Abstract
We investigated the relative importance of dispersal and vicariance in forming the Madagascar insect fauna, sequencing approximately 2300 bp from three rRNA gene regions to investigate the phylogeny of Afrotropical small minnow mayflies (Ephemeroptera: Baetidae). Six lineages contained trans-oceanic sister taxa, and variation in genetic divergence between sister taxa revealed relationships that range from very recent dispersal to ancient vicariance. Dispersal was most recent and frequent in species that spend the larval stage in standing water, adding to evidence that these evolutionarily unstable habitats may select for ecological traits that increase dispersal in insects. Ancestral state likelihood analysis suggested at least one Afrotropical lineage had its origin in Madagascar, demonstrating that unidirectional dispersal from a continental source may be too simplistic. We conclude that the Malagasy mayfly fauna should be considered in a biogeographical context that extends beyond Madagascar itself, encompassing trans-oceanic dispersal within multiple lineages.
Keywords: Africa, ancestral state, biogeography, dispersal, genetic, phylogeny
1. Introduction
Madagascar is one of the most biologically diverse areas on Earth and is well recognized as a discrete, globally important centre of evolution (Goodman & Benstead 2003; de Wit 2003). While large parts of the fauna and flora have evolved over more than 80 million years of isolation from all other landmasses (Lourenço 1996; Goodman & Benstead 2003), an increasing number of studies highlight the important impact of more recent colonizations (Cibois et al. 1999; Douady et al. 2002; Nagy et al. 2003; Yoder et al. 2003; Sparks 2004). In particular, recent molecular phylogenetic studies of reptiles (Raxworthy et al. 2002; Vences et al. 2003) and birds (Jansa et al. 1999; Groombridge et al. 2002) have begun to establish a closer link of Malagasy lineages with groups elsewhere, where trans-marine migrations occur within geographically widespread radiations. To date, these studies have been confined to vertebrates.
Insects comprise a large proportion of faunal biodiversity in Madagascar (Paulian & Viette 2003). Insects are highly diverse in their ecological attributes affecting dispersal propensity, and therefore their ability to colonize islands, but we are aware of no formal studies of the age and origin of Malagasy insect groups. The global biogeography of many insect groups is attributed to vicariance processes (e.g. Gauld & Wahl 2002; Sanmartin & Ronquist 2004). Single dispersal events are sometimes invoked to link speciose allopatric lineages, e.g. Tricoptera (Johanson 1998) and families of Coleoptera (Sequeira & Farrell 2001; Davis et al. 2002), but wide-ranging and repeated dispersal is thought to occur for only the more vagile groups, such as Lepidoptera (de Jong 2003; Zakharov et al. 2004). Nonetheless, many Malagasy insect groups are taxonomically more similar to Africa than Asia or Australia (e.g. Cassola 2003; Donnelly & Parr 2003). This suggests that geographical proximity and trans-oceanic dispersal may be an important determinant of the Malagasy insect fauna.
Mayflies (Ephemeroptera) are well suited for biogeographical studies because of their ancient origins, global distribution, limited dispersal powers and strict larval habitat affinity (Sartori et al. 2000). Mayfly fossils date from the Carboniferous (ca 300 Ma; Hubbard & Kukalova-Peck 1980), thus pre-dating Gondwanan vicariance. Their global diversification has been thought to be the result of ancient continental separations (Edmunds 1972, 1975), including their presence on islands (e.g. Gerlach 2001). Long before theories of continental drift were well established, the presence of mayflies on the Seychelles was taken as evidence that the islands were of continental origin (see Scott 1932; Perkins 1933). Mayfly dispersal is thought to be very limited (Brittain 1982). A number of studies of small minnow mayflies (Baetidae) suggest that dispersal is largely limited to the same or nearby water bodies (Hershey et al. 1993; Monaghan et al. 2002; Caudill 2003; Hughes et al. 2003). This is partly due to the fact that most species live only a few hours as winged adults, relying for energy in the adult stage entirely on reserves built up during the larval stage. However, some ovoviviparous females may live up to two weeks in the adult phase (Gillies 1949). Larvae of most species are restricted to either lentic (e.g. lakes, ponds) or lotic (streams, rivers) freshwaters.
For the mayflies, taxonomic similarity among former parts of Gondwanaland appears more related to present-day geographical distance rather than to vicariant history (Sartori et al. 2000). Of 24 Baetidae genera in Madagascar, 16 are shared with Africa (separated 165 Ma). Of these 16 shared genera, only three occur in India (separated 88 Ma), and two in Australia (separated 110 Ma) (see Gattolliat & Sartori 2003). All species of mayfly in Madagascar are endemic, except for Cloeon smaeleni (Gattolliat & Rabeantoandro 2002; Elouard et al. 2003). The Malagasy fauna of mayflies therefore constitutes a puzzle in that affinities with the African continent are apparent, but their seemingly poor dispersal ability would argue against trans-ocean exchange. Testing the origin and relatedness of Malagasy lineages, and their sister relationships elsewhere, would be a major step towards the understanding of global diversity of the Ephemeroptera.
Here we investigate the relative importance of trans-oceanic exchange, vicariance and endemic radiations in the formation of the Malagasy fauna, using a group of insects thought to have limited powers of dispersal. We conducted a phylogenetic analysis of all major Malagasy groups of Baetidae and their African counterparts, to include representatives of 26 genera. Our specific goals were to determine the number of lineages shared between Madagascar and Africa and whether lineage origins could be attributed to either area. The results show a complex pattern, from very recent dispersal to events of ancient vicariance, and indicate that a traditional scenario of unidirectional dispersal from a continental source is too simplistic.
2. Material and methods
(a) Sampling
Of the 24 Malagasy Baetidae genera, eight are endemic, 13 are restricted to the Afrotropical region, one is pantropical (Cloeodes) and two are cosmopolitan (Labiobaetis/Pseudocloeon and Cloeon) (Gattolliat & Sartori 2003). We focussed on 10 genera found in both Madagascar and Africa, and on six Malagasy endemic genera. Ten additional Afrotropical genera, as well as the Palaearctic and Afrotropical Baetis were added to the analysis to examine basal relationships among lineages of Baetidae. Labiobaetis/Pseudocloeon from Borneo and New Guinea were included in the analysis, as were Cloeon from Europe. Samples were collected in May–June 2003 by the authors or taken from collections of the Museum of Zoology in Lausanne. DNA was extracted from thoracic muscles using a Qiagen Dneasy Tissue Kit. Mitochondrial 12S and 16S ribosomal subunits were amplified using primers 12Sai and 12Sbi (Simon et al. 1994), and 16Sar (Simon et al. 1994) and 16S2 (Giessler et al. 1999). Two fragments of nuclear 18S rRNA were amplified using 18S5′, 18Sb5.0, and 18S1.0, 18Sbi, 18S2.0, and 18S3′ (Whiting et al. 1997; Shull et al. 2001). Both strands were sequenced using PCR primers and analysed with an ABI 3700 automated sequencer. For the choice of out-group taxa, molecular evidence to date indicates Baetidae are the sister taxon to all mayflies, and that Odonata are the most closely related extant insects (Hovmöller et al. 2002; Ogden & Whiting 2003). Thus, four mayfly species from the family Tricorythidae were analysed for this study and sequences from the dragonfly Libellula saturata (Odonata, Libellulidae) were taken from Genbank (accession numbers: 12S AY282562; 16S AF037181; 18S AY338717). Multiple individuals of 38 in-group species were sequenced to test for errors, contamination, and mislabelling, but removed from final phylogenetic analysis. All specimens were given a unique number for the study and extracted DNA is stored at the Natural History Museum, London in the frozen collection database (BMNH 704056–704132 and BMNH 704630–704678).
(b) Phylogenetic Analysis
All four gene fragments were length-variable (two 18S fragments combined: 1444–1449 bp; 12S: 326–338 bp; 16S: 498–510 bp) and thus we used three general approaches to phylogeny reconstruction: direct optimization as implemented in Poy v. 3.0.11 (Gladstein & Wheeler 1999) and parsimony and maximum likelihood searches of static multiple alignments. For direct optimization, an initial alignment was performed manually for 18S regions and the sequences were separated into one conserved fragment and two variable fragments for ease of analysis. Poy searches were performed under equal weight of all character changes including indels, conducting 10 replicates and holding a maximum of 10 trees each replicate. Alternative gap cost parameters were explored with no substantial effects on topology. Complete command lines and implied alignments can be obtained from the corresponding author. Bremer support was calculated using a heuristic procedure implemented in Poy on the best output tree.
For parsimony and likelihood searches, we first examined gap opening penalties (from 1 to 15) for congruence of length-variable regions using an incongruence length difference (ILD) test (Farris et al. 1994, 1995). Multiple alignments were assembled using ClustalW (using web servers provided by Major Linux and Institut Pasteur). Tree searches were conducted with Paup* v. 4.0b10 (Swofford 2002) using random addition sequences, 1000 replicates and gaps coded as a fifth character state, holding 50 trees at each replicate. The tree with lowest ILD was found using gap penalties of 10 for all three ribosomal markers. Parsimony tree searches on this alignment (2385 characters; 12S: 365 bp, 16S: 554 bp, 18S: 1466 bp) were conducted with Paup* as above, with the multi-trees option. Congruence of mtDNA and nDNA markers was examined by comparing each partition with the total evidence tree. Mapped on the total evidence tree, mtDNA tree length increased by 0.1% (from 7076 to 7084) and nDNA by 7% (597–641), and there was no incongruence within the seven well supported lineages (see below). Based on these results, we present only the total evidence tree. Data were bootstrapped (1000 replicates) with Paup*. Inferred indels were treated as distinct character states (Phillips et al. 2000), but coding them as missing data had no effect on tree topologies within well supported lineages. We constructed a maximum likelihood topology under a GTR+I+G model (selected in Modeltest 3.06; Posada & Crandall 1998) with all parameters estimated from the data using Phyml (Guindon & Gascuel 2003).
(c) Ancestral state likelihood
Using likelihood methods (Pagel 1999; Belshaw et al. 2000), we estimated the ancestral state for each well resolved clade joining African–Malagasy sister taxa. Geographic area was treated as a multi-state character (Africa, Madagascar, Seychelles, Asia, Europe) and we used Multistate v. 0.8 to calculate tree likelihoods (10 replicates) with single nodes fixed at either Africa or Madagascar as a state. Using the highest log-likelihood calculated for each state, differences greater than 2.0 were considered significant (Schluter et al. 1997), with the higher likelihood considered to be the most well supported ancestral state.
3. Results
(a) Phylogenetic analysis
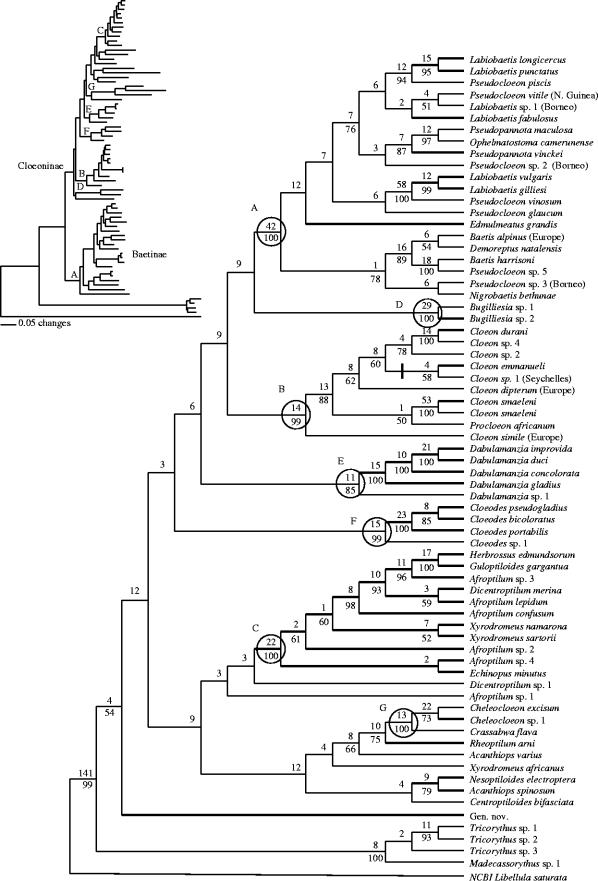
Direct optimization produced a single tree (figure 1). This topology differed from that of the shortest parsimony tree search on static alignments by only a single node within the Cloeon lineage (clade B, figure 1). Six well supported clades (figure 1, clades A, B, D, E, F, G) contained both Malagasy and African taxa and a seventh (figure 1, clade C) was composed entirely of Malagasy species. Support for deeper nodes within the tree was weaker than at the tips, with very low support among the seven major clades. Maximum likelihood recovered the same seven lineages with only a single change within clade B: Afrotropical Cloeon was monophyletic, with Procloeon and the two European Cloeon species basal to the Afrotropical clade (figure 2b). The likelihood topology was different from direct optimization and parsimony at deeper nodes, notably by separating Baetidae into two basal sister groups (see figure 1 inset).
Figure 1.
Phylogenetic reconstruction of Afrotropical Baetidae based on the single resulting tree from direct optimization of 12S, 16S, and two 18S rRNA gene regions using Poy. The vertical bar indicates the alternate placement of Cloeon sp. 2 using parsimony reconstruction. Values above branches indicate Bremer Support and values below branches indicate parsimony bootstrap percentage (if above 50%). Letters A–G indicate well supported lineages (see text). Branches are thickened for Malagasy species and the origin of non-Afrotropical taxa is in parentheses. The maximum likelihood topology is summarized in the upper left, with the corresponding lineages marked.
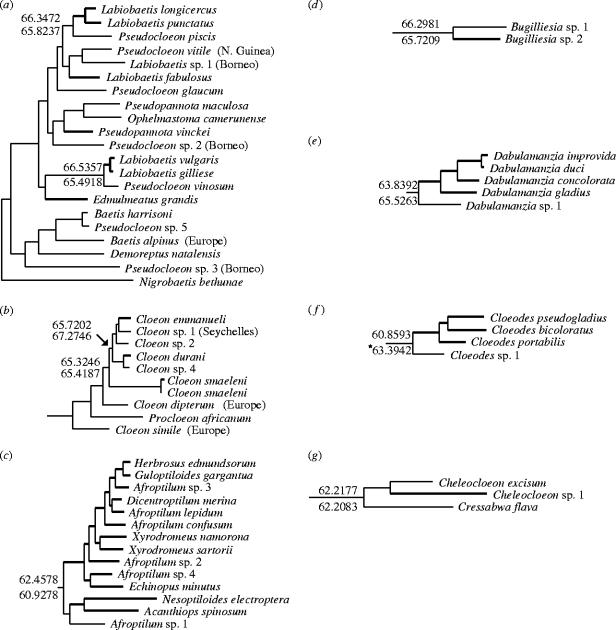
Figure 2.
Likelihood tree topologies, branch lengths and ancestral state reconstructions for the seven well supported lineages within Afrotropical Baetidae (a--g corresponding to clades A--G in figure 1), taken from the maximum likelihood tree. Branch thickness and letters corresponding to nodes are as in figure 1. Ancestral state likelihoods are shown for Africa (above branch leading to node if interest) and Madagascar (below branch). Significantly higher likelihood is marked by asterisk.
The Labiobaetis/Pseudocloeon/Baetis lineage (clade A) was the most geographically widespread and our results highlight the taxonomic uncertainty of the group. Historically, adults with no hind wing and double intercalary veins on the forewing were assigned to the cosmopolitan genus Pseudocloeon. Waltz & McCafferty (1985) restricted the concept of Pseudocloeon to the type species, and Lugo-Ortiz et al. (1999) assigned all Labiobaetis to Pseudocloeon. Malagasy species described subsequently were assigned to Labiobaetis (Gattolliat 2001a). To avoid confusion, we hereafter refer to this lineage as Labiobaetis/Pseudocloeon. African and Malagasy members of Labiobaetis/Pseudocloeon appeared polyphyletic, with each well supported clade containing two Malagasy and one African species. Asian (New Guinea, Borneo) Labiobaetis/Pseudocloeon clustered together weakly but were not supported as sister to an Afrotropical clade. Likelihood branch lengths suggest a wide range of genetic divergence between over-ocean sister taxa within the group (figure 2a).
The Cloeon and Procloeon lineage (clade B, figure 1) displayed three clear instances of trans-oceanic sister relationships, including very closely related C. smaeleni in both Africa and Madagascar. For the taxa studied, Africa–Madagascar divergence, based on branch length differences, appeared less than Seychelles–Madagascar divergence (figure 2). This pattern does not correspond with geological age (165 Ma and 65–80 Ma, respectively). A third major clade (clade C) consisted entirely of Malagasy species. Eight Malagasy species of Afroptilum, Dicentroptilum and Xyrodromeus occurred within clade C, and the fact that their African congeners occurred in other clades makes these genera paraphyletic. The four smaller lineages (figure 1, clades D, E, F, G) each contained African and Malagasy sister taxa. Malagasy species within these clades always were monophyletic, and branch lengths revealed a wide range of trans-oceanic genetic divergence (figure 2).
(b) Ancestral distribution
Ancestral state reconstructions were conducted separately for nine nodes within the likelihood tree where species from Madagascar and Africa occurred in lineages (figure 2). Likelihood estimations testing for a significantly higher probability for Africa or Madagascar as the ancestral state found that only the Cloeodes (clade F) lineage clearly discriminated between both possibilities, with Madagascar as the more likely ancestral state (figure 2). No significant differences were found between likelihoods in analyses of the remaining lineages.
4. Discussion and conclusions
Most species of Baetidae in Madagascar could be grouped into seven well supported lineages. One lineage was entirely endemic, four were Afrotropical (i.e. composed of Malagasy and African mainland species), and two included Asian and European species. Phylogenetic support was inconclusive for basal relationships among the seven lineages; nonetheless, a number of inconsistencies with proposed species complexes are clear from the data. Bugilliesia (Lugo-Ortiz & McCafferty 1996), Centroptiloides (Lugo-Ortiz & McCafferty 1998a) and Cloeodes (Lugo-Ortiz & McCafferty 1998b) species complexes were all polyphyletic based on our molecular reconstruction. Likelihood analysis recovered two major lineages within the family and provided support for the hypothesis that the Afrotropical Baetidae is composed of two subfamilies, Baetinae and Cloeoninae (Gillies 1991). The inclusion of Asian and European taxa in these two proposed subfamilies indicates they may represent a deep subdivision of the Baetidae globally. Several genera were polyphyletic, suggesting that taxonomic revision is needed. This was particularly the case for the Labiobaetis/Pseudocloeon lineage and for species within the Malagasy endemic lineage that are assigned to African genera (see below).
The phylogenetic reconstruction suggests that the Malagasy Baetidae fauna is not the result of simple vicariance or unidirectional mainland–island dispersal. In many instances, Malagasy species had closest relatives in Africa, and clades also included closely related Asian species (e.g. within clade A). The large number of trans-oceanic sister groups and the wide range of genetic relatedness is strong evidence that Madagascar is part of a larger geographical network of lineage evolution and exchange that includes the African continent (sensu Raxworthy et al. 2002), Indian Ocean Islands (Seychelles in our study; see Groombridge et al. 2002; Vences et al. 2003) and southern Asia. The wide range of genetic divergence between African and Malagasy sister taxa (estimated from likelihood branch length) indicates a lack of synchrony in divergence events and shows that trans-oceanic exchange has occurred repeatedly.
It may be appropriate to consider the evolution of the Malagasy insect fauna in a biogeographical context that extends beyond Madagascar itself, and that trans-oceanic dispersal may be more common than thought for a broad range of insects (Trewick 2000). Zakharov et al. (2004) recently discussed genetic evidence that the highly vagile swallowtail butterflies (Papilio spp.) have made repeated trans-oceanic dispersal. Our estimates of ancestral states were largely inconclusive with regard to the origin of Malagasy lineages, except for Cloeodes, which our results indicate to be ancestrally a Malagasy lineage with descendents now confined to Africa. Further sampling of African taxa is required to confirm such a result (Emerson 2002), but it casts doubt on any hypothesis of unidirectional, mainland–island dispersal. The widespread nature of several lineages also requires a more thorough sampling and assessment of the Indian subcontinent and Asia as potential ancestral areas.
For the two most widespread lineages (Cloeon and Labiobaetis/Pseudocloeon), close trans-oceanic sister relationships preclude any significant substructure within the Afrotropical region, including Madagascar. For example, the very small genetic difference between trans-oceanic C. smaeleni, and its wide sub-Saharan and southern Arabian distribution (Gillies 1985) suggest a large, continuous range and frequent dispersal, even across the ocean. Species of Cloeon possess several attributes that may act alone or in combination to increase their dispersal success and therefore their range size. Eggs can reach complete development in the adult female and hatch upon contact with water (Gillies 1949), and unmated females can produce fully reproductive offspring (Harker 1997). Larvae can tolerate periods of anoxia and unusually high water temperature (e.g. Nagell 1980) as well as high salinity (Forbes 1968; Forbes & Allanson 1970). Not all species possess all of these characteristics, but singly or in combination, these traits are likely to increase the success of active or passive long-distance dispersal. We are unaware of any such mechanisms in Labiobaetis/Pseudocloeon, although some data suggest that the specific habitat type in which larvae live may be related to their range size (see below).
Only in one case (clade C) was a major radiation entirely composed of species endemic to Madagascar. It is an ecologically important group, because one or more members of this clade often are present in high abundance (e.g. Benstead et al. 2003) and members of the group are found in most rivers in Madagascar (authors', personal observation). Interestingly, every feeding behaviour known for Baetidae (collector–gatherer, scraper, predator) occurred in this lineage except for detritus ‘shredding,’ which is poorly represented in the tropics generally (Dudgeon 1999; Dobson et al. 2002). This lineage is most likely the result of a radiation from an ancestor that was a collector–gatherer such as Afroptilum. Both Malagasy Xyrodromeus exhibit a high degree of convergence to African Xyrodromeus africanus, with specialized mouthparts for scraping epilithic algae (Gattolliat & Sartori 2003). This character is also present in several other Malagasy genera outside of this lineage, including all species of Rheoptilum and Scutoptilum (Gattolliat 2001b, 2002), and in a single species each of Cloeodes and Dabulamanzia (Gattolliat & Sartori 2000; Gattolliat 2001c), indicating it has evolved repeatedly. Interestingly, Malagasy Herbrossus and Guloptiloides are predators, a relatively rare life strategy among mayflies (Gattolliat & Sartori 2001). These were recovered as sister taxa in our analysis, and members of the endemic clade; however, predation was paraphyletic in Madagascar based on the phylogenetic position of a third predator, Nesoptiloides. A fourth, African carnivore (Centroptiloides) appeared within yet another lineage, strongly suggesting predation has evolved independently several times.
The spatial scale of lineage evolution within the Baetidae supports the hypothesis that habitat type is an important predictor of aquatic insect range size (Ribera & Vogler 2000). For aquatic beetles (Coleoptera), Ribera et al. (2001, 2003) found that standing-water (lentic) species had larger ranges than species living in running waters, and hypothesized that standing water bodies of the size inhabited by most insects are short-lived at the scale of decades, and hence long-term persistence of populations is only possible through dispersal. Consistent with these predictions, the predominantly lentic clade of Cloeon was least structured geographically, and hence the most dispersive lineage in our study. Labiobaetis/Pseudocloeon was the only other lineage with closely related trans-oceanic sister taxa. Interestingly, larval Labiobaetis/Pseudocloeon species live in running waters, but many species are confined to slow-moving sections of rivers and are found in aquatic vegetation where water movement is slow (personal observation). Based on this finding, we hypothesize that the two other standing-water Afrotropical genera, Demoulinia and Potamocloeon (Gattolliat 2003), also have undergone recent trans-oceanic dispersal events.
In conclusion, our study contributes to recent evidence that dispersal has greatly affected the faunal composition of Madagascar, and proposes that the geographical extent of lineages may be predicted by ecological traits of organisms that are principally determined by their habitat type. The results demonstrate the high vagility of insects, even for mayflies (e.g. Johnson 1969), whose brief winged phase and strict habitat fidelity would seem to prohibit trans-oceanic dispersal (Brittain 1982; Brittain & Sartori 2003). It is also evident that dispersal is not necessarily unidirectional; our results show a high likelihood that one lineage originated in Madagascar, and an equal likelihood of a Malagasy or African origin for six other lineages. With such regularities in phylogenetic and biogeographical patterns emerging (e.g. Raxworthy et al. 2002), the challenge is now to determine what factors may promote dispersal. Mayflies associated with ponds and small standing water bodies had the widest range and showed the most recent, and presumably most frequent, trans-oceanic exchange, adding to a growing body of evidence that strategies for survival in evolutionarily unstable habitats may select for greater dispersal abilities in any taxonomic group (Ribera et al. 2001). As a predictive framework for identifying deeply separated, endemic lineages from those of more recent origin, habitat affinity also could be broadly used to set conservation priorities in the endangered fauna of Madagascar.
Acknowledgments
Research was funded by the Museum of Zoology in Lausanne, the UK Biotechnology and Biological Sciences Research Council and by a Swiss National Science Foundation fellowship (no. 68592) to M.T.M. We wish to thank Joseph Rakotonarivo, Gabrielle Randria, Sophie Z. Rabeantoandro, Ranalison Oliarinony and Francois Jarrige at IRD in Antananarivo. We thank staff of the Kruger National Park, in particular Hendrik Sithole, Bruce Leslie, Velly Ndlouy, Sipho Mokgalaka and Thomas Ndoy. We are also indebted to Mick Angliss, Limpopo Province Department of Environmental Affairs, and Stephan Foord, University of Venda for guiding us to suitable collecting sites within the Limpopo Province, South Africa. Valuable specimens were kindly provided by Mike Dobson (East Africa), Justin Gerlach (Seychelles), and Katayo Sagata (New Guinea). We thank Joan Pons for help with data analysis and comments from Michael Balke and three anonymous reviewers improved the manuscript.
References
- Belshaw R, Dowton M, Quicke D.L.J, Austin A.D. Estimating ancestral geographical distributions: a Gondwanan origin for aphid parasitoids? Proc. R. Soc. B. 2000;267:491–496. doi: 10.1098/rspb.2000.1027. 10.1098/rspb.2000.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benstead J.P, Douglas M.M, Pringle C.M. Relationships of stream invertebrate communities to deforestation in eastern Madagascar. Ecol. Appl. 2003;13:1473–1490. [Google Scholar]
- Brittain J.E. Biology of mayflies. Annu. Rev. Entomol. 1982;27:119–147. [Google Scholar]
- Brittain J.E, Sartori M. Ephemeroptera. In: Resh V.H, Cardé R.T, editors. Encyclopedia of insects. Academic Press; Amstrerdam: 2003. pp. 373–380. [Google Scholar]
- Cassola F. Coleoptera: Cicindelidae, tiger beetles (Studies of tiger beetles CXI) In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago Press; 2003. pp. 669–677. [Google Scholar]
- Caudill C.C. Measuring dispersal in a metapopulation using stable isotope enrichment: high rates of sex-biased dispersal between patches in a mayfly metapopulation. Oikos. 2003;101:624–630. [Google Scholar]
- Cibois A, Pasquet E, Schulenberg T.S. Molecular systematics of the Malagasy babblers (Passeriformes: Timaliidae) and warblers (Passeriformes: Sylviidae), based on cytochrome b and 16S rRNA sequences. Mol. Phylogenet. Evol. 1999;13:581–595. doi: 10.1006/mpev.1999.0684. 10.1006/mpev.1999.0684 [DOI] [PubMed] [Google Scholar]
- Davis A.L.V, Scholtz C.H, Philips T.K. Historical biogeography of scarabaeine dung beetles. J. Biogeogr. 2002;29:1217–1256. [Google Scholar]
- de Jong R. Are there butterflies with Gondwanan ancestry in the Australian region? Invertebr. Syst. 2003;17:143–156. [Google Scholar]
- de Wit M.J. Madagascar: heads it's a continent, tails it's an island. Annu. Rev. Earth Planet. Sci. 2003;31:213–248. [Google Scholar]
- Dobson M, Magana A, Mathooko J.M, Ndegwa F.K. Detritivores in Kenyan highland streams: more evidence for the paucity of shredders in the tropics? Freshw. Biol. 2002;47:909–919. [Google Scholar]
- Donnelly T.W, Parr M.J. Odonata, dragonflies and damselflies. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago; 2003. pp. 645–654. [Google Scholar]
- Douady C.J, Catzeflis F, Kao D.J, Springer M.S, Stanhope M.J. Molecular evidence for the monophyly of tenrecidae (mammalia) and the timing of the colonization of Madagascar by Malagasy tenrecs. Mol. Phylogenet. Evol. 2002;22:357–363. doi: 10.1006/mpev.2001.1055. 10.1006/mpev.2001.1055 [DOI] [PubMed] [Google Scholar]
- Dudgeon D. Hong Kong University Press; Hong Kong: 1999. Tropical Asian streams: zoobenthos, ecology and conservation. [Google Scholar]
- Edmunds G.F. Biogeography and evolution of Ephemeroptera. Annu. Rev. Entomol. 1972;17:21–42. [Google Scholar]
- Edmunds G.F. Phylogenetic biogeography of mayflies. Ann. Mo. Bot. Gard. 1975;62:251–263. [Google Scholar]
- Elouard J.-M, Gattolliat J.-L, Sartori M. Ephemeroptera, mayflies. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago Press; 2003. pp. 639–645. [Google Scholar]
- Emerson B.C. Evolution on oceanic islands: molecular phylogenetic approaches to understanding pattern and process. Mol. Ecol. 2002;11:2451–2451. doi: 10.1046/j.1365-294x.2002.01507.x. [DOI] [PubMed] [Google Scholar]
- Farris J.S, Kallersjo M, Kluge A.G, Bult C. Testing significance of incongruence. Cladistics. 1994;10:315–319. [Google Scholar]
- Farris J.S, Kallersjo M, Kluge A.G, Bult C. Constructing a significance test for incongruence. Syst. Biol. 1995;44:570–572. [Google Scholar]
- Forbes, A. T. 1968 Contributions to the ecology of the Sundays River. MSc thesis, Rhodes University.
- Forbes A.T, Allanson B.R. Ecology of the Sundays River. Part 2. Osmoregulation in some mayfly nymphs (Ephemeroptera: Baetidae) Hydrobiologia. 1970;369:489–503. [Google Scholar]
- Gattolliat J.L. Six new species of Labiobaetis Novikova & Kluge (Ephemeroptera: Baetidae) from Madagascar with comments on the validity of the genus. Ann. Limnol. 2001a;37:97–123. [Google Scholar]
- Gattolliat J.L. Rheoptilum: a new genus of two-tailed Baetidae (Ephemeroptera) from Madagascar. Aquat. Insects. 2001b;23:67–81. 10.1076/aqin.23.1.67.4932 [Google Scholar]
- Gattolliat J.L. The genus Cloeodes (Ephemeroptera: Baetidae) in Madagascar. Rev. Suisse. Zool. 2001c;108:387–402. [Google Scholar]
- Gattolliat J.L. Two new genera of Baetidae (Ephemeroptera; Insecta) from Madagascar. Aquat. Insects. 2002;24:143–159. 10.1076/aqin.24.2.143.4903 [Google Scholar]
- Gattolliat J.-L. The genera Demoulinia Gillies and Potamocloeon Gillies (Ephemeroptera: Baetidae) in Madagascar. Zootaxa. 2003;184:1–18. [Google Scholar]
- Gattolliat J.-L, Rabeantoandro S.Z. The genus Cloeon (Ephemeroptera, Baetidae) in Madagascar. Mitteilungen der Schweizerischen Entomologischen Gesselschaft, Bulletin de la Société Entomologique Suisse. 2002;74:195–209. [Google Scholar]
- Gattolliat J.L, Sartori M. Contribution to the systematics of the genus Dabulamanzia (Ephemeroptera: Baetidae) in Madagascar. Rev. Suisse Zool. 2000;107:561–577. [Google Scholar]
- Gattolliat J.-L, Sartori M. Predaceous Baetidae in Madagascar: an uncommon and unsuspected high diversity. In: Dominguez E, editor. Trends in research in Ephemeroptera and Plecoptera. Kluwer Academic/Plenum Publishers; New York: 2001. pp. 321–330. [Google Scholar]
- Gattolliat J.-L, Sartori M. An overview of the Baetidae of Madagascar. In: Gaino E, editor. Research update on Ephemeroptera and Plecoptera. University of Perugia; 2003. pp. 135–144. [Google Scholar]
- Gauld I.D, Wahl D.B. The Eucerotinae: a Gondwanan origin for a cosmopolitan group of Ichneumonidae? J. Nat. Hist. 2002;36:2229–2248. [Google Scholar]
- Gerlach J. The mayflies of Seychelles: morphology, distribution and ecology. Phelsuma. 2001;9:67–70. [Google Scholar]
- Giessler S, Mader E, Schwenk K. Morphological evolution and genetic differentiation in Daphnia species complexes. J. Evol. Biol. 1999;12:710–723. [Google Scholar]
- Gillies M.T. Notes on some Ephemeroptera Baetidae from India and South-East Asia. Trans. R. Entomol. Soc. Lond. 1949;100:161–177. [Google Scholar]
- Gillies M.T. A preliminary account of the East-African species of Cloeon Leach and Rhithrocloeon Gen-N (Ephemeroptera) Aquat. Insects. 1985;7:1–17. [Google Scholar]
- Gillies M.T. A diphyletic origin for the two-tailed Baetid mayflies occurring in East African stony streams with a description of the new genus and species Tanzaniella spinosa Gen. Nov. Sp. Nov. In: Alba-Tercador J, Sanchez-Ortega A, editors. Overview and strategies of Ephemeroptera and Plecoptera. Sandhill Crane Press; Gainesville, FL: 1991. pp. 175–187. [Google Scholar]
- Gladstein D, Wheeler W.C. American Museum of Natural History; New York: 1999. POY. Program and documentation. Freely available from http://research.amnh.org/scicomp/projects/poy.php. [Google Scholar]
- Goodman S.M, Benstead J.P. University of Chicago Press; 2003. The natural history of Madagascar. [Google Scholar]
- Groombridge J.J, Jones C.G, Bayes M.K, van Zyl A.J, Carrillo J, Nichols R.A, Bruford M.W. A molecular phylogeny of African kestrels with reference to divergence across the Indian Ocean. Mol. Phylogenet. Evol. 2002;25:267–277. doi: 10.1016/s1055-7903(02)00254-3. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Harker J.E. The role of parthenogenesis in the biology of two species of mayfly (Ephemeroptera) Freshw. Biol. 1997;37:287–297. [Google Scholar]
- Hershey A.E, Pastor J, Peterson B.J, Kling G.W. Stable isotopes resolve the drift paradox for Baetis mayflies in an Arctic River. Ecology. 1993;74:2315–2325. [Google Scholar]
- Hovmöller R, Pape T, Källersjö M. The Paleoptera problem: basal pterygote phylogeny inferred from 18S and 28S rDNA sequences. Cladistics. 2002;18:313–323. doi: 10.1111/j.1096-0031.2002.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Hubbard M.D, Kukalova-Peck J. Permian mayfly nymphs: new taxa and systematic characters. In: Flannagan J.F, Marshall K.E, editors. Advances in Ephemeroptera biology. Plenum Press; New York: 1980. pp. 19–31. [Google Scholar]
- Hughes J.M, Mather P.B, Hillyer M.J, Cleary C, Peckarsky B. Genetic structure in a montane mayfly Baetis bicaudatus (Ephemeroptera: Baetidae), from the Rocky Mountains, Colorado. Freshw. Biol. 2003;48:2149–2162. [Google Scholar]
- Jansa S.A, Goodman S.M, Tucker P.K. Molecular phylogeny and biogeography of the native rodents of Madagascar (Muridae: Nesomyinae): a test of the single-origin hypothesis. Cladistics. 1999;15:253–270. doi: 10.1111/j.1096-0031.1999.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Johanson K.A. Phylogenetic and biogeographic analysis of the family Helicopsychidae (Insecta: Trichoptera) Entomol. Scand. Suppl. 1998;53:6–170. [Google Scholar]
- Johnson C.G. Methuen & Co; London: 1969. Migration and dispersal of insects by flight. [Google Scholar]
- Lourenço W.R, editor. Biogeography of Madagascar. Orstom; Paris: 1996. [Google Scholar]
- Lugo-Ortiz C.R, McCafferty W.P. The Bugilliesia complex of African Baetidae (Ephemeroptera) Trans. Am. Entomol. Soc. 1996;122:175–197. [Google Scholar]
- Lugo-Ortiz C.R, McCafferty W.P. The Centroptiloides complex of Afrotropical small minnow mayflies (Ephemeroptera: Baetidae) Ann. Entomol. Soc. Am. 1998a;91:1–26. [Google Scholar]
- Lugo-Ortiz C.R, McCafferty W.P. Phylogeny and biogeography of Nesydemius n. gen., and related Afrotropical genera (Insecta: Ephemeroptera: Baetidae) Ann. Limnol. 1998b;34:7–12. [Google Scholar]
- Lugo-Ortiz C.R, McCafferty W.P, Waltz R.D. Definition and reorganization of the genus Pseudocloeon (Ephemeroptera: Baetidae) with new species descriptions and combinations. Trans. Am. Entomol. Soc. 1999;125:1–37. [Google Scholar]
- Monaghan M.T, Spaak P, Robinson C.T, Ward J.V. Population genetic structure of 3 Alpine stream insects: influences of gene flow, demographics, and habitat fragmentation. J. North Am. Benthol. Soc. 2002;21:114–131. [Google Scholar]
- Nagell B. Overwintering strategy of Cloeon dipterum (L.) larvae. In: Flannagan J.F, Marshall K.E, editors. Advances in Ephemeroptera biology. Plenum Press; New York: 1980. pp. 259–264. [Google Scholar]
- Nagy Z.T, Joger U, Wink M, Glaw F, Vences M. Multiple colonization of Madagascar and Socotra by colubrid snakes: evidence from nuclear and mitochondrial gene phylogenies. Proc. R. Soc. B. 2003;270:2613–2621. doi: 10.1098/rspb.2003.2547. 10.1098/rspb.2003.2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden T.H, Whiting M.F. The problem with “the Paleopera problem:” sense and sensitivity. Cladistics. 2003;19:432–442. doi: 10.1111/j.1096-0031.2003.tb00313.x. [DOI] [PubMed] [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999;48:612–622. [Google Scholar]
- Paulian R, Viette P. An introduction to terrestrial and freshwater invertebrates. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago Press; 2003. pp. 503–511. [Google Scholar]
- Perkins R.C.L. Insect fauna of the Seychelles. Nature. 1933;132:192–193. [Google Scholar]
- Phillips A, Janies D, Wheeler W. Multiple sequence alignment in phylogenetic analysis. Mol. Phylogenet. Evol. 2000;16:317–330. doi: 10.1006/mpev.2000.0785. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Raxworthy C.J, Forstner M.R.J, Nussbaum R.A. Chameleon radiation by oceanic dispersal. Nature. 2002;415:784–787. doi: 10.1038/415784a. [DOI] [PubMed] [Google Scholar]
- Ribera I, Vogler A.P. Habitat type as a determinant of species range sizes: the example of lotic–lentic differences in aquatic Coleoptera. Biol. J. Linn. Soc. 2000;71:33–52. [Google Scholar]
- Ribera I, Barraclough T.G, Vogler A. The effect of habitat type on speciation rates and range movements in aquatic beetles: inferences from species-level phylogenies. Mol. Ecol. 2001;10:721–735. doi: 10.1046/j.1365-294x.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- Ribera I, Foster G.N, Vogler A.P. Does habitat use explain large scale species richness patterns of aquatic beetles in Europe? Ecography. 2003;26:145–152. [Google Scholar]
- Sanmartin I, Ronquist F. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Syst. Biol. 2004;53:216–243. doi: 10.1080/10635150490423430. [DOI] [PubMed] [Google Scholar]
- Sartori M, Gattolliat J.-L, Oliarinony R, Elouard J.-M. Biogeography of Malagasy mayflies (Insecta, Ephemeroptera): preliminary results. In: Lourenço W.R, Goodman S.M, editors. Diversité et endémisme à Madagascar. Mémoires de la Société de Biogéographie; Paris: 2000. pp. 307–317. [Google Scholar]
- Schluter D, Price T, Mooers A.O, Ludwig D. Likelihood of ancestor states in adaptive radiation. Evolution. 1997;51:1699–1711. doi: 10.1111/j.1558-5646.1997.tb05095.x. [DOI] [PubMed] [Google Scholar]
- Scott H. Summary and general conclusions regarding the insect fauna of the Seychelles and adjacent islands. Proc. Linn. Soc. Lond. 1932:136–140. Session 144, part IV. [Google Scholar]
- Sequeira A.S, Farrell B.D. Evolutionary origins of Gondwanan interactions: how old are Araucaria beetle herbivores? Biol. J. Linn. Soc. 2001;74:459–474. [Google Scholar]
- Shull V.L, Vogler A.P, Baker M.D, Maddison D.R, Hammond P.M. Sequence alignment of 18S ribosomal RNA and the basal relationships of Adephagan beetles: evidence for monophyly of aquatic families and the placement of Trachypachidae. Syst. Biol. 2001;50:945–969. doi: 10.1080/106351501753462894. [DOI] [PubMed] [Google Scholar]
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994;87:651–701. [Google Scholar]
- Sparks J.S. Molecular phylogeny and biogeography of the Malagasy and South Asian cichlids (Teleostei: Perciformes: Cichlidae) Mol. Phylogenet. Evol. 2004;30:599–614. doi: 10.1016/S1055-7903(03)00225-2. 10.1016/S1055-7903(03)00225-2 [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4.0b10. [Google Scholar]
- Trewick S.A. Molecular evidence for dispersal rather than vicariance as the origin of flightless insect species on the Chatham Islands. N. Z. J. Biogeogr. 2000;27:1189–1200. [Google Scholar]
- Vences M, Vieites D.R, Glaw F, Brinkmann H, Kosuch J, Veith M, Meyer A. Multiple overseas dispersal in amphibians. Proc. R. Soc. B. 2003;270:2435–2442. doi: 10.1098/rspb.2003.2516. 10.1098/rspb.2003.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz R.D, McCafferty W.P. Redescription and new lectotype designation for the type species of Pseudocloeon, P. kraepelini Klapalek (Ephemeroptera: Baetidae) Proc. Entomol. Soc. Wash. 1985;87:800–804. [Google Scholar]
- Whiting M.F, Carpenter J.C, Wheeler Q.D, Wheeler W.C. The strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst. Biol. 1997;46:1–68. doi: 10.1093/sysbio/46.1.1. [DOI] [PubMed] [Google Scholar]
- Yoder A.D, Burns M.M, Zehr S, Delefosse T, Veron G, Goodman S.M, Flynn J.J. Single origin of Malagasy Carnivora from an African ancestor. Nature. 2003;421:734–737. doi: 10.1038/nature01303. [DOI] [PubMed] [Google Scholar]
- Zakharov E.V, Smith C.R, Lees D.C, Cameron A, Vane-Wright R.I, Sperling F.A. Independent gene phylogenies and morphology demonstrate a Malagasy origin for a wide-ranging group of swallowtail butterflies. Evolution. 2004;58:2763–2782. doi: 10.1111/j.0014-3820.2004.tb01628.x. [DOI] [PubMed] [Google Scholar]