correct, dietary modifications could be linked to changes in sound parameters.
Acknowledgments
The authors would like to thank J.M. Ouin and his Aqua-Lab team (Institut Halieutique et des Sciences Marines, Tuléar University) for helping to collect fishes and the institute for providing hospitality and laboratory facilities. We thank also P. and R. Coutant, and P. Morinière for helping during the recordings in the Aquarium of La Rochelle. D. Lepièce (FUNDP) helped with the sound file acquisition. This work is supported by grant no. 2.4574.01. and by ‘Bourse de séjour scientifique’ no. 2.4558.03 from ‘Fonds National de la Recherche Scientifique de Belgique’.
References
- Akamatsu T, Okumura T, Novarini N, Yan H.Y. Empirical refinements applicable to the recording of fish sounds in small tanks. J. Acoust. Soc. Am. 2002;112:3073–3082. doi: 10.1121/1.1515799. [DOI] [PubMed] [Google Scholar]
- Amorim M.C.P. Sound production in the blue–green damselfish, Chromis viridis (Cuvier, 1830) (Pomacentridae) Bioacoustics. 1996;6:265–272. [Google Scholar]
- Baker M.C, Cunningham M.A. The biology of bird-song dialects. Behav. Brain Sci. 1985;8:85–133. [Google Scholar]
- Bazua-Duran C, Au W.W.L. Geographic variations in the whistles of spinner dolphins (Stenella longirostris) of the Main Hawai'ian Islands. J. Acoust. Soc. Am. 2004;116:3757–3769. doi: 10.1121/1.1785672. [DOI] [PubMed] [Google Scholar]
- Bell L.J, Meyer J.T, Numach K. Morphological and genetic variation in Japanese populations of the anemone fish (Amphiprion clarkii) Mar. Biol. 1982;72:99–108. [Google Scholar]
- Bernardi G, Holbrook S.J, Schmitt R.J. Gene flow at three spatial scales in a coral reef fish, the three-spot dascyllus, Dascyllus trimaculatus. Mar. Biol. 2001;138:457–465. [Google Scholar]
- Bjorgesaeter A, Ugland K.I. Geographic variation and acoustic structure of the underwater vocalization of harbor seal (Phoca vitulina) in Norway, Sweden and Scotland. J. Acoust. Soc. Am. 2004;116:2459–2468. doi: 10.1121/1.1782933. [DOI] [PubMed] [Google Scholar]
- Catchpole C.K, Slater P.J.B. Cambridge University Press; Cambridge, UK: 1995. Bird song: biological themes and variations. [Google Scholar]
- Chen K.C, Mok H.K. Sound production in the anemonefishes, Amphiprion clarkii and A. frenatus (Pomacentridae), in captivity. Jpn. J. Ichthyol. 1988;35:90–97. [Google Scholar]
- Cocroft R.B, Ryan M.J. Patterns of advertisement call evolution in toads and chorus frogs. Anim. Behav. 1995;49:283–303. [Google Scholar]
- Doherty P.J, Planes S, Mather P. Gene flow and larval duration in seven species of fish from the Great Barrier Reef. Ecology. 1995;76:2373–2391. [Google Scholar]
- Fauvelot C, Planes S. Understanding origins of present day genetic structure in marine fish: biologically or historically driven patterns? Mar. Biol. 2002;141:773–788. [Google Scholar]
- Fauvelot C, Bernardi G, Planes S. Reductions in the mitochondrial DNA diversity of coral reef fish provide evidence of population bottlenecks resulting from Holocene sea-level change. Evolution. 2003;57:1571–1583. doi: 10.1111/j.0014-3820.2003.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Fine M.L. Seasonal and geographical variation of the mating call of the oyster toadfish Opsanus tau L. Oecologia. 1978a;36:45–57. doi: 10.1007/BF00344570. [DOI] [PubMed] [Google Scholar]
- Fine M.L. Geographical variation in sound production evoked by brain stimulation in the oyster toadfish. Naturwissenschaften. 1978b;65:493. [Google Scholar]
- Fine M.L, Winn H.E, Olla B.L. Communication in fishes. In: Seboek T.A, editor. How animals communicate. Indiana University press; Bloomington and London: 1977. pp. 472–518. [Google Scholar]
- Fricke H.W. Mating system, resource defense and sex change in the anemonefish Amphiprion akallopisos. Z. Tierpsychol. 1979;50:313–326. [Google Scholar]
- Froese R, Pauly D, editors. FishBase. 2004. World Wide Web electronic publication. www.fishbase.org, version (10/2004). [Google Scholar]
- Grant B.R, Grant P.R. Cultural inheritance of song and its role in the evolution of Darwin's finches. Evolution. 1996;50:2471–2487. doi: 10.1111/j.1558-5646.1996.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Higgins L.A, Waugaman R.D. Sexual selection and variation: a multivariate approach to species-specific calls and preferences. Anim. Behav. 2004;68:1139–1153. [Google Scholar]
- Kenyon T.N. The significance of sound interception to males of the bicolor damselfish, Pomacentrus partitus, during courtship. Environ. Biol. Fishes. 1994;40:391–405. [Google Scholar]
- Lagardère J.P, Fonteneau G, Mariani A, Morinière P. Les émissions sonores du poisson-clown mouffette Amphiprion akallopisos, Bleeker 1853 (Pomacentridae), enregistrées dans l'aquarium de la Rochelle. Ann. Soc. Sci. Nat. Charente-Marit. 2003;9:281–288. [Google Scholar]
- Leis J.M. The pelagic stages of reef fishes: the larval biology of coral reef fishes. In: Sale P.F, editor. The ecology of fishes on coral reefs. Academic Press; San Diego: 1991. pp. 183–227. [Google Scholar]
- Leis J.M. Pacific coral-reef fishes: the implications of behaviour and ecology of larvae for biodiversity and conservation, and a reassessment of the open population paradigm. Environ. Biol. Fishes. 2002;65:199–208. [Google Scholar]
- Luh H.K, Mok H.K. Sound production in the domino damselfish, Dascyllus trimaculatus (Pomacentridae) under laboratory conditions. Jpn. J. Ichthyol. 1986;33:70–74. [Google Scholar]
- Mann D.A, Lobel P.S. Acoustic behavior of the damselfish Dascyllus albisella: behavioral and geographic variation. Environ. Biol. Fishes. 1998;51:421–428. [Google Scholar]
- Mayr E. Fayard; Paris: 1989. Histoire de la Biologie. Diversité, évolution et hérédité. [Google Scholar]
- McCafferty S, Bermingham E, Quenouille B, Planes S, Hoelzer G, Asoh K. Historical biogeography and molecular systematics of the Indo-Pacific genus Dascyllus (Teleostei: Pomacentridae) Mol. Ecol. 2002;11:1377–1392. doi: 10.1046/j.1365-294x.2002.01533.x. [DOI] [PubMed] [Google Scholar]
- Munro J.L, Williams D.M. Assessment and management of coral reef fisheries: biological, environmental, and socio-economic aspects. Proc. Fifth Int. Coral Reef Congr., Tahiti. 1985;4:544–578. [Google Scholar]
- Myrberg A.A., Jr Ethology of the bicolour damselfish Eupomacentrus partitus (Pisces: Pomacentridae): a comparative analysis of laboratory and field behaviour. Anim. Behav. Monogr. 1972;5:197–283. [Google Scholar]
- Myrberg A.A, Jr, Mohler M, Catala J. Sound production by males of a coral reef fish (Pomacentrus partitus): its significance to females. Anim. Behav. 1986;34:913–923. [Google Scholar]
- Nelson J.S, Hoddell R.J, Chou L.M, Chan W.K, Phang V.P.E. Phylogeographic structure of false clownfish, Amphiprion ocellaris, explained by sea level changes on the Sunda shelf. Mar. Biol. 2000;37:727–736. [Google Scholar]
- Peters G, Tonkin-Leyhausen B.A. Evolution of acoustic communication signals of mammals: friendly close-range vocalizations in Felidae (Carnivora) J. Mammal. Evol. 1999;6:129–159. [Google Scholar]
- Rice A.N, Lobel P.S. The pharyngeal jaw apparatus of the Cichlidae and Pomacentridae: function in feeding and sound production. Rev. Fish Biol. Fish. 2004;13:433–444. [Google Scholar]
- Shaklee J.B. Genetic variation and population structure in the damselfish, Stegastes fasciolatus, throughout the Hawaiian Archipelago. Copeia. 1984;1984:629–640. [Google Scholar]
- Simmons L.W, Zuk M, Rotenberry J.T. Geographic variation in female preference functions and male songs of the field cricket Teleogryllus oceanicus. Evolution. 2001;55:1386–1394. doi: 10.1111/j.0014-3820.2001.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Smiths T.B. Bird song, ecology and speciation. Phil. Trans. R. Soc. B. 2002;357:493–503. doi: 10.1098/rstb.2001.1056. 10.1098/rstb.2001.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura A. Acoustic behavior of clownfishes (Amphiprion spp.) Bull. Fac. Fish. Nagasaki Univ. 1983;54:21–27. [Google Scholar]