Abstract
Changes in cell–cell and cell–matrix adhesion accompany the transition from benign tumours to invasive, malignant cancer and the subsequent metastatic dissemination of tumour cells. This review discusses a possible role of cell adhesion molecules not only in redirecting a tumour cell’s adhesive capabilities but also in modulating intracellular signalling, and with it, tumour malignancy.
Keywords: cadherin/cell adhesion/integrin/metastasis/signalling
Introduction
Cancer is still one of the prime causes of human morbidity and mortality, and ∼90% of all cancer deaths arise from metastasis formation. Of all the processes involved in tumour progression, local invasion and the formation of tumour metastases are clinically the most relevant but least well understood at the molecular level, and represent one of the great challenges in exploratory cancer research.
It is now well established that alterations in the expression and function of cell–cell and cell–matrix adhesion molecules correlate with the progression to tumour malignancy. For example, functional experiments with cultured tumour cells and transgenic mouse models have indicated that the loss of the cell adhesion molecule E-cadherin is causally involved in the formation of epithelial cancers (carcinomas; reviewed by Christofori and Semb, 1999). More recently, it has been observed that the function of E-cadherin during tumour progression is frequently replaced or even overruled by the expression of mesenchymal cadherins, such as N-cadherin or cadherin-11 (reviewed by Cavallaro et al., 2002). The functional implication of such a ‘cadherin switch’ remains to be elucidated. Yet recent experimental results, demonstrating an interaction of cadherins with receptor tyrosine kinases (RTKs), suggest that changes in cadherin expression may not only modulate tumour cell adhesion, but also affect signal transduction and hence tumour malignancy.
A physical and/or functional interaction between other cell adhesion molecules of varying nature and RTKs has also been demonstrated. For example, neural cell adhesion molecule (NCAM), a cell adhesion molecule of the immunoglobulin (Ig) gene family, associates with and stimulates fibroblast growth factor receptors (FGFRs), thereby inducing signal transduction cascades that eventually modulate cell–matrix adhesion and thus cell motility and invasion (Cavallaro and Christofori, 2001). Another member of the cadherin family, endothelial cell-specific VE-cadherin, has been shown to communicate with vascular endothelial growth factor (VEGF) receptor-2 signalling (Dejana et al., 2000). Moreover, the hyaluronan receptor CD44 (Ponta et al., 2003) associates with RTKs, including the epidermal growth factor receptor (EGFR) family member ErbB4 and the hepatocyte growth factor (HGF) receptor c-Met. Finally, integrins, cell surface receptors that mediate adhesion to the extracellular matrix, have been shown to associate with RTKs, including c-Met, EGFR and ErbB2, and to cooperate with ligand-induced signalling.
Together, the data indicate that cell adhesion molecules not only play an important role in mediating cell–cell or cell–matrix adhesion but are also critical in modulating intracellular signalling. In a bidirectional crosstalk, cell adhesion and signalling receptors are able to affect each other’s functions: cell adhesion molecules stimulate or augment receptor function and, conversely, receptor signalling modulates the function of cell adhesion molecules, either by promoting or repressing the function of cell adhesion molecules. Here, I discuss some of the available evidence for the signalling role of adhesion molecules and the potential functions in physiological and pathological processes.
The cadherin switch
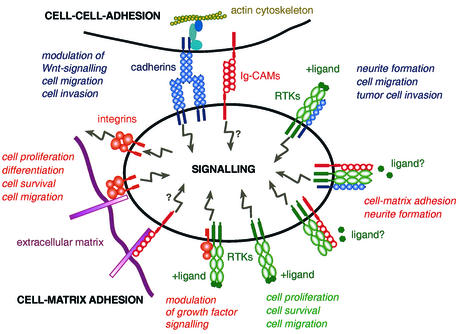
Cadherins play a critical role in establishing adherens-type junctions by mediating Ca2+-dependent cell–cell adhesion (Takeichi, 1995; Gumbiner, 2000; Yagi and Takeichi, 2000). While epithelial cadherins, with E-cadherin as the prototype family member, are critical in the formation and maintenance of epithelial structures, mesenchymal cadherins, such as N-cadherin, are preferentially expressed in migratory cells and in cells of connective tissue. Classical cadherins are single-span transmembrane-domain glycoproteins which, via their extracellular cadherin domains, mediate homophilic protein–protein interactions in a zipper-like fashion (Figure 1). The intracellular domains of classical cadherins interact with β-catenin, γ-catenin (also called plakoglobin) and p120ctn to assemble the cytoplasmic cell adhesion complex (CCC) that is critical for the formation of extracellular cell–cell adhesion. β-catenin and γ-catenin bind directly to α-catenin, which links the CCC to the actin cytoskeleton.
Fig 1. Schematic representation of potential interactions between cadherins, Ig-CAMs, integrins and RTKs that may modulate intracellular signal transduction (see text for details).
In most, if not all, cancers of epithelial origin, E-cadherin-mediated cell–cell adhesion is lost concomitant with progression towards malignancy, and it has been proposed that the loss of E-cadherin-mediated cell–cell adhesion is a prerequisite for tumour cell invasion and metastasis formation (Birchmeier and Behrens, 1994). Re-establishing the functional cadherin complex, e.g. by forced expression of E-cadherin, results in a reversion from an invasive, mesenchymal, to a benign, epithelial phenotype of cultured tumour cells (Vleminckx et al., 1991; Birchmeier and Behrens, 1994). Using a transgenic mouse model of pancreatic β-cell carcinogenesis (Rip1Tag2), we have previously demonstrated that the loss of E-cadherin-mediated cell–cell adhesion is one rate-limiting step in the progression from adenoma to carcinoma in vivo, further highlighting the role of E-cadherin as a suppressor of tumour invasion (Perl et al., 1998).
In a number of human cancer types which have lost E-cadherin expression, de novo expression of mesenchymal cadherins, such as N-cadherin and cadherin-11, has been observed (Li and Herlyn, 2000; Tomita et al., 2000). N-cadherin has been shown to promote cell motility and migration, thus showing an opposite effect as compared with E-cadherin (Islam et al., 1996; Tran et al., 1999; Hazan et al., 2000; Li et al., 2001). N-cadherin-induced tumour cell invasion can even overcome E-cadherin-mediated cell–cell adhesion (Nieman et al., 1999; Hazan et al., 2000). This cadherin conversion recapitulates a well characterized phenomenon occurring during embryonic development, e.g. when epiblast cells switch from E- to N-cadherin in order to ingress the primitive streak or when primordial germ cells migrate to populate the genital ridge (Edelman et al., 1983; Hatta and Takeichi, 1986; Bendel-Stenzel et al., 2000). Based on these observations, a novel concept has been formulated that a ‘cadherin switch’ is involved not only in delamination and migration of epithelial cells during embryonic development but also during the transition from a benign to an invasive, malignant tumour phenotype (Li and Herlyn, 2000; Tomita et al., 2000).
E-cadherin and N-cadherin are both classical cadherins and on first sight seem to involve similar mechanisms of cell–cell adhesion. Hence, the functional implication of the ‘cadherin switch’ for tumour progression is not obvious. One possibility is that the change from E- to N-cadherin expression may provide a tumour cell with a new ‘homing address’ to find new ‘neighbours’. Unlike E-cadherin, N-cadherin (and, presumably, other mesen chymal cadherins) promotes a dynamic adhesion state in tumour cells, not only allowing the dissociation of single cells from the tumour mass but also their interactions with endothelial and stromal components (Hazan et al., 2000; Li and Herlyn, 2000; Tomita et al., 2000). On the other hand, N-cadherin may provide a pro-migratory signal to the cells expressing it (Figure 1). In fact, N-cadherin-mediated induction of FGFR signalling has been demonstrated to occur in neurons, where it supports neurite outgrowth, an event strictly related to cell migration and invasion (Doherty and Walsh, 1996). Work in our laboratory has revealed a physical association between N-cadherin and different members of the FGFR family in a variety of non-transformed and tumour cell types (Cavallaro et al., 2001), and a functional cooperation between N-cadherin and FGFR signalling has been demonstrated by Hazan and co-workers in breast cancer cells (Nieman et al., 1999; Suyama et al., 2002). It is thought that N-cadherin facilitates binding of FGFs to the receptor but prevents ligand-induced internalization of the receptor, thus leading to an increased cell-surface receptor level and sustained MEK/MAPK signalling, increased cellular motility, and invasion (Suyama et al., 2002).
Association of another member of the cadherin family with an RTK has been reported for the endothelial cell-specific VE-cadherin with the major signalling receptor for VEGF, VEGFR-2 (Dejana et al., 2000). Similarly to N-cadherin and FGFR, VE-cadherin is able to enhance VEGF-induced VEGFR signalling and, in the absence of VE-cadherin, endothelial cells are unable to respond to VEGF and undergo apotosis (Carmeliet et al., 1999). Conversely, VE-cadherin, β-catenin, γ-catenin, and p120ctn, but not α-catenin, are phosphorylated on tyrosine by activated VEGFR-2 (Esser et al., 1998). However, it is not clear whether tyrosine phosphorylation of these proteins is an earmark for their degradation, as is the case for E-cadherin (see below). Since VEGF is a potent angiogenic factor and VE-cadherin (and N-cadherin) is expressed by endothelial cells during ongoing angiogenesis, these cadherins may play an important role in the fine-tuning of physiological and pathological angiogenesis, not only by modulating endothelial cell adhesion but also by influencing the activity of angiogenic growth factor signalling via RTKs.
Cadherin-mediated cell–cell adhesion can also affect the Wnt-signalling pathway (Bienz and Clevers, 2000; Polakis, 2000). β-catenin (and γ-catenin) is usually sequestered by cadherins in the CCC. Upon loss of E-cadherin function, non-sequestered, free β-catenin is usually phosphorylated by glycogen synthase kinase 3β (GSK-3β) in the adenomatous polyposis coli (APC)– axin–GSK-3β complex and subsequently degraded by the ubiquitin–proteasome pathway. In many cancer cells, loss of function of the tumour suppressor APC, mutations in β-catenin or inhibition of GSK-3β by the activated Wnt-signalling pathway leads to the stabilization of β-catenin in the cytoplasm. Subsequently, it translocates to the nucleus, where it binds to members of the Tcf/Lef-1 family of transcription factors and modulates expression of Tcf/Lef-1-target genes, including the proto-oncogene c-Myc and cyclin D1.
Cadherins also appear to directly affect each other’s function. Suppression of N-cadherin function in invasive squamous carcinoma cells results in the induction of E- and P-cadherin expression and in the reversion to an epithelial phenotype. In contrast, forced expression of N-cadherin in epithelial-like squamous cells causes downregulation of E- and P-cadherin and the acquisition of an invasive phenotype (Islam et al., 1996). This implies that the expression of N-cadherin during tumour progression might be equally necessary and sufficient to overcome E-cadherin-mediated cell–cell adhesion and to promote malignant tumour progression.
NCAM and N-cadherin-mediated FGFR signalling
Besides cadherins, members of the Ig family of cell adhesion molecules (CAMs) also play an important role in the progression to tumour malignancy. For example, in various cancer types, expression of NCAM shifts from the adult 120 kDa isoform to the embryonic 140 and 180 kDa isoforms together with a general downregulation of expression (reviewed by Cavallaro and Christofori, 2001). A correlation between reduced NCAM expression and poor prognosis has been reported for a few cancer types (Fogar et al., 1997; Roesler et al., 1997; Tezel et al., 2001). Using the Rip1Tag2 transgenic mouse model of pancreatic β-cell carcinogenesis, our laboratory has previously reported that abrogation of NCAM expression in pancreatic β-cell tumours results in a dramatic increase in the incidence of metastases, predominantly to local lymph nodes (Perl et al., 1999). Detailed phenotypic analysis of cultured tumour cell lines revealed an impaired cell– substrate adhesion of NCAM-deficient cells, while cell– cell adhesion was not affected by the lack of NCAM (Cavallaro et al., 2001).
The findings described above suggest that CAMs and cadherins modulate cellular functions that are critical in tumour progression. Until recently, NCAM and N-cadherin functions have been studied mainly in neurons, where a functional interaction between NCAM and FGFR in inducing neurite outgrowth has been demonstrated (Doherty and Walsh, 1996). Interfering with FGFR signalling by means of dominant-negative FGFR mutants or chemical inhibitors prevented neurite outgrowth induced by NCAM-mediated homophilic binding. These observations were also confirmed for another Ig-CAM, L1, and for N-cadherin (Saffell et al., 1997). Although specific motifs in the extracellular domain of both protein families were proposed to mediate the direct binding between CAMs and FGFR, a physical association remains to be demonstrated.
Our finding that NCAM modulates tumour cell–matrix adhesion implies that NCAM is somehow able to modulate integrin function (Cavallaro et al., 2001). Biochemical analyses have identified the signalling pathway(s) linking NCAM to integrin activation: NCAM interacts with and activates members of the FGFR family. NCAM-mediated FGFR activation results in the assembly of a classical signalling complex, including the adaptor molecule FRS-2, the non-receptor tyrosine kinase c-Src, and the downstream effector molecules cortactin and phospholipase Cγ (PLCγ). Inhibition of FGFR signalling by specific inhibitors or dominant-negative FGFR constructs, as well as neutralizing antibodies to β1 integrin, repressed NCAM-induced cell–substrate adhesion, further supporting the idea that NCAM directly modulates β1 integrin function and thus cell–matrix adhesion (Cavallaro et al., 2001). Notably, further analysis also revealed that NCAM’s association with FGFR recruits N-cadherin into the signalling complex (Figure 1). However, it remains unknown whether N-cadherin and NCAM act together in the stimulation of FGFR signalling or whether they elicit qualitatively or quantitatively different signal transduction cascades. Moreover, the requirement of FGFs in the activation of FGFRs that are complexed with NCAM and/or N-cadherin remains elusive: while FGFs were not required for NCAM-mediated FGFR signalling (Cavallaro et al., 2001), N-cadherin synergized with FGF2 in the stimulation of FGFR signalling (Suyama et al., 2002).
Of course, the observation that N-cadherin is able to induce FGFR signal transduction supports the idea that upregulated N-cadherin expression during tumour progression (the cadherin switch; see above) exerts its function not only by changing a tumour cell’s adhesive repertoire but also by stimulating classical signal transduction pathways. Future experimentation will be required to determine in more detail how N-cadherin, NCAM and FGFR signalling act together to modulate tumour cell– matrix adhesion and thereby tumour cell migration and invasion. Moreover, it will be interesting to see whether other cadherins and CAMs induce signalling by FGFRs or even other RTKs in physiological and pathological processes.
CD44-mediated growth factor signalling
Hepatocyte growth factor (HGF) and its cognate receptor c-Met are known inducers of cell scattering, migration, invasion, proliferation as well as epithelial–mesenchymal transition, tubular organization and morphogenesis. Their involvement in cancer progression, mainly by affecting the invasive behaviour of tumour cells, has been amply demonstrated in various experimental systems. In human cancers, the c-Met gene has frequently been found to be amplified, mutated or overexpressed (reviewed in Birchmeier and Gherardi, 1998).
Together with c-Met, expression of the hyaluronan receptor CD44 is frequently upregulated in cancers (for a review, see Ponta et al., 2003). Based on extensive alternative splicing of exon v1–v10, various isoforms exist which are further diversified by additional post-translational modifications. Notably, the v6 isoform of CD44 seems to play a critical role in tumour metastasis: ectopic expression of v6-containing CD44 isoforms or treatment with anti-v6 monoclonal antibodies modulates metastasis formation of cancer cells in animal models in vivo and tumour cell invasiveness in vitro (Herrlich et al., 1998; Ponta et al., 1998). Moreover, the v6 isoform of CD44 seems to be required for HGF-induced c-Met activation, and CD44v6 and c-Met are found to interact physically. While the extracellular domain of CD44v6 is required and sufficient to allow HGF-induced autophosphorylation of c-Met, transfer of the signal to downstream effectors, such as MEK and MAPK, depends on the presence of the cytoplasmic tail of CD44v6 (Orian-Rousseau et al., 2002).
Another splice variant of CD44, CD44v3, contains Ser-Gly repeats that support covalent attachment of heparan sulfate proteoglycans. CD44v3 binds a number of heparin-binding growth factors, including members of the FGF family and heparin-binding epidermal growth factor (HB-EGF). Here also, a physical association between a cell adhesion molecule and an RTK has been demonstrated: in the presence of CD44v3, binding of HB-EGF to its cognate receptor, the EGFR family member ErbB4, is facilitated (Yu et al., 2002). Moreover, CD44v3 recruits active matrix metalloprotease 7 (MMP7; matrilysin), which then proteolytically converts HB-EGF from the precursor to its active receptor binding form. Subsequent stimulation of the receptor results in increased cell proliferation, migration and survival. This cell surface complex between CD44v3, HB-EGF, ErbB4 and MMP7 is found on tumour cells in vitro, and in uterine epithelium and lactating mammary gland epithelium in vivo (Yu et al., 2002). Notably, CD44, via the interaction of its cytoplasmic domain with ERM (ezrin–radixin–moesin) proteins, is also connected to the actin cytoskeleton, thereby modulating cell migration and cell shape (reviewed in Ponta et al., 2003).
Modulation of RTK signalling by integrins
Integrins, the prime mediators of cell–matrix adhesion (Eliceiri, 2001; Hynes, 2002), have also been found to crosstalk with RTKs in a cell- and integrin-type-dependent manner. Indeed, integrins are known to be involved in tumour invasion and metastasis; however, their actual function varies between different integrin family members and different tumour types. In various model systems, the direct association of integrins and RTKs has been demonstrated conclusively by biochemical techniques (Figure 1). Given its ability to control cell proliferation, migration and invasion, the integrin/RTK crosstalk is likely to play a key role in the onset and progression of various tumour types. Typical examples are the association of the integrins α6β4 and α6β1 with the EGFR family member ErbB2 (Falcioni et al., 1997). Activation of these integrins by specific antibodies results in ErbB2 phosphorylation, and co-expression of integrins with ErbB2 stimulates cell proliferation and invasiveness. Notably, the β4 cytoplasmic domain as a monomer is necessary and sufficient to associate with ErbB2, whereas the extracellular domain, and hence matrix binding, is not required for this function (Gambaletta et al., 2000). Integrin α6β4 has also been shown to cooperate with the function of HGF and its receptor c-Met in carcinoma cells, resulting in altered invasive growth properties (Trusolino et al., 2001). HGF-induced invasive growth requires both c-Met and integrin: upon c-Met activation, the integrin is phosphorylated on the β4 cytoplasmic domain, which subsequently forms a complex with Shc and PI3K, thereby potentiating Ras and PI3K signalling. Again, binding of the extracellular domain to its ligand laminin is not required, suggesting an adhesion-independent function for this integrin. Finally, EGFR has been shown to interact with β1 integrin in a reciprocal crosstalk involving the MAPK pathway to modulate invasive growth in three-dimensional culture systems. Antibody-mediated inhibition of either β1 or EGFR activity represses, whereas activation of EGFR induces, invasive growth (Wang et al., 1998).
Together, these data indicate that different cell adhesion molecules, CAMs, cadherins, CD44 and integrins alike, are able to modulate classical signal transduction pathways. Besides mediating cell adhesion, these properties may be criticial functions that cell adhesion molecules play during tumour progression.
Growth factor signalling affecting cell adhesion molecules
Loss of E-cadherin function during tumour progression can be caused by a variety of genetic or epigenetic mechanisms, including mutational inactivation, chromosomal aberrations, transcriptional repression of the E-cadherin gene, e.g. by the repressors Snail and Sip-1, and subsequent promoter hypermethylation and chromatin rearrangements (Hirohashi, 1998; Christofori and Semb, 1999; Batlle et al., 2000; Cano et al., 2000; Comijn et al., 2001). Tyrosine phosphorylation has also previously been implicated in the regulation of cadherin function: RTKs, such as EGFR, c-Met and FGFR, and the non-receptor tyrosine kinase, c-Src, phosphorylate E-cadherin, N-cadherin, β-catenin, γ-catenin and p120ctn, resulting in the disassembly of the cytoplasmic adhesion complex and a disruption of cadherin-mediated cell adhesion and cell scattering (Behrens et al., 1993; Hamaguchi et al., 1993; Fujita et al., 2002). Upon autophosphorylation, RTKs are often ubiquitylated by E3 ligases, such as c-Cbl, which associate with phosphorylated RTKs, resulting in the degradation of the RTK by the proteasome (Joazeiro et al., 1999; Levkowitz et al., 1999). In a very elegant study, the group of Walter Birchmeier has recently identified a novel E3 ligase, termed Hakai (Japanese for destruction) that binds tyrosine-phosphorylated E-cadherin (but not N-cadherin), and by ubiquitylation earmarks it for endo cytosis and proteasome-mediated degradation (Fujita et al., 2002). Notably, Hakai is structurally and functionally related to c-Cbl. Consistent with the function of RTKs in the degradation of E-cadherin protein, treatment of cells with phosphatase inhibitors causes the dissociation of α- and β-catenin from the adhesion complex, as well as the concomitant decrease in E-cadherin-mediated cell adhesion (Ozawa and Kemler, 1998). Interestingly, the cadherin switch in epiblast cells can be recapitulated in vitro by treating the cells with HGF, further under scoring a critical role of RTK-mediated changes in cell adhesion during physiological processes (DeLuca et al., 1999).
Activation of the insulin-like growth factor receptor 1 (IGFR1) by its ligands IGF-I and II can induce epithelial– mesenchymal transition, also exemplified by the loss of E-cadherin function. Curiously, IGF1R has been found in a complex with E-cadherin and β-catenin and, upon IGF stimulation, E-cadherin is internalized and degraded, whereas β-catenin translocates from the plasma membrane to the nucleus, resulting in the modulation of β-catenin/Tcf target gene expression (Morali et al., 2001). In the RipTag2 transgenic mouse model of pancreatic β-cell carcinogenesis, transgenic overexpression of IGF1R results in a dramatic acceleration of tumour progression from benign adenoma to invasive carcinoma and the formation of metastasis concomitant with the loss of E-cadherin function (see above; Lopez and Hanahan, 2002). Here also, growth factor signalling seems to directly affect the function of a cell adhesion molecule. How IGF1R modulates E-cadherin function, i.e. whether Hakai or another E3 ligase is involved in the IGF-mediated degradation of E-cadherin, remains to be elucidated.
Conclusions
There is now an increasing body of evidence that cell adhesion molecules of varying classes and functions, including cadherins, Ig-CAMs, CD44 and integrins, can induce or modulate classical signalling pathways by associating with and activating RTKs. Conversely, a number of RTKs have been shown to use cell adhesion molecules as substrates, thereby modulating their function (Figure 1). These observations add another level of complexity to the field of signal transduction. We have to leave behind the idea of signalling specificities being merely determined by the fact that RTKs are activated exclusively by their appropriate ligands. Extracellular matrix, neighbouring cells and intracellular signalling components, tacitly accepted as ‘cellular context’, are critical determinants of signalling specificities as well.
While today we do not know how general and widespread these mechanisms are, it will be interesting to learn about their functional role in physiology and disease, in particular in the regulation of tumour cell migration, invasion and metastasis formation.
Acknowledgments
Acknowledgements
I thank the members of my laboratory, past and present, for their excellent work and their dedication. I am grateful to Ugo Cavallaro and Birgit Schaffhauser for Figure 1 and for critically reading the manuscript. I apologize to all those colleagues whose important work I could not cite due to space limitations.
References
- Batlle E., Sancho,E., Franci,C., Dominguez,D., Monfar,M., Baulida,J. and Garcia De Herreros,A. (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol., 2, 84–89. [DOI] [PubMed] [Google Scholar]
- Behrens J., Vakaet,L., Friis,R., Winterhager,E., Van Roy,F., Mareel,M.M. and Birchmeier,W. (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol., 120, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel-Stenzel M.R., Gomperts,M., Anderson,R., Heasman,J. and Wylie,C. (2000) The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech. Dev., 91, 143–152. [DOI] [PubMed] [Google Scholar]
- Bienz M. and Clevers,H. (2000) Linking colorectal cancer to Wnt signaling. Cell, 103, 311–320. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. and Gherardi,E. (1998) Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol., 8, 404–410. [DOI] [PubMed] [Google Scholar]
- Birchmeier W. and Behrens,J. (1994) Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta, 1198, 11–26. [DOI] [PubMed] [Google Scholar]
- Cano A., Perez-Moreno,M.A., Rodrigo,I., Locascio,A., Blanco,M.J., del Barrio,M.G., Portillo,F. and Nieto,M.A. (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol., 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell, 98, 147–157. [DOI] [PubMed] [Google Scholar]
- Cavallaro U. and Christofori,G. (2001) Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim. Biophys. Acta, 1552, 39–45. [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Niedermeyer,J., Fuxa,M. and Christofori,G. (2001) N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol., 3, 650–657. [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Schaffhauser,B. and Christofori,G. (2002) Cadherins and the tumour progression: is it all in a switch? Cancer Lett., 176, 123–128. [DOI] [PubMed] [Google Scholar]
- Christofori G. and Semb,H. (1999) The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci., 24, 73–76. [DOI] [PubMed] [Google Scholar]
- Comijn J., Berx,G., Vermassen,P., Verschueren,K., van Grunsven,L., Bruyneel,E., Mareel,M., Huylebroeck,D. and van Roy,F. (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell, 7, 1267–1278. [DOI] [PubMed] [Google Scholar]
- Dejana E., Lampugnani,M.G., Martinez-Estrada,O. and Bazzoni,G. (2000) The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int. J. Dev. Biol., 44, 743–748. [PubMed] [Google Scholar]
- DeLuca S.M., Gerhart,J., Cochran,E., Simak,E., Blitz,J., Mattiacci-Paessler,M., Knudsen,K. and George-Weinstein,M. (1999) Hepatocyte growth factor/scatter factor promotes a switch from E- to N-cadherin in chick embryo epiblast cells. Exp. Cell Res., 251, 3–15. [DOI] [PubMed] [Google Scholar]
- Doherty P. and Walsh,F.S. (1996) CAM-FGF receptor interactions: a model for axonal growth. Mol. Cell. Neurosci., 8, 99–111. [DOI] [PubMed] [Google Scholar]
- Edelman G.M., Gallin,W.J., Delouvee,A., Cunningham,B.A. and Thiery,J.P. (1983) Early epochal maps of two different cell adhesion molecules. Proc. Natl Acad. Sci. USA, 80, 4384–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri B.P. (2001) Integrin and growth factor receptor crosstalk. Circ. Res., 89, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Esser S., Lampugnani,M.G., Corada,M., Dejana,E. and Risau,W. (1998) Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci., 111, 1853–1865. [DOI] [PubMed] [Google Scholar]
- Falcioni R., Antonini,A., Nistico,P., Di Stefano,S., Crescenzi,M., Natali,P.G. and Sacchi,A. (1997) α6β4 and α6β1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell Res., 236, 76–85. [DOI] [PubMed] [Google Scholar]
- Fogar P., Basso,D., Pasquali,C., De Paoli,M., Sperti,C., Roveroni,G., Pedrazzoli,S. and Plebani,M. (1997) Neural cell adhesion molecule (N-CAM) in gastrointestinal neoplasias. Anticancer Res., 17, 1227–1230. [PubMed] [Google Scholar]
- Fujita Y., Krause,G., Scheffner,M., Zechner,D., Leddy,H.E., Behrens,J., Sommer,T. and Birchmeier,W. (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol., 4, 222–231. [DOI] [PubMed] [Google Scholar]
- Gambaletta D., Marchetti,A., Benedetti,L., Mercurio,A.M., Sacchi,A. and Falcioni,R. (2000) Cooperative signaling between α6β4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J. Biol. Chem., 275, 10604–10610. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. (2000) Regulation of cadherin adhesive activity. J. Cell Biol., 148, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Matsuyoshi,N., Ohnishi,Y., Gotoh,B., Takeichi,M. and Nagai,Y. (1993) p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J., 12, 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K. and Takeichi,M. (1986) Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature, 320, 447–449. [DOI] [PubMed] [Google Scholar]
- Hazan R.B., Phillips,G.R., Qiao,R.F., Norton,L. and Aaronson,S.A. (2000) Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion and metastasis. J. Cell Biol., 148, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Sleeman,J., Wainwright,D., Konig,H., Sherman,L., Hilberg,F. and Ponta,H. (1998) How tumor cells make use of CD44. Cell. Adhes. Commun., 6, 141–147. [DOI] [PubMed] [Google Scholar]
- Hirohashi S. (1998) Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am. J. Pathol., 153, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell, 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Islam S., Carey,T.E., Wolf,G.T., Wheelock,M.J. and Johnson,K.R. (1996) Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell–cell adhesion. J. Cell Biol., 135, 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro C.A., Wing,S.S., Huang,H., Leverson,J.D., Hunter,T. and Liu,Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin–protein ligase. Science, 286, 309–312. [DOI] [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Li G. and Herlyn,M. (2000) Dynamics of intercellular communication during melanoma development. Mol. Med. Today, 6, 163–169. [DOI] [PubMed] [Google Scholar]
- Li G., Satyamoorthy,K. and Herlyn,M. (2001) N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res., 61, 3819–3825. [PubMed] [Google Scholar]
- Lopez T. and Hanahan,D. (2002) Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell, 1, 339–353. [DOI] [PubMed] [Google Scholar]
- Morali O.G., Delmas,V., Moore,R., Jeanney,C., Thiery,J.P. and Larue,L. (2001) IGF-II induces rapid β-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene, 20, 4942–4950. [DOI] [PubMed] [Google Scholar]
- Nieman M.T., Prudoff,R.S., Johnson,K.R. and Wheelock,M.J. (1999) N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol., 147, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V., Chen,L., Sleeman,J.P., Herrlich,P. and Ponta,H. (2002) CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev., 16, 3074–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M. and Kemler,R. (1998) Altered cell adhesion activity by pervanadate due to the dissociation of α-catenin from the E-cadherin–catenin complex. J. Biol. Chem., 273, 6166–6170. [DOI] [PubMed] [Google Scholar]
- Perl A.K., Wilgenbus,P., Dahl,U., Semb,H. and Christofori,G. (1998) A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature, 392, 190–193. [DOI] [PubMed] [Google Scholar]
- Perl A.K., Dahl,U., Wilgenbus,P., Cremer,H., Semb,H. and Christofori,G. (1999) Reduced expression of neural cell adhesion molecule induces metastatic dissemination of pancreatic β tumor cells. Nat. Med., 5, 286–291. [DOI] [PubMed] [Google Scholar]
- Polakis P. (2000) Wnt signaling and cancer. Genes Dev., 14, 1837–1851. [PubMed] [Google Scholar]
- Ponta H., Wainwright,D. and Herrlich,P. (1998) The CD44 protein family. Int. J. Biochem. Cell Biol., 30, 299–305. [DOI] [PubMed] [Google Scholar]
- Ponta H., Sherman,L. and Herrlich,P.A. (2003) CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol., 4, 33–45. [DOI] [PubMed] [Google Scholar]
- Roesler J., Srivatsan,E., Moatamed,F., Peters,J. and Livingston,E.H. (1997) Tumor suppressor activity of neural cell adhesion molecule in colon carcinoma. Am. J. Surg., 174, 251–257. [DOI] [PubMed] [Google Scholar]
- Saffell J.L., Williams,E.J., Mason,I.J., Walsh,F.S. and Doherty,P. (1997) Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron, 18, 231–242. [DOI] [PubMed] [Google Scholar]
- Suyama K., Shapiro,I., Guttman,M. and Hazan,R.B. (2002) A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell, 2, 301–314. [DOI] [PubMed] [Google Scholar]
- Takeichi M. (1995) Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol., 7, 619–627. [DOI] [PubMed] [Google Scholar]
- Tezel E., Kawase,Y., Takeda,S., Oshima,K. and Nakao,A. (2001) Expression of neural cell adhesion molecule in pancreatic cancer. Pancreas, 22, 122–125. [DOI] [PubMed] [Google Scholar]
- Tomita K., van Bokhoven,A., van Leenders,G.J., Ruijter,E.T., Jansen,C.F., Bussemakers,M.J. and Schalken,J.A. (2000) Cadherin switching in human prostate cancer progression. Cancer Res., 60, 3650–3654. [PubMed] [Google Scholar]
- Tran N.L., Nagle,R.B., Cress,A.E. and Heimark,R.L. (1999) N-cadherin expression in human prostate carcinoma cell lines. An epithelial–mesenchymal transformation mediating adhesion with stromal cells. Am. J. Pathol., 155, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L., Bertotti,A. and Comoglio,P.M. (2001) A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell, 107, 643–654. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet,L.,Jr, Mareel,M., Fiers,W. and van Roy,F. (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell, 66, 107–119. [DOI] [PubMed] [Google Scholar]
- Wang F., Weaver,V.M., Petersen,O.W., Larabell,C.A., Dedhar,S., Briand,P., Lupu,R. and Bissell,M.J. (1998) Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl Acad. Sci. USA, 95, 14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T. and Takeichi,M. (2000) Cadherin superfamily genes: functions, genomic organization and neurologic diversity. Genes Dev., 14, 1169–1180. [PubMed] [Google Scholar]
- Yu W.H., Woessner,J.F.,Jr, McNeish,J.D. and Stamenkovic,I. (2002) CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev., 16, 307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]