Abstract
We studied the relationship between one component of immune function and basal metabolic rate (BMR), an indicator of the ‘pace-of-life syndrome’, among 12 tropical bird species and among individuals of the tropical house wren (Troglodytes aedon), to gain insights into functional connections between life history and physiology. To assess constitutive innate immunity we introduced a new technique in the field of ecological and evolutionary immunology that quantifies the bactericidal activity of whole blood. This in vitro assay utilises a single blood sample to provide a functional, integrated measure of constitutive innate immunity. We found that the bactericidal activity of whole blood varied considerably among species and among individuals within a species. This variation was not correlated with body mass or whole-organism BMR. However, among species, bacteria killing activity was negatively correlated with mass-adjusted BMR, suggesting that species with a slower pace-of-life have evolved a more robust constitutive innate immune capability. Among individuals of a single species, the house wren, bacteria killing activity was positively correlated with mass-adjusted BMR, pointing to physiological differences in individual quality on which natural selection potentially could act.
Keywords: life history, immune function, metabolic rate, tropics, bactericidal activity
1. Introduction
The most fundamental trade-off in life-history evolution is the inverse relationship between reproductive success (fecundity) and adult survival rate (Cody 1966; Charnov 1993; Ricklefs 2000). Attempts to explain this relationship have vacillated between emphasis on ecological factors such as risk of predation, food availability and density dependence, and physiological parameters, notably energy expenditure (Lack 1947; Skutch 1949; Ashmole 1963; Drent & Daan 1980; Martin 2004; Speakman et al. 2004; Wiersma et al. 2004). Recently, interest has focused on connecting demographic parameters related to survival and reproduction, and various physiological attributes, including metabolism and immune function, all subsumed within the ‘pace-of-life syndrome’ (Ricklefs & Wikelski 2002). Understanding the evolutionary processes that shape the pace-of-life syndrome requires insights into evolved responses, which reflect historical constraints and adaptation by natural selection, and individual responses, which reveal both the potential for selection and physiological constraints on the response to selection. Comparative studies of physiological correlates of life histories are largely restricted to metabolism: low metabolic rates are thought to be associated with low reproductive investment and high survival rate (Pearl 1928; Daan et al. 1990; Zera & Harshman 2001; Ricklefs & Wikelski 2002). In comparisons within species, investigations into the trade-off between survival and reproduction have focused on the costs of increased work loads, especially during reproduction, elucidating damaging effects of oxidative stress and compromised immune function in individual animals (Deerenberg et al. 1997; Wiersma et al. 2004).
Development, maintenance and use of the immune system incur costs in terms of both time and nutrients (Klasing & Leshchinsky 1999). Accordingly, interspecific variation in immune function might be related to longevity in such a way that higher levels or a better quality of immune response evolved in longer-lived species having increased investment in self-maintenance over reproduction. An alternative, though not mutually exclusive, hypothesis relates immunocompetence to environmental conditions, such as risk of disease, and suggests that birds in relatively disease-free environments, such as marine and arctic habitats, have evolved less robust immune defenses (Piersma 1997). In both scenarios, immune function is an important physiological attribute in the evolution of life histories. Within species, variation in immune function might indicate fitness-related individual ‘quality’ differences. In particular, individuals having better body condition might support a higher level of metabolism and a more robust immune system (Norris & Evans 2000).
Comparative ecological and evolutionary immunology faces the problem of defining a functional measure of overall immunity to infectious challenges that allows for comparisons among species. Most assays of immune function require species-specific reagents that preclude comparisons across a range of species, and laborious protocols that are not practical in field studies. Additional complications arise from the multifaceted nature of the immune system which has led to the development of an array of techniques that highlight single, often non-comparable components of the immune system (Norris & Evans 2000; Adamo 2004). Finally, some tests of immune function are difficult to interpret because excessive responses may indicate immunopathology, making it possibly difficult to infer quality of response from quantity of response.
We introduce a technique novel to physiological ecology that presents a functional, integrated and comparative measure of constitutive innate immunity by quantifying the bactericidal activity of whole blood. Constitutive innate immunity, a mixture of humoral (e.g. natural antibodies, complement, acute phase proteins) and cellular components (e.g. macrophages, heterophils, thrombocytes), establishes the first line of defence against invading pathogens. Its functioning is ostensibly unaffected by previous challenges and the development of pathogen-specific antibodies, making it ideal for studies across species or individuals and across environments with differing abundances and diversities of pathogens. In addition, components of the innate immune system, including natural antibodies, are encoded in the germ-line genome, thus providing genetic variation for evolution, and they play an essential role in activating the acquired arm of the immune system for specific defences against infection (Carroll & Prodeus 1998; Ochsenbein & Zinkernagel 2000). The bactericidal activity of whole blood is an excellent predictor of the susceptibility of humans to a variety of bacterial infections (Keusch et al. 1975). Keusch et al. (1975) measured intracellular bactericidal activity of leukocytes in whole blood to diagnose patients with an inherited disorder of phagocytic cells, characterized by the inability of phagocytes to kill certain types of bacteria and fungi, and resulting in recurrent life-threatening bacterial and fungal infection.
To test the hypothesis that the immune response is an integral part of the evolved life history, we described the relationship between constitutive innate immunity and basal metabolic rate (BMR), an indicator of the pace-of-life, in an interspecific comparison of tropical birds. We predicted that species with low BMR would have higher levels of investment in immunity, including the constitutive innate arm. In addition, to determine whether constitutive innate immunity might indicate fitness-related quality differences among individuals within a species, and to compare inter- and intraspecific patterns, we explored the relationship between bacteria killing activity of blood and BMR among individual tropical house wrens (Troglodytes aedon).
2. Methods
(a) Animals
We mist-netted individuals of 12 bird species during March–July 2004 in the tropical humid lowland rainforest of Soberania National Park and around Gamboa, Panama (table 1). Upon capture, we immediately took a blood sample for the immune assay, and transported blood and birds to our laboratory. All birds were housed in cages at an ambient temperature of about 25 °C, and provided with fruit, mealworms, re-hydrated crickets and/or seeds, depending on a species' diet. Usually we measured metabolism the night after capture, but occasionally we kept birds for up to 3 days. All house wrens for the intraspecific comparisons were feeding 12 day old nestlings when we captured them for blood sampling and BMR-measurements. After metabolism measurements, we released them before sunrise at their nest sites; in all cases birds resumed feeding their offspring. By restricting our study to one geographic location, and by taking advantage of the wide variety of life histories among tropical birds, we minimized the effect of environment as confounding factor in our analysis.
Table 1.
Body mass, mass-corrected BMR (average±s.e.) and bactericidal activity of whole blood (average % of colony forming units, CFU, killed±s.e.) for 12 species of tropical birds.
| species | body mass (g) | BMR (kJ d−1 g−0.638) | n | bactericidal activity (% CFU killed) | n | |
|---|---|---|---|---|---|---|
| bicoloured antbird | Gymnopithys leucaspis | 27.2 | 3.4±0.11 | 8 | 99.9±0.04 | 5 |
| blue-crowned motmot | Momotus momota | 99.7 | 2.2±0.15 | 4 | 99.1±0.38 | 8 |
| blue–gray tanager | Thraupis episcopus | 30.4 | 4.1±0.11 | 8 | 11.2±6.66 | 15 |
| clay-coloured robin | Turdus grayi | 72.0 | 3.8±0.16 | 10 | 82.9±4.20 | 30 |
| crimson-backed tanager | Ramphoceles dimidiatus | 26.4 | 4.0±0.12 | 8 | 49.0±9.53 | 11 |
| dusky antbird | Cercomacra tyrannina | 14.0 | 3.0±0.25 | 3 | 93.3±6.50 | 2 |
| grey-headed tanager | Eucometis penicillatus | 30.9 | 3.7±0.23 | 3 | 90.9±4.90 | 7 |
| house wren | Troglodytes aedon | 13.3 | 3.5±0.07 | 28 | 89.0±3.06 | 27 |
| red-capped manakin | Pipra mentalis | 14.7 | 4.1±0.17 | 7 | −3.4±8.92 | 6 |
| ruddy ground dove | Columbina talpacoti | 44.8 | 3.1±0.56 | 7 | −3.2±5.60 | 18 |
| slaty antshrike | Thamnophilus punctatus | 20.5 | 3.5±0.41 | 3 | 99.6±0.27 | 3 |
| spotted antbird | Hylophylax naevioides | 16.9 | 3.6±0.16 | 6 | 94.6±2.56 | 10 |
(b) Bacteria killing assay
We assessed the bactericidal activity of fresh whole blood in vitro. We collected blood samples of birds in the field within 5 min of capture, a period previously shown to minimize the effect of capture stress on our bacteria killing assay (Matson et al., unpublished work). We sterilized the area around the wing vein with 70% ETOH, allowed the ETOH to evaporate, then punctured the wing vein with a sterile needle, and collected 50–100 μl of blood in sterile heparinized haematocrit tubes (Fisher Scientific). Haematocrit tubes were transported in sterilized plastic boxes to the laboratory, where the blood was processed within half an hour after obtaining the sample.
A laminar flow hood (AC600, AirClean Systems, Raleigh, NC) provided a sterile working environment in the laboratory. For interspecific comparisons, we diluted 20 μl of blood with 180 μl CO2-independent media (#18045, Gibco-Invitrogen, Carlsbad, CA) enriched with 5% complement-inactivated foetal calf serum and 4 mM l-glutamine. To increase the resolution of our antibacterial assay for house wrens, a species with a relatively high killing activity, we diluted 10 μl of blood in 190 μl media. To each dilution we added 20 μl of a suspension of living Escherichia coli (ATCC #8739). The E. coli were reconstituted from lyophilized pellets (Epower Microorganisms #0483E7, MicroBioLogics, St Cloud, MN) in phosphate buffered saline (Sigma, St Louis, MO) following company instructions and adjusted to a concentration of 150–200 colonies per 75 μl of diluted blood–bacteria mixture. The mixture of diluted blood and E. coli was incubated for 30 min at 41 °C, and then 75 μl was spread onto agar plates (d-ifco soybean-casein digest agar, USP, Fisher catalog #DF0369176). Each sample was plated in duplicate. The number of bacteria in the added bacteria suspension was quantified with control dilutions consisting of 200 μl of media mixed with 20 μl of bacteria-suspension. We made 1–5 control dilutions per session, and used the average when n>1. Plates were incubated upside down at ambient temperature (25–30 °C) and the number of colony-forming units was counted the following day.
We tested for variation among control plates by making 8 control dilutions from the same bacteria suspension (20 μl of suspension in 200 μl of media) and plating each dilution in duplicate. Following our normal procedure, we calculated the average of each pair of control plates. We found that the coefficient of variation among controls was 3.8% (average number of colonies±s.d.: 120.8±4.60, n=8). Our normal control dilutions were plated directly, without incubating them. When we compared 27 pairs of unincubated control dilutions with dilutions that had been incubated for 30 min at 41 °C, we found a 7.8% increase in number of colonies after incubation (unincubated±s.d.: 148±43.2; incubated±s.d.: 159±51.5; paired-t=2.66, p=0.013, n=27). For calculations of bactericidal activity, we used the number of colonies from unincubated control plates because they reflect the initial situation at the instant that the blood starts acting on the bacteria. Components within blood can prevent bacteria growth or kill bacterial cells, in either of which case bacteria will not grow. However, bacterial cells may divide in blood as indicated by negative killing values. The bactericidal activity of blood was expressed as the number of colony-forming units remaining after incubation of the blood–bacteria mixture relative to the number inoculated (control plates).
(c) Oxygen consumption
We measured rates of oxygen consumption for post-absorptive birds during their nocturnal phase by standard flow-through respirometry methods. Birds were placed in stainless steel metabolism chambers that had an air-tight Lexan lid, formed by a thick rubber gasket. Birds perched on a wire-mesh platform over a layer of mineral oil that trapped feces. The chambers sat in a large cool-box with a Peltier device (Sable Systems, Pelt-4) to control ambient temperature (Ta)±0.1 °C. We measured birds at 31–32 °C, which is in their thermoneutral zone, as determined by measurements over a range of Tas for each species.
Compressed air coursed through columns of Drierite to remove water, through previously calibrated (Levy 1964) Mykrolis mass flow controllers (FC-2900; 2 SLPM) set between 500 and 700 ml min−1 (STP), depending on species, and then into the chamber. Exiting air passed through an Edgetech dewpoint hygrometer (Dewprime II, factory recalibration August 2003). A subsample was then routed through silica gel, Ascarite, and silica gel to remove water and CO2 before measuring the fractional concentration of O2 with an Applied Electrochemistry oxygen analyser (S3A-II). We calibrated the entire system for measuring O2-consumption by infusing pure O2 into our chamber using a syringe pump with a previously determined flow rate while simultaneously pushing room air through another port into the chamber at a known rate. Comparisons of known O2 influx via the syringe pump with calculated influx showed a mean difference of ±2% for our 10 trials.
After a 3 h equilibration period, we recorded O2-concentration, the dewpoints of inlet and outlet air, the temperature of the dewpoint hygrometer, and Ta in the chamber, using a data logger (Campbell Scientific, model CR23X). When, during the fourth hour of measurements, the traces for O2-consumption were stable for at least 10 min, we noted these times and used these data for calculations. After completing the metabolism measurements, we immediately measured the body temperature of birds with a Physitemp thermometer (Model Batt-12) and a 36 gauge copper–constantan thermocouple. The relative humidity of outlet air was always between 10 and 40%. Oxygen consumption was calculated with eqn (4) of Hill (1972). We used 20.08 J ml−1 O2 to convert oxygen consumption to heat production (Schmidt-Nielsen 1997).
(d) Statistics
Bactericidal activity was expressed in percentages that varied from below zero, when bacteria proliferated during the incubation, to 100%, when all of the bacteria were killed. The negative values complicated data transformation, and together with the nonlinear relationships between variables, prompted us to use non-parametric Spearman rank correlation analysis as implemented in Spss 12.0. Metabolism data were analysed using linear regression analysis.
Interspecific correlations may be statistically biased if closely related species resemble each other as a result of common ancestry (Felsenstein 1985; Harvey & Pagel 1991). We made a phylogeny based on the DNA-hybridization relationships determined by Sibley & Ahlquist (1990) and used Abouheif's (Abouheif 1999; Rheindt et al. 2004) Test for Serial Independence to determine whether a phylogenetic effect was present in our data for mass, BMR and bactericidal activity. None of these variables was significantly autocorrelated (BMR p=0.33, mass p=0.12, bactericidal activity p=0.33), and therefore we did not include phylogeny in our analyses.
3. Results
(a) Interspecific relationships of bactericidal activity, body mass and BMR
Average bactericidal activity varied among species between −3% and +100% (table 1). We found no significant relationship between bactericidal activity and body mass (ρs=−0.007, n=12, p=0.98) or between bactericidal activity and whole-animal BMR (ρs=−0.19, n=12, p=0.56). We adjusted BMR for body mass by dividing BMR by mass0.638, the exponent taken from an allometric equation relating BMR to body mass in birds (Tieleman & Williams 2000). Relating mass-adjusted BMR and bactericidal activity for all species we found a significant negative correlation (ρs=−0.59, p=0.042, figure 1a). Because our data set included only two non-passerines (blue-crowned motmot and ruddy ground dove), we verified that restricting the analysis to passerines only did not alter the results; the correlation between mass-adjusted BMR and bactericidal activity was maintained (ρs=−0.87, n=10, p=0.001). These data suggest that species with a BMR higher than predicted for their body mass have a lower bactericidal activity of their blood than species with a relatively low BMR.
Figure 1.
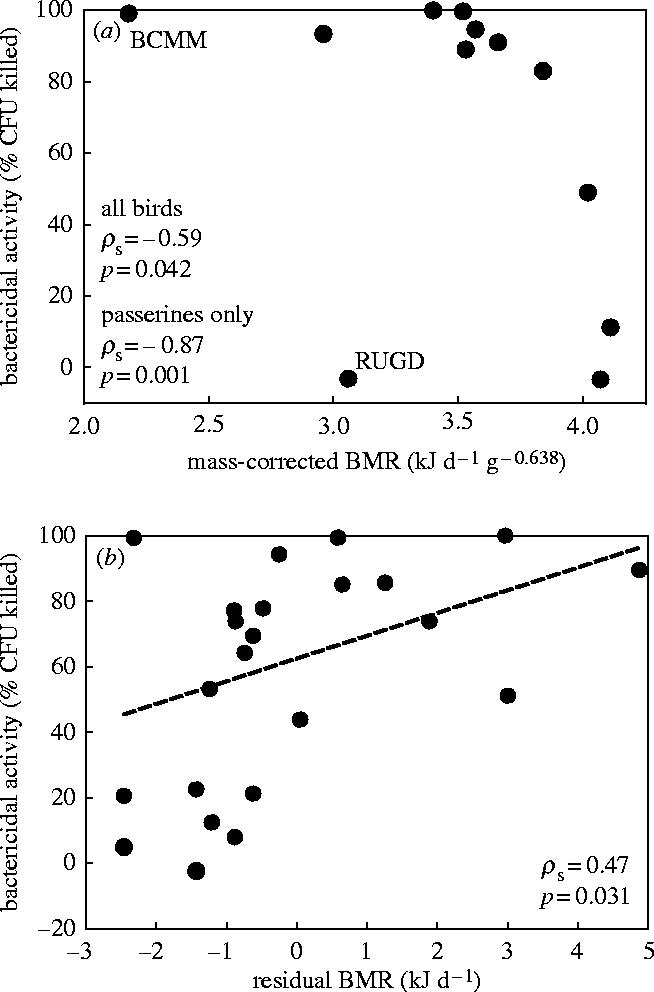
(a) Relationship between mass-adjusted basal metabolic rate (BMR) and bactericidal activity (% colony forming units, CFU, killed) of whole blood among 12 species of tropical birds. The two non-passerine species are marked BCMM (blue-crowned motmot) and RUGD (ruddy ground dove). (b) Relationship between residual BMR and bactericidal activity of whole blood among individuals of the house wren.
(b) Intraspecific relationships of bactericidal activity, body mass and BMR
In house wrens, BMR was positively related with body mass according to the regression equation BMR (kJ d−1)=1.215 (s.e. 0.403) mass (g)+2.227 (s.e. 5.376; r2=0.26, d.f.=27, pslope=0.006, n=13 males, 15 females). Bactericidal activity (measured for 21 birds) was not significantly correlated with body mass (ρs=−0.14, n=21, p=0.53) or whole-animal BMR (ρs=0.29, n=21, p=0.21). To correct for body mass we calculated residual BMR (=measured−predicted by the regression equation relating BMR and mass), and found a significant positive correlation between residual BMR and bactericidal activity (ρs=0.47, n=21, p=0.031, figure 1b).
4. Discussion
Constitutive innate immunity was assessed as the ability of whole blood to kill bacteria. Interpretation of bactericidal activity is unambiguous because the principal job of the immune system is to kill potential pathogens and our assay is a direct measurement of this crucial function. Bactericidal activity varied substantially among 12 species of tropical birds and among individuals within one species, the house wren, and covaried with a physiological measure of the ‘intensity of living’. Bactericidal activity of blood was not related to mass or to whole-animal BMR, but correlated with mass-corrected BMR. If BMR were inversely related to lifespan, then the negative interspecific correlation between immune function and BMR would support our hypothesis that the level of investment in constitutive innate immunity can be understood in the context of life-history evolution. In this case, our data are consistent with the idea that species with longer average lifespans invest more into immune function to protect future opportunities to reproduce. In any case, the association of high immune competency with low metabolic rate might reflect a basic trade-off between rate of activity and self-maintenance functions.
The ruddy ground dove, with low mass-corrected BMR and low bactericidal activity of its blood, deviated markedly from the overall negative correlation between these variables. We hypothesize that Columbiformes might have evolved a different evolutionary strategy to resist or control infections, with apparent small investments in constitutive innate immunity, but a strong systemic inflammatory response to infections as indicated by high levels of acute phase proteins after inoculation with heat-killed E. coli (K. C. Klasing & M. Wikelski, unpublished data). In agreement with this idea, Matson et al. (2004) recently reported that the mourning dove (Zenaida macroura), another member of the Columbiformes, had the lowest constitutive complement and natural antibody levels in a comparative study on constitutive innate humoral immunity of 11 avian species. Comparing pigeons and doves with other bird species may therefore provide interesting evolutionary insights into trade-offs between the constitutive innate arm, the acute phase response, and the adaptive arm of the immune system (Norris & Evans 2000; Lee & Klasing 2004). We wonder if these immunological trade-offs might be related to the unusual life histories of pigeons and doves, characterized by year-round reproduction in most environments, and the unique passive transfer of immunoglobulin to chicks via crop secretions (Goudswaard et al. 1978).
The interspecific and intraspecific relationships between the bactericidal activity of blood and mass-corrected BMR differed. The positive intraspecific correlation between bactericidal activity and mass-corrected BMR in house wrens, opposite to the interspecific pattern, might reflect fitness-related quality differences among individuals. Accordingly, high quality individuals might have a relatively high BMR that allows them to work hard during the nestling period while maintaining high body condition, reflected in high immunocompetence. Alternatively, low quality individuals might have relatively high BMR and increased bactericidal activity because of active infections. The acute phase response to pathogens results in increased BMR and increased levels of bactericidal acute phase proteins in the blood (Gabay & Kushner 1999). Future experimental and/or mechanistic work on the link between metabolism and constitutive innate immunity should yield insights into the validity of these ideas.
The bactericidal activity of whole blood represents a functional, integrated measure of constitutive innate immunity previously missing from the tools available to ecological and evolutionary immunology (Norris & Evans 2000; Merchant et al. 2003). While the current study presents results on the ability of whole blood to kill one strain of E. coli, this assay can be extended to other bacteria and yeast species or strains with different characteristics, to increase the robustness of the overall measure of innate immunity. Additional potential improvements include modifying the blood dilution or the incubation time of the blood–bacteria mixture in order to increase the resolution of the results at the extremes of the range. Nonetheless, our results show that this measure has relevance in both comparative and intraspecific studies. In vitro bactericidal activity of whole blood provides a functional and field compatible measure of constitutive innate immune function.
Acknowledgments
We thank the Smithsonian Tropical Research Institute for logistic help throughout the study, Jennie Bennett for developing the bacteria killing assay for use in wild birds, Thomas Dijkstra for practical help in the field, and Kevin Matson for practical help and comments on a previous draft. This research was funded by National Science Foundation grant IBN-0212587.
Footnotes
Present address: Animal Ecology Group, Centre for Ecological and Evolutionary Studies, University of Groningen, PO Box 14, 9750 AA Haren, The Netherlands.
References
- Abouheif E. A method for testing the assumption of phylogenetic independence in comparative data. Evol. Ecol. Res. 1999;1:895–909. [Google Scholar]
- Adamo S.A. How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 2004;68:1443–1449. [Google Scholar]
- Ashmole N.P. The regulation of numbers of tropical oceanic birds. Ibis. 1963;103:458–473. [Google Scholar]
- Carroll M.C, Prodeus A.P. Linkages of innate and adaptive immunity. Curr. Opin. Immunol. 1998;10:36–40. doi: 10.1016/s0952-7915(98)80028-9. [DOI] [PubMed] [Google Scholar]
- Charnov E.L. Oxford University Press; New York: 1993. Life history invariants. Some explorations of symmetry in evolutionary biology. [Google Scholar]
- Cody M.L. A general theory of clutch size. Evolution. 1966;20:174–184. doi: 10.1111/j.1558-5646.1966.tb03353.x. [DOI] [PubMed] [Google Scholar]
- Daan S, Masman D, Groenewold A. Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am. J. Physiol. 1990;259:R333–R340. doi: 10.1152/ajpregu.1990.259.2.R333. [DOI] [PubMed] [Google Scholar]
- Deerenberg C, Apanius V, Daan S, Bos N. Reproductive effort decreases antibody responsiveness. Proc. R. Soc. B. 1997;264:1021–1029. 10.1098/rspb.1997.0141 [Google Scholar]
- Drent R.H, Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Goudswaard J, Noordzij A, Stam J.W. Pigeon IgA: a major antigen in pigeon breeder's disease. Immunol. Commun. 1978;7:661–668. doi: 10.3109/08820137809068726. [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hill R.N. Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J. Appl. Physiol. 1972;33:261–263. doi: 10.1152/jappl.1972.33.2.261. [DOI] [PubMed] [Google Scholar]
- Keusch G.T, Douglas S.D, Ugurbil K. Intracellular bactericidal activity of leukocytes in whole blood for the diagnosis of chronic granulomatous disease of childhood. J. Infect. Dis. 1975;131:584–587. doi: 10.1093/infdis/131.5.584. [DOI] [PubMed] [Google Scholar]
- Klasing K.C, Leshchinsky T.V. Functions, costs, and benefits of the immune system during development and growth. In: Adams N.J, Slotow R.H, editors. Proceedings of 22nd International Ornithological Congress, Durban. BirdLife South Africa; Johannesburg: 1999. pp. 2817–2835. [Google Scholar]
- Lack D. The significance of clutch size. Ibis. 1947;89:302–352. [Google Scholar]
- Lee K.A, Klasing K.C. A role for immunology in invasion biology. Trends Ecol. Evol. 2004;19:523–529. doi: 10.1016/j.tree.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Levy A. The accuracy of the bubble meter for gas flow measurements. J. Sci. Instrum. 1964;41:449–453. [Google Scholar]
- Martin T.E. Avian life-history evolution has an eminent past: does it have a bright future? Auk. 2004;21:239–301. [Google Scholar]
- Matson K.D, Ricklefs R.E, Klasing K.C. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 2004:1–12. doi: 10.1016/j.dci.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Merchant M.E, Roche C, Elsey R.M, Prudhomme J. Antibacterial properties of serum from the American alligator (Alligator mississippiensis) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003;136:505–513. doi: 10.1016/s1096-4959(03)00256-2. [DOI] [PubMed] [Google Scholar]
- Norris K, Evans M.R. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. [Google Scholar]
- Ochsenbein A.F, Zinkernagel R.M. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- Pearl R. Alfred Knopf; New York: 1928. The rate of living: being an account of some experimental studies on the biology of life duration. [Google Scholar]
- Piersma T. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos. 1997;80:623–631. [Google Scholar]
- Rheindt F.E, Grafe T.U, Abouheif E. Rapidly evolving traits and the comparative method: how important is testing for phylogenetic signal? Evol. Ecol. Res. 2004;6:377–396. [Google Scholar]
- Ricklefs R.E. Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor. 2000;102:9–22. [Google Scholar]
- Ricklefs R.E, Wikelski M. The physiology/life history nexus. Trends Ecol. Evol. 2002;17:462–468. [Google Scholar]
- Schmidt-Nielsen K.Animal physiology: adaptation and environment5th edn1997Cambridge University Press [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds: a study in molecular evolution. [Google Scholar]
- Skutch A.F. Do tropical birds rear as many young as they can nourish? Ibis. 1949;91:430–455. [Google Scholar]
- Speakman J.R, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Tieleman B.I, Williams J.B. The adjustment of avian metabolic rates and water flux to desert environments. Physiol. Biochem. Zool. 2000;73:461–479. doi: 10.1086/317740. [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman J.R, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. B. 2004;271(Suppl. 5):S360–S363. doi: 10.1098/rsbl.2004.0171. 10.1098/rsbl.2004.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera A.J, Harshman L.G. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 2001;32:95–126. [Google Scholar]


