Abstract
Mounting an immune response requires a relatively substantial investment of energy and marked reductions in energy availability can suppress immune function and presumably increase disease susceptibility. We have previously demonstrated that a moderate reduction in energy stores via partial surgical lipectomy (LIPx) impairs humoural immunity of Siberian hamsters (Phodopus sungorus). Here we tested the hypothesis that LIPx-induced decreases in immunity are mediated by changes in the adipose tissue hormone leptin. Hamsters received bilateral surgical removal of inguinal white adipose tissue (IWATx) or sham surgeries (Sham). Half the animals in each group received osmotic minipumps containing murine leptin (0.5 μl h−1 for 10 days) whereas the remaining animals received minipumps containing vehicle alone; all animals were subsequently challenged with the novel antigen keyhole limpet haemocyanin (KLH). In general, serum leptin and anti-KLH antibodies were significantly correlated with one another with higher levels generally indicating enhanced immunity. In addition, IWATx hamsters had significantly lower serum anti-KLH IgG compared with sham animals. Exogenous leptin, however, attenuated LIPx-induced immune suppression but did not affect humoural immunity in sham animals. These results suggest that reductions in energy availability lead to impairments in humoural immunity and that leptin can serve as a neuroendocrine signal between body fat and immunity regulating humoural immune responses.
Keywords: body fat, adipose tissue, seasonal, immune, energetics
1. Introduction
For any organism to survive, it must maintain a balanced energy budget where energy intake is equal to or greater than energy expenditure. For most non-tropical animals, maintenance of a positive energetic budget can become challenging during times of increased energetic demands, especially when food availability is at its nadir (e.g. during winter). In order to cope with the energetic bottleneck of winter, individuals of many seasonally breeding mammalian species have evolved specific morphological, physiological and behavioural adaptations to maintain energy homeostasis, either by curtailing energetically costly processes (e.g. breeding) or by invoking energy-saving responses (e.g. induction of torpor and/or hibernation) (Bronson & Heideman 1994). Among the suite of physiological adaptations, many rodents undergo marked seasonal fluctuations in body mass, primarily in the form of changes in body fat (Bartness & Wade 1985). For example, Siberian hamsters (P. sungorus) housed in short ‘winter-like’ photoperiods (i.e. less than 12 h of light per day) undergo a marked (approximately 20–30%) reduction in total body fat. Reduced body fat in winter is presumably adaptive in that it reduces body size and thus energy demands because small bodies require less food than large bodies, and heat loss is reduced as body surface area is reduced with respect to total volume of the animal. In addition to decreases in body fat, Siberian hamsters also display reduced humoural and cell-mediated immune function in short compared with long days (Demas et al. 1997; Yellon et al. 1999; Demas 2002; Drazen et al. 2001).
Immunity, as with all biological processes, requires substantial energy to maintain ‘optimal’ functioning (Sheldon & Verhulst 1996; Demas 2004). For example, mounting a humoural immune response to a specific, non-replicating antigen results in a significant increase in energy metabolism (Demas et al. 1997; Lochmiller & Deerenberg 2000; Norris & Evans 2000; Zuk & Stoehr 2002). More extreme immune challenges (e.g. infection or sepsis) can increase metabolic rate by approximately 30–60% in humans (reviewed in Lochmiller & Deerenberg 2000) and starvation impairs immunity and increases susceptibility to disease or death (Chandra 1996; Moret & Schmid-Hempel 2000).
It has been suggested that trade-offs among immune function and other energetically expensive processes have evolved such that individuals are able to tolerate minor reductions in immunity at times when the energetic costs of mounting an immune response outweigh the benefits (Nelson & Demas 1996; Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Nelson et al. 2002). Despite the apparent link between energy availability and immunity, relatively little is known regarding changes in total body fat and immune function. Marked reductions in energy availability without concomitant reductions in energy output can lead to substantial suppression of immunity by decreasing the availability of utilisable fuels (Chandra 2002; Nova et al. 2002). Free fatty acids (FFAs), one of the primary constituents of adipose tissue, provide a major fuel source for lymphocytes and may be used preferentially over glucose (Ardawi & Newsholme 1985). Furthermore, FFAs can enhance mitogen-induced proliferation of rodent and human lymphocytes in vitro (reviewed in Pond 1996). Alternatively, decreases in body fat stores may affect immunity indirectly via changes in endocrine signalling with the immune system. Adipose tissue, rather than simply serving as an inert reservoir for lipid storage, also functions as an important endocrine organ responsible for the synthesis and secretion of several metabolic hormones and proteins (Ahima & Flier 2000). For example, the peptide hormone leptin (ob protein) is secreted primarily by adipose tissue and has been shown to enhance a variety of immunological parameters in mammals (Lord et al. 1998; Faggioni et al. 2001) and leptin deficiency can increase susceptibility to infections (Faggioni et al. 2001). In addition, changes in serum leptin concentrations are positively correlated with measures of immune function in rodents (Drazen et al. 2000).
A recent study from our laboratory has provided initial support for the hypothesis that energetic regulation of immunity is mediated by the neuroendocrine system (Drazen et al. 2001). Specifically, Siberian hamsters housed in short days for 10 weeks displayed significant reductions in body fat, as well as reduced humoural immunity. Administration of exogenous leptin via osmotic minipumps restored short-day reductions in immunity to levels comparable to long day animals; leptin, however, had no effect on immunity in long-day animals (Drazen et al. 2001). These results suggest that short-day decreases in humoural immunity in Siberian hamsters are due, at least in part, to reductions in body fat and probably decreased leptin.
In the present study, we tested the hypothesis that decreased humoural immunity in response to decreased energy stores (i.e. body fat) is mediated by the adipose tissue hormone leptin in Siberian hamsters. This species was chosen because individuals display robust and reliable seasonal fluctuations in both body fat and humoural immunity (Nelson et al. 1996; Sinclair & Lochmiller 2000). Specifically, total body fat was manipulated by surgical removal of body fat (partial lipectomy; LIPx) as described previously (Demas et al. 2003). Partial LIPx reduces total body fat by reducing fat cell number, thus decreasing available energy stored as lipid in adipose tissue provides a valuable model for studying the effects of energy availability on specific physiological responses (Dark et al. 1985; Mauer & Bartness 1994, 1997; Mauer et al. 2001). In addition, circulating leptin concentrations were manipulated via exogenous administration of the hormone. If reduced energy availability suppresses humoural immunity in this species, then LIPx should reduce serum antibody levels, as previously reported (Demas et al. 2003). Furthermore, if serum leptin concentrations provide an endocrine signal to the immune system indicating total body fat reserves, antibody levels should remain unaffected in LIPx hamsters treated with exogenous leptin. Alternatively, if the total amount of metabolizable fuels (e.g. FFAs, glycerol) is the critical parameter, LIPx should lead to immunosuppression independent of circulating leptin concentrations.
2. Material and Methods
Forty adult (more than 60 days of age) female Siberian hamsters (P. sungorus) were obtained from our breeding colony maintained at Indiana University. The progenitors of these animals were generously provided by Dr Randy Nelson (Ohio State University) and Dr Timothy Bartness (Georgia State University). All animals were initially group-housed (2–4 per cage with same sex siblings upon weaning at 21 days of age). Two weeks before the start of the experiment, animals were housed individually in polypropylene cages (28×17×12 cm3) in colony rooms with a 24 h light : dark (LD) 16 : 8 cycle (lights on 06:00 h EST). Temperature was kept constant at 20±2 °C and relative humidity was maintained at 50±5%. Food (Prolab 2000) and tap water were available ad libitum throughout the experiment. All animals were treated in accordance with the Indiana University Institutional Animal Care and Use Committee (IACUC).
(a) Experimental procedures
All animals were housed in a colony room with a long-day LD 16 : 8 photoperiod (lights on at 06:00 h EST). One half of the animals (n=20) received bilateral lipectomy of inguinal white adipose tissue (IWATx) whereas the remaining animals (n=20) received sham surgeries. Surgeries were performed under deep anaesthesia following administration of 0.3 ml/100 g body mass of ketamine (21 mg)+xylazine (2.4 mg) as described previously (Demas et al. 2003). In IWATx hamsters, bilateral dorsal incisions were made, IWAT was removed by surgical dissection and the skin closed with surgical staples. Sham IWATx animals received incisions through the skin and the IWAT was freed from the skin, irrigated with saline and the skin closed. All hamsters were returned to the colony room and allowed to recover. Two weeks later, half of the IWATx animals (n=10) and half of the sham animals (n=10) were randomly assigned to receive surgically implanted osmotic minipumps (Alzet 2002; 200 μl volume; 0.5 μl h−1 delivery rate; Alza Scientific, Mountain View, CA) containing leptin (2.6 μg μl−1 leptin; Peprotech, Inc., Rocky Hill, NJ) dissolved in 0.5 M Tris buffer. The remaining animals received minipumps containing vehicle (0.5 M Tris buffer). Minipumps were implanted subcutaneously (s.c.) in the intrascapular region of the animals. Three days after implantations of minipumps (Day 0), all hamsters received a single subcutaneous injection of 100 μg of the antigen keyhole limpet haemocyanin (KLH), to which all animals were previously naive, suspended in 0.1 ml sterile saline and were then returned to the colony room. KLH is an innocuous respiratory protein derived from the giant keyhole limpet (Megathura crenulata). KLH was used because it generates a robust antigenic response in rodents, but does not make the animals sick (e.g. prolonged inflammation or fever). Blood was drawn from the retro-orbital sinus 10 days post-immunization; this sampling period was chosen in order to capture peak immunoglobulin G (IgG) production (the predominant Ig class present in blood) during the course of the immune response to KLH (Demas et al. 1997; Drazen et al. 2000). On the day of sampling, animals were brought into the surgery room, lightly anaesthetized with anhydrous diethyl ether vapours (Sigma Chemical, St Louis, MO), and blood samples (500 μl) were drawn from the retro-orbital sinus between 1000 and 1200 h. Samples were allowed to clot for 1 h, the clots were removed, and the samples centrifuged (at 4 °C) for 30 min at 2500 r.p.m. Serum aliquots were aspirated and stored in sealable polypropylene microcentrifuge tubes at −80 °C until assayed for IgG. Immediately, following blood sampling animals were killed by cervical dislocation and spleens, paired testes, as well as epididymal WAT (EWAT), retroperitoneal WAT (RWAT) and IWAT pads (in sham-operated animals) were removed from all hamsters. All tissues were cleaned of connective tissue and weighed to the nearest 0.1 mg by laboratory assistants who were naive to the experimental hypotheses and treatment assignments. Two LIPx hamsters died before blood samples could be drawn post-immunization; thus, data from these animals were not included in any subsequent analyses.
(b) Assessment of humoural immunity
To assess humoural immunity, serum anti-KLH IgG concentrations were assayed using an enzyme-linked immunosorbent assay (ELISA) as previously described (Demas 2002). Specifically, microtitre plates were coated with antigen by incubating overnight at 4 °C with 0.5 mg ml−1 KLH in sodium bicarbonate buffer (pH 9.6), washed with phosphate buffered saline (PBS; pH 7.4) containing 0.05% Tween 20 (PBS-T; pH 7.4), then blocked with 5% non-fat dry milk in PBS-T overnight at 4 °C to reduce non-specific binding, and washed again with PBS-T. Thawed serum samples were diluted 1 : 20 with PBS-T, and 150 μl of each serum dilution was added in duplicate to the wells of the antigen-coated plates. Positive control samples (pooled sera from hamsters previously determined to have high levels of anti-KLH antibody, similarly diluted with PBS-T) and negative control samples (pooled sera from KLH-naive hamsters, similarly diluted with PBS-T) were also added in duplicate to each plate; plates were sealed, incubated at 37 °C for 3 h, then washed with PBS-T. Secondary antibody (alkaline phosphatase-conjugated anti-mouse IgG diluted 1 : 2000 with PBS-T; Cappel, Durham, NC) was added to the wells, and the plates were sealed and incubated for 1 h at 37 °C. Plates were washed again with PBS-T and 150 μl of the enzyme substrate p-nitrophenyl phosphate (Sigma Chemical, St Louis, MO; 1 mg ml−1 in diethanolamine substrate buffer) was added to each well. Plates were protected from light during the enzyme–substrate reaction, which was terminated after 20 min by adding 50 μl of 1.5 M NaOH to each well. The optical density (OD) of each well was determined using a plate reader (Bio-Rad, Benchmark; Richmond, CA) equipped with a 405 nm wavelength filter, and the mean OD for each set of duplicate wells was calculated. To minimize intra-assay variability, the mean OD for each sample was expressed as a percent of its plate positive control OD for statistical analyses.
(i) Leptin enzyme immunoassay
Serum leptin concentrations were determined in Day 10 serum samples using a single enzyme immunoassay (EIA) from a commercially prepared murine kit (TiterZyme, Assay Designs, Ann Arbor, MI). This assay was previously validated for use with Siberian hamsters (G. E. Demas, unpublished data). The antiserum used is highly specific for leptin; cross-reactivity with other cytokines is less than 0.1%. The sensitivity of the assay is 4.76 pg ml−1. Intra-assay variability was low with a coefficient of variability less than 10%.
(c) Statistical analyses
Differences among all dependent measures were determined using separate two-way (surgery×hormone treatment) analyses of variance (ANOVA) (SPSS, Chicago, IL). Post hoc comparisons between pair-wise means were conducted using Tukey-HSD tests when the overall ANOVAs were statistically significant. The correlations between serum leptin and anti-KLH IgG was determined using a Pearson product-moment test with linear regression (SPSS). In all cases, differences between group means were considered statistically significant if p<0.05.
3. Results
Serum leptin concentrations were significantly elevated in both IWATx and sham-operated hamsters treated with exogenous leptin compared with vehicle-treated hamsters (F1,29=10.53; p<0.05) (figure 1). IWATx vehicle-treated hamsters had leptin concentrations below sham-operated animals, although this difference was not statistically significant. Importantly, leptin concentrations in IWATx leptin-treated did not differ from sham-operated, vehicle-treated hamsters, suggesting that exogenous leptin treatment sufficiently mimicked control levels of the hormone (figure 1).
Figure 1.
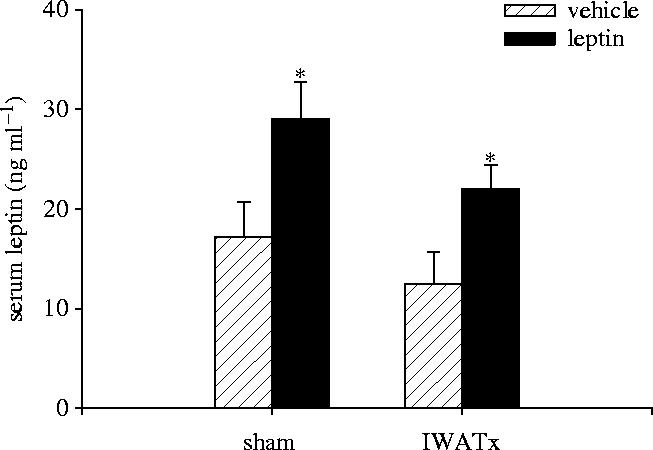
Mean (±s.e.m.) serum leptin concentrations in Siberian hamsters receiving bilateral IWAT lipectomies (IWATx), or sham surgeries (sham) and treated with exogenous leptin or vehicle alone. Significant differences between pair-wise means are indicated by an asterisk if p<0.05.
IWATx hamsters had significantly reduced anti-KLH IgG compared with sham-operated animals (F1,29=11.57; p<0.05; figure 2). IWATx hamsters treated within leptin, however, had anti-KLH IgG levels not significantly different from sham-operated animals (p>0.05). Sham-operated hamsters treated with exogenous leptin did not have significantly different serum anti-KLH IgG concentrations compared with control animals receiving vehicle (p>0.05; figure 2). Serum anti-KLH IgG was significantly correlated with serum leptin concentrations across all animals (R2=0.480; p<0.05; figure 3)
Figure 2.
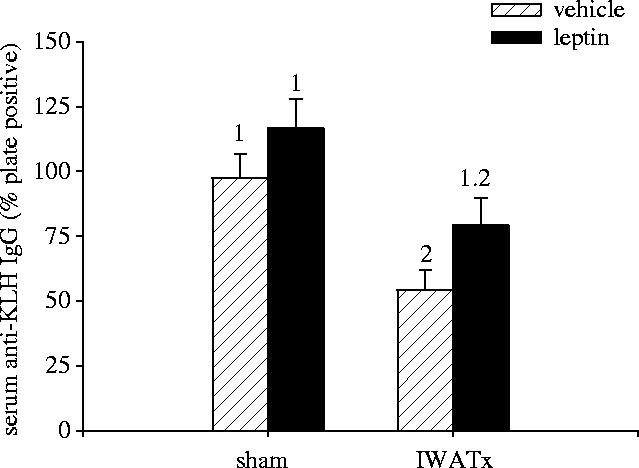
Mean (±s.e.m.) serum anti-KLH immunoglobulin G (IgG) levels in Siberian hamsters receiving bilateral IWAT lipectomies (IWATx), or sham surgeries (sham) and treated with exogenous leptin or vehicle alone. Groups with different numbers indicate statistically significant differences between groups means (p<0.05); groups sharing the same number are statistically equivalent.
Figure 3.
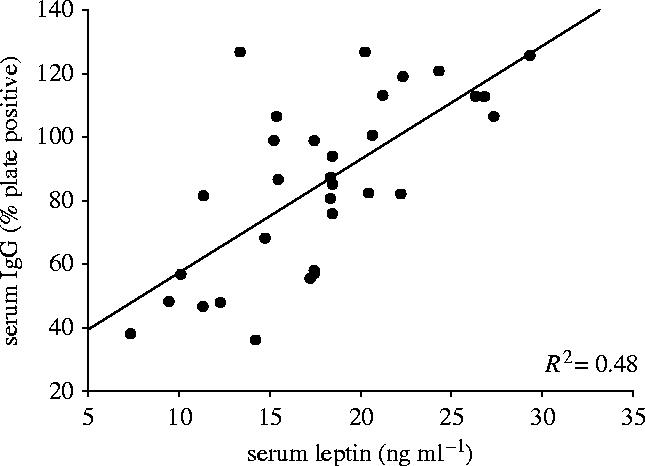
Relationship between serum leptin (ng ml−1) and anti-KLH concentrations (% plate positive) of Siberian hamsters (p<0.05).
Body masses did not differ among any of the experimental groups (p>0.05; figure 4a). There were no significant differences in EWAT or RWAT pad masses between IWATx and sham-operated animals (p>0.05), demonstrating that these WAT pads did not experience compensatory hypertrophy in response to IWATx (figure 4b). Exogenous leptin had no effect on EWAT or IWAT pad mass in any of the experimental conditions (p>0.05; figure 4b). There were no differences in splenic or paired testes masses among any of the experimental groups (p>0.05; data not shown).
Figure 4.
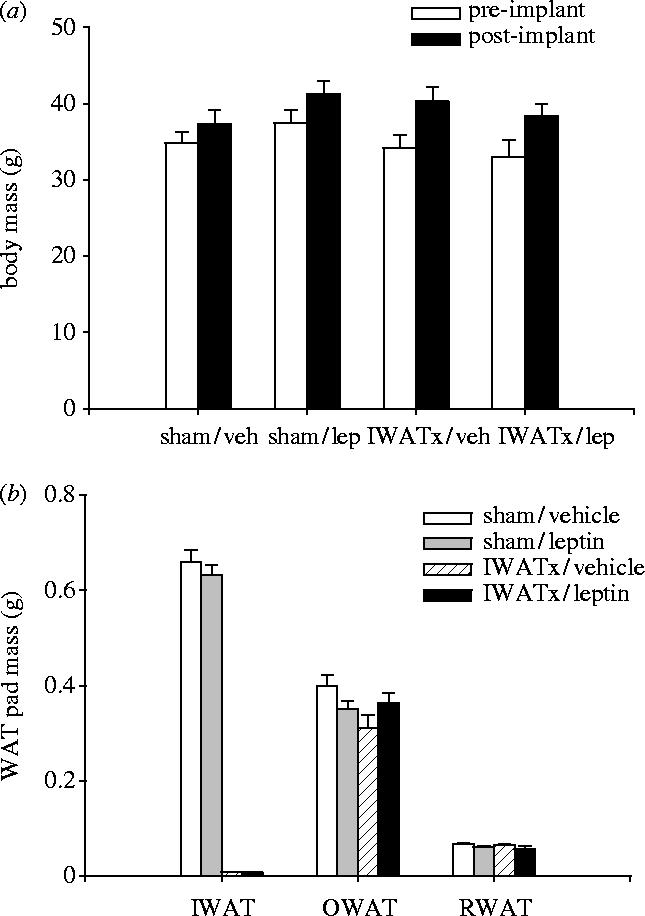
Mean (±s.e.m.) (a) body masses and (b) white adipose tissue pad masses [epididymal (EWAT), inguinal (IWAT) and retroperotoneal (RWAT)] in Siberian hamsters receiving bilateral IWAT lipectomies (IWATx), or sham surgeries (sham) and treated with exogenous leptin or vehicle alone.
4. Discussion
The results of the present study are consistent with previous studies demonstrating that moderate reductions in total body fat impair humoural immunity (Demas et al. 2003). Specifically, surgical removal of IWAT reduced humoural immunity in Siberian hamsters. Exogenous administration of the adipose tissue hormone leptin, however, attenuated the LIPx-induced decrease in antibody production, although serum anti-KLH antibodies were still below sham-operated animals. These results support previous findings that reductions in total body fat can impair humoural immunity and provide support for the hypothesis that these changes in immune function may be regulated, at least in part, by changes in serum leptin. Alternatively, it is possible that the experimental treatments employed in this study altered the timing of the immune response so that, although the absolute magnitude of the peak response did not differ, these peaks occurred on different days among the different groups. This possibility is unlikely, however, because previous research in Siberian hamsters suggests that the kinetics of the anti-KLH IgG response is unaffected by LIPx (Demas et al. 2003). Collectively, the present results suggest that leptin acts as a neuroendocrine signal communicating information about total body fat to the immune system.
One important finding of the present study is that these results suggest that adipose tissue serves as an important endocrine organ linking current energy availability with immune function. Adipose tissue probably serves at least two important physiological functions. First, adipose tissue provides storage for excess energy (food) intake in the form of triglycerides, which can be broken down to FFAs and glycerol during times of heightened energetic demands. A second, equally important but traditionally overlooked function of adipose tissue is as an endocrine organ (Ahima & Flier 2000). In fact, an increasing number of hormones have been identified as being synthesized and secreted by adipose tissue; these hormones play an important neuroendocrine role in signalling the status of current energy reserves to the brain, as well as other peripheral tissues. One important goal of the present study was to uncouple the contribution of these two potential physiological mechanisms to the energetic regulation of humoural immune responses. The present results provide initial support for the latter mechanism; leptin secreted by adipose tissue appears to play a role in the regulation of humoural immunity in that decreases in circulating leptin via LIPx impair antibody responses to KLH, whereas exogenous leptin can attenuate this effect.
It is important to note, however, that leptin treatment only partially restored impaired immune function in LIPx animals, suggesting that factors other than leptin may also play an important role in regulating immune system responses. As mentioned above, adipose tissue synthesizes and secretes a variety of substances in addition to leptin, including adiponectin, as well as a variety of cytokines (e.g. interleukin 1[IL-1]; IL-6)(Ahima & Flier 2000). Although significantly less is known regarding the precise physiological roles of these factors, it is quite plausible that they also play an important role in the interactions between the immune system and energy balance. Alternatively, several hormones of non-adipose tissue origin (e.g. insulin, amylin) have also been identified as possible adiposity signals (Woods & Seeley 2000); these hormones also serve as important endocrine candidates for the link between energy stores and immunity. Lastly, it is possible that, although a ‘false signal’ of energy stores was provided in the exogenous leptin-treated animals in the present study, the lack of an adequate amount of actual adipose tissue in LIPx animals may have prevented the full development of an antigen-stimulated antibody response. If true, this would support the idea that the availability of utilisable lipid in WAT, and not simply the endocrine correlates of body fat, is also an important factor in the development of a humoural immune response.
In common with previous reports suggesting that decreases in energy availability can impair immunity, the results of the present study support the notion of a trade-off between energy availability and immune function to the extent that immunity is compromised at times when long-term energy stores are reduced. Surgical removal of IWAT, as in the present study, typically results in a lipid deficit of approximately 10% in IWATx animals (Mauer & Bartness 1994). Because these relatively small amounts are not necessarily a substantial proportion of the total body fat of Siberian hamsters, it may be argued that partial surgical lipectomy is not a biologically meaningful manipulation of total body fat (cf Mauer & Bartness 1994). Given that these modest reductions in body fat elicited marked reductions in antibody production, we would argue that these rodents are clearly responsive to the manipulations and, thus, LIPx provides a meaningful, biologically relevant model with which to examine the interactions between total body fat and immunity. Although greater immunological impairments may occur with additional lipid deficits, these manipulations would require more invasive surgeries (e.g. removal of more internally located fat pads) that could possibly compromise survival of the animals.
The precise mechanisms by which leptin mediates changes in immune function are not known; however, one likely candidate is via the action of this hormone on the sympathetic nervous system (SNS). Although many of the effects of leptin on the regulation total body fat are due to the effects of this hormone on food intake, an increasing number of studies suggest that the actions of leptin on energy balance are due, at least in part, to activation of the SNS and subsequent increases in metabolism (Mizuno et al. 1998; Scarpace et al. 2000; Elmquist 2001; Rayner 2001). Recently, it has been demonstrated that, similar to leptin's role in regulating energy balance, the effects of leptin on immune function may also involve the SNS (Okamoto et al. 2000). Specifically, intracerebroventricular (i.c.v.) administration of leptin to mice reduces splenocyte proliferation in response to the T-cell mitogen concanavalin A (Con A). This effect, however, is abolished by surgical denervation of the spleen (Okamoto et al. 2000).
More recently, we evaluated the role of sympathetic denervation of lymphoid tissue in exogenous leptin-induced increases in humoural immunity in Siberian hamsters (Demas 2002). Specifically, hamsters were housed in long or short days and half of the animals in each photoperiod were administered exogenous leptin via osmotic minipumps. Half of the animals in each hormonal condition received surgical sympathetic denervation of the spleen (Demas 2002) whereas the remaining animals were left intact. As previously reported (Drazen et al. 2000) anti-KLH antibodies were reduced in short days and the short-day reduction in humoural immunity was reversed by exogenous leptin administration. Surgical denervation of the spleen, however, attenuated the immune-enhancing properties of exogenous leptin and short-day denervated hamsters receiving leptin displayed suppressed immunity comparable to short-day control animals not receiving the hormone (Demas 2002). Because more than 95% of the efferent innervation of the spleen is sympathetic in origin (Elenkov et al. 2000) the results of these studies are consistent with the idea that leptin acts via the SNS to mediate immune responses. Alternatively, it is possible that leptin affects immune response via direct actions of the hormone on lymphoid tissues. In support of this idea, leptin receptors have been identified on circulating lymphocytes (Martin-Romero et al. 2000) and exogenous leptin treatment can affect in vitro immune responses (Lord et al. 1998; Martin-Romero et al. 2000).
The results of the present study suggest that modest reductions in energy availability in the form of reduced body fat can reduce humoural immunity and that the effects of body fat on immune function are mediated, at least in part, by circulating leptin. Given that changes in immunity can have marked consequences for disease resistance, theses findings support the idea that reduced energy availability can lead to increased disease susceptibility in a variety of mammalian species. Although this idea is intriguing, not all changes in immunity correlate with changes in disease susceptibility (Adamo 2004); thus, future studies that employ experimental pathogens will be required to test this idea. The present results provide an important step towards an understanding of the neuroendocrine mechanisms by which mammalian immunity is regulated by energy availability.
Acknowledgments
The authors acknowledge Amber Cravens and Tracy Estes and for expert animal care and Dr Lance Kriegsfeld for helpful comments on the manuscript. This study was presented in preliminary form at the annual meeting of the Society for Behavioural Neuroendocrinology (SBN) in July 2004. This research was supported in part by a North American Association for the Study of Obesity (NAASO) Young Investigator grant and by Indiana University.
References
- Adamo S.A. How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 2004;68:1443–1449. [Google Scholar]
- Ahima R.S, Flier J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Ardawi M.S.M, Newsholme E.A. Metabolism in lymphocytes and its importance in the immune response. Essays Biochem. 1985;21:1–43. [PubMed] [Google Scholar]
- Bartness T.J, Wade G.N. Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci. Biobehav. Rev. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Bronson F, Heideman P.D. Seasonal regulation of reproduction in mammals. In: Knobil K, Neill J.D, editors. The physiology of reproduction. Raven Press; New York: 1994. pp. 541–584. [Google Scholar]
- Chandra R.K. Nutrition, immunity and infection: from basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc. Natl Acad. Sci. USA. 1996;93:14 304–14 307. doi: 10.1073/pnas.93.25.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002;56(Suppl. 3):73–76. doi: 10.1038/sj.ejcn.1601492. [DOI] [PubMed] [Google Scholar]
- Dark J, Forger N.G, Stern J.S, Zucker I. Recovery of lipid mass after removal of adipose tissue in ground squirrels. Am. J. Physiol. 1985;249:R73–R78. doi: 10.1152/ajpregu.1985.249.1.R73. [DOI] [PubMed] [Google Scholar]
- Demas G.E. Splenic denervation blocks leptin-induced enhancement of humoral immunity in Siberian hamsters (Phodopus sungorus) Neuroendocrinology. 2002;76:178–184. doi: 10.1159/000064527. [DOI] [PubMed] [Google Scholar]
- Demas G.E. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 2004;43:75–80. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Demas G.E, Chefer V, Talan M.I, Nelson R.J. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Demas G.E, Drazen D.L, Nelson R.J. Reductions in total body decrease humoral immunity. Proc. R. Soc. B. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. 10.1098/rspb.2003.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen D.L, Kriegsfeld L.J, Schneider J.E, Nelson R.J. Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am. J. Physiol. 2000;278:R1401–R1407. doi: 10.1152/ajpregu.2000.278.6.R1401. [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Demas G.E, Nelson R.J. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus) Endocrinology. 2001;142:2768–2775. doi: 10.1210/endo.142.7.8271. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J, Wilder R.L, Chrousos G.P, Vizi E.S. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Elmquist J.K. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol. Behav. 2001;74:703–708. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Feingold K.R, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? OIKOS. 2000;88:87–98. [Google Scholar]
- Lord G.M, Matarese G, Howard J.K, Baker R.J, Bloom S.R, Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- Mauer M.M, Bartness T.J. Body fat regulation after partial lipectomy in Siberian hamsters is photoperiod dependent and fat pad specific. Am. J. Physiol. 1994;266:R870–R878. doi: 10.1152/ajpregu.1994.266.3.R870. [DOI] [PubMed] [Google Scholar]
- Mauer M.M, Bartness T.J. Fat pad-specific compensatory mass increases after varying degrees of lipectomy in Siberian hamsters. Am. J. Physiol. 1997;273:R2117–R2123. doi: 10.1152/ajpregu.1997.273.6.R2117. [DOI] [PubMed] [Google Scholar]
- Mauer M.M, Harris R, Bartness The regulation of total body fat: lessons learned from lipectomy studies. Neurosci. Biobehav. Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Murakami T, Otani S, Kuwajima M, Shima K. Leptin affects pancreatic endocrine functions through the sympathetic nervous system. Endocrinology. 1998;139:3863–3870. doi: 10.1210/endo.139.9.6201. [DOI] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E. Seasonal changes in immunity. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Fine J.B, Demas G.E, Moffatt C.A. Photoperiod and population density interact to affect reproductive and immune function in male prairie voles. Am. J. Physiol. 1996;270:R571–R577. doi: 10.1152/ajpregu.1996.270.3.R571. [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E, Klein S.L, Kriegsfeld L.K. Cambridge University Press; 2002. Seasonal patterns of stress, immune function and disease. [Google Scholar]
- Norris K, Evans M.R. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. [Google Scholar]
- Nova E, Samartin S, Gomez S, Morande G, Marcos A. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur. J. Clin. Nutr. 2002;56(Suppl. 3):34–37. doi: 10.1038/sj.ejcn.1601482. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Irie Y, Ishikawa I, Kimura K, Saito M. Central leptin suppresses lymphocyte functions through activation of the corticotrophin-releasing hormone-sympathetic nervous system. Brain Res. 2000;855:192–197. doi: 10.1016/s0006-8993(99)02409-9. [DOI] [PubMed] [Google Scholar]
- Pond C. Interactions between adipose tissue and the immune system. Proc. Nutr. Soc. 1996;55:111–126. doi: 10.1079/pns19960014. [DOI] [PubMed] [Google Scholar]
- Rayner D.V. The sympathetic nervous system in white adipose tissue regulation. Proc. Nutr. Soc. 2001;60:357–364. doi: 10.1079/pns2001101. [DOI] [PubMed] [Google Scholar]
- Scarpace P.J, Matheny M, Moore R.L, Kumar M.V. Modulation of uncoupling protein 2 and uncoupling protein 3: regulation by denervation, leptin and retinoic acid treatment. J. Endocrinol. 2000;164:331–337. doi: 10.1677/joe.0.1640331. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. TREE. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Sinclair J.A, Lochmiller R. The winter immunoenhancement hypothesis: associations among immunity, density, and survival in prairie vole (Microtus ochrogaster) populations. Can. J. Zool. 2000;78:254–264. [Google Scholar]
- Woods S.C, Seeley R.J. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16:894–902. doi: 10.1016/s0899-9007(00)00454-8. [DOI] [PubMed] [Google Scholar]
- Yellon S.M, Teasley L.A, Fagoaga O.R, Nguyen H.C, Truong H.N, Nehlsen-Cannarella L. Role of photoperiod and the pineal gland in T cell-dependent humoral immune reactivity in the Siberian hamster. J. Pineal Res. 1999;27:243–248. doi: 10.1111/j.1600-079x.1999.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160(Suppl.):9–22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]


