Abstract
In polygynous (multiple queens per nest) ants, queen dispersal is often limited with young queens being recruited within the parental colony. This mode of dispersal leads to local resource competition between nestmate queens and is frequently associated with extremely male-biased sex ratios at the population level. The queen-replenishment hypothesis has been recently proposed to explain colony sex ratio investment under such conditions. It predicts that colonies containing many queens (subject to high local resource competition) should only produce males, whereas colonies hosting few queens (reduced or no local resource competition) should produce new queens in addition to males. We experimentally tested this hypothesis in the ant Formica exsecta by manipulating queen number over three consecutive years in 120 colonies of a highly polygynous population. Queens were transferred from 40 colonies into another 40 colonies while queen number was not manipulated in 40 control colonies. Genetic analyses of worker offspring revealed that our treatment significantly changed the number of reproductive queens. The sex ratio of colonies was significantly different between treatments in the third breeding season following the experiment initiation. We found that, as predicted by the queen-replenishment hypothesis, queen removal resulted in a significant increase in the proportion of colonies that produced new queens. These results provide the first experimental evidence for the queen-replenishment hypothesis, which might account for sex ratio specialization in many highly polygynous ant species.
Keywords: sex ratio, queen-replenishment hypothesis, local resource competition, polygyny, ants
1. Introduction
Studies of sex allocation in social Hymenoptera provide opportunities to test evolutionary theories of inclusive fitness, and the sex-ratio and parent-offspring conflict (Nonacs 1986; Bourke & Franks 1995; Crozier & Pamilo 1996; Queller & Strassmann 1998; Chapuisat & Keller 1999; Keller & Reeve 2002; Mehdiabadi et al. 2003). Social Hymenoptera have a haplodiploid sex-determination system where haploid eggs develop into males and diploid eggs into females (Crozier 1971; Evans et al. 2004). As a result, sisters are more closely related to each other (r=0.75) than to their brothers (r=0.25). This relatedness asymmetry within colonies decreases when queens mate with several males or when nests contain several related queens (Boomsma & Grafen 1990, 1991; Boomsma 1993; Bourke & Chan 1994). If relatedness asymmetry varies between colonies within a population, theory predicts that colony sex allocation should be bimodally distributed. Colonies in which the relatedness asymmetry is higher than the population average should predominantly produce new queens (gynes) while colonies with a relatedness asymmetry lower than the population average should specialize in male production (Boomsma & Grafen 1990, 1991; Boomsma 1993; Ratnieks & Boomsma 1997).
That variance in relatedness asymmetry correlates with sex ratio specialization has been demonstrated in many species of social Hymenoptera (Queller & Strassmann 1998; Hammond et al. 2002). However, recent studies have found that colony sex ratio specialization occurs in ant species where relatedness asymmetry does not vary between colonies (Pearson et al. 1997; Helms 1999; Passera et al. 2001; Fournier et al. 2003; Helms et al. 2004). Moreover, some other species exhibit variation in colony relatedness asymmetry but sex ratio is not associated with this variation (Brown & Keller 2000; Fjerdingstad et al. 2002).
Recently a new hypothesis has been proposed to account for sex ratio specialization in highly polygynous ants (i.e. species where nests contain numerous queens). The queen-replenishment hypothesis proposes that colonies containing many queens should only produce males, whereas colonies hosting few queens should produce new queens in addition to males (Brown & Keller 2000). This hypothesis is based on the observation that, in highly polygynous species, dispersal of queens is often limited with young newly mated queens being recruited back into their parental colony from which they may eventually disperse with workers to initiate new colonies nearby (Bourke & Franks 1995; Keller 1995). This mode of reproduction may lead to intense local resource competition (Clark 1978) and is often associated with extremely male-biased sex ratios (Bourke & Franks 1995; Crozier & Pamilo 1996). According to the queen-replenishment hypothesis, colonies containing relatively few queens are those benefiting most from recruiting new queens. This is because as queen numbers decrease, local resource competition between queens is reduced and below a certain threshold, there is a premium on recruiting new queens to enhance colony survival and productivity. This threshold and the value of new queens will depend on resource availability relative to the current number of queens. Because queen lifespan is typically limited in polygynous ants (Keller & Genoud 1997), it may take only a few years for gyne-producing colonies to reach the threshold where they benefit to produce and recruit again new gynes.
Formica exsecta has become an important model system for studying intraspecific variation in sex ratios (Pamilo & Rosengren 1983; Sundström et al. 1996; Brown & Keller 2000; Brown et al. 2002). This species is known to form two different social systems. In one, colonies are mostly headed by a single queen (monogynous social system), which can be singly or multiply mated (Sundström et al. 1996). In the other, colonies are usually headed by multiple queens (polygynous social system, Cherix et al. 1980). In both social systems, colony sex ratios are split, but apparently for different reasons. In monogynous populations, split sex ratios are correlated with the mating status of the queen that determines the degree of relatedness asymmetry (Sundström et al. 1996). In a highly polygynous population in the Swiss Jura mountains, the available data on split sex ratios are consistent with the predictions of the queen-replenishment hypothesis (Brown & Keller 2002; Brown et al. 2002). Colonies producing only males have greater effective queen number than female-producing colonies but do not have lower relatedness asymmetry from the perspective of the adult workers that rear the brood (Brown & Keller 2000). Moreover, colonies that produce gynes increase their effective queen number and are significantly more likely to specialize in male production the following year (Brown & Keller 2002). However, these data are correlative and the queen-replenishment hypothesis' predictions have not yet been tested experimentally.
The aim of this study is to experimentally determine whether manipulation of queen number in the field results in a shift in sex ratio as predicted by the queen-replenishment hypothesis. We chose 120 colonies in a F. exsecta population in the Swiss Jura mountains where colonies are known to be polygynous (Cherix et al. 1980; Liautard & Keller 2001) and colony sex ratios are bimodally distributed (Liautard et al. 2003). Queens were transferred from 40 colonies into another 40 colonies while queen number was not manipulated in 40 control colonies. We used polymorphic microsatellites to verify whether queen transfer was successful. We colleted data on colony sex ratio specialization to test the prediction that colonies from which queens are removed will preferentially produce gynes while colonies in which queens are added will specialize in male production.
2. Material and Methods
(a) Queen transfer
The experiment was conducted at Pré Nouveau, a cattle pasture at 1120 m altitude in the Swiss Jura mountains. Approximately 140 polygynous colonies occur at this site. For the experiment, 120 colonies were marked with an identification number on a wooden stake and a transponder with an electronic identification number (Trovan, EURO I.D. Identification Systems, Germany). Colonies were randomly assigned to one of three groups (40 colonies per group). One group consisted of colonies where queens were removed (R-colonies), a second of colonies where queens were added (A-colonies), and a third of control colonies where queen number was not experimentally manipulated (C-colonies). Queens were removed from R-colonies and added into A-colonies in all the assigned colonies during three different periods (fall (autumn) 2001: 24th/25th September; spring 2002: 5th/6th April and 18th/19th April; spring 2003: 16th/17th April and 23rd/24th April). Two observations suggested that queens transferred between colonies would be adopted. First, nestmate recognition appears poorly developed in F. exsecta in our study population (Brown et al. 2003). Second, multiple queen F. exsecta colonies in nature appear derived from the addition of new queens to established colonies (Liautard & Keller 2001). Queen transfer was discontinued in the spring of 2004 because none of the treated colonies (R- and A- colonies) produced gynes in 2003. The R- and A-colonies were arranged in pairs such that queens removed from a given R-colony were always added into the same A-colony. However, a small number of nests were abandoned during the course of the experiment. Partner nests from the deserted nests were regrouped into new pairs. In order to collect queens, we removed approximately 25% of the above-ground mound material on sunny days in the spring when queens are often near the mound surface. The nest material was placed into a shallow fluon-lined box and carefully searched for queens, which were removed and kept in perforated plastic vials until they were transferred. The removed mound material was placed back onto the parent mound within a few minutes after queens were collected. To transfer queens, we made a small excavation into the mound of the A-colonies, put the queens into the opening and immediately covered them with nest material. All queens were transferred within a few hours of collection.
(b) Reproductive status of transferred queens
In order to check whether transferred queens were inseminated and reproductively active, we collected 113 queens from 15 control colonies (7.5±2.2; mean±s.d.) in May 2004 just before the end of the experiment. These queens were dissected to determine their reproductive status by the presence or absence of sperm in the spermatheca and eggs in the oviduct. Queens were removed and dissected from control colonies to avoid any impact on colony sex ratios within the two manipulative treatments (R- and A- colonies) and this was done near the end of the experiment to minimize the probability that their removal would have any effect on control colony sex ratios. We assume that insemination rate and reproductive activity of queens remain constant across years and, therefore, our estimates should reflect the reproductive status of queens transferred throughout the experiment.
(c) Colony sex ratio
For each colony we collected 50 pupae in September 2001, July 2002, June 2003 and July 2004. Pupae from the summer broods (2002–2004) were used to determine the colony sex and caste ratio by moistening pupae in ethanol and observing the size, eye shape and genitalia of late-stage pupa (Brown & Keller 2000; Liautard et al. 2003). Colonies were considered as gyne-producing when any gyne pupae were present (usually along with male and worker brood). Male-producing colonies, produced only male and worker pupae. Because gyne brood make up a large proportion of the brood in gyne-producing colonies (Brown et al. 2003), there is only a small probability of wrongly classifying gyne-producing colonies as male-producers, and this probability will not vary across experimental treatments. Only colonies that produced reproductive brood (gynes and/or males) were included in the sex ratio analysis. Samples from 2001 and 2004 were prepared for genetic analysis and stored at −20 °C.
(d) Offspring genotyping
To test whether the queen transfer impacted the genetic structure of experimental colonies, we genotyped worker pupae just before (September 2001) and just after the experiment (July 2004). These pupae were available for 79 colonies (30 R-colonies, 25 A-colonies and 24 C-colonies). We genotyped four pupae per nest for each period. Genotypes were determined at five microsatellite loci. In 2001, we conducted an analysis of the brood of 24 colonies at the loci FE17, FE37, FE38, FE42, FE49 (Gyllenstrand et al. 2002). All other samples were genotyped in 2004 (55 colonies from 2001 and 79 colonies from 2004) at the loci FE17, FE19, FE37, FE21, FE51 (Gyllenstrand et al. 2002). DNA was extracted from the entire pupae in 500 ml of 5% Chelex and incubated for 10 min at 90 °C, vortexed, and incubated for another 10 min at 90 °C.
Two multiplex-PCRs were used: (i) multiplex-PCR1 comprised FE19, FE21 and FE51; (ii) multiplex-PCR2 combined FE17 and FE37. The loci FE38, FE42 and FE49 were amplified in simplex-PCR following the protocol of Liautard et al. (unpublished data). Multiplex-PCR amplifications were carried out in 10 μl reaction volumes containing 2 μl of DNA (diluted 5×), 0.15–0.6 μM of each primer (concentration was optimized for each primer pair individually), 2× PCR-buffer, 1× Q-solution (Qiagen), 2 mM MgCl2 and 0.5 U of Taq-polymerase (Qiagen). PCR products were mixed and run on an automatic sequencer (ABI Prism 377XL). Number of alleles per locus ranged from 4 to 18 (9.8±5.8; mean±s.d.) with expected heterozygosity between 0.40 and 0.81.
(e) Statistical analysis
We estimated relatedness among worker brood by nest and locus using the computer program RELATEDNESS 5.0.8 (Goodnight & Queller 1994). Relatedness among brood is negatively correlated with the number of queens that contribute to brood production (Ross 1993). Thus, removing queens from colonies should increase relatedness among brood, while adding queens to colonies should decrease relatedness among brood. To test these predictions, we calculated the difference between the relatedness estimate prior to the experiment (2001) and the relatedness estimate after the experiment (2004) as rdiff=r2001−r2004 for all colonies. If the queen transfer impacted the colony relatedness structure we would expect rdiff to be negative for colonies where we removed queens and rdiff to be positive for colonies where we added queens, while rdiff should be intermediate for control colonies. We normalized the relatedness values by a logarithmic transformation. Because we did not analyse the same loci in all of the colonies in 2001, we first tested whether loci contributed differentially to the colony relatedness level and found that they did not (one-way ANOVA: F7,371=0.424, p=0.89). We then carried out a one-tailed t-test to determine whether the change in relatedness (rdiff) was significantly lower in colonies where queens were removed than in those where queens were added.
To test whether the number of female-producing colonies differed between the three treatments (R-, A- and C-colonies), we conducted a G-test of independence with William's correction (Sokal & Rohlf 1995) for each study year (2002–2004). Pairwise post hoc comparisons between treatments were conducted with the sequential Bonferroni method (Rice 1989).
3. Results
Over the course of the experiment, 29 of the 120 experimental colony nests were abandoned and excluded from analyses. The rate of nest abandonment (6 R-colonies, 12 A-colonies, and 11 C-colonies) was not significantly different between experimental treatments (chi-square test: χ22=2.14, p=0.34).
(a) Reproductive status of transferred queens
The dissection of queens showed that 95 out of 113 were inseminated; the proportion of inseminated queens per colony was 0.85±0.11 (mean±s.d.). All but one of 113 queens had developed ovaries and eggs in the oviduct, demonstrating that the majority of transferred queens were inseminated and reproductively active.
(b) Queen transfer and impact on colony genetic structure
The number of queens found and transferred varied greatly between periods and colonies (fall 2001: 2.2±7.9; spring 2002: 7.8±9.1; spring 2003: 10.7±13.4; mean ±s.d.). We transferred a total of 751 queens.
The change in relatedness between the periods before and after the experiment was negative for R-colonies, positive for A-colonies and at an intermediate level for C-colonies (figure 1). As predicted, the difference in relatedness was significantly lower for R-colonies than for A-colonies (one-tailed t-test: t53=1.80, p=0.039), indicating that our manipulation of queen number was successful.
Figure 1.
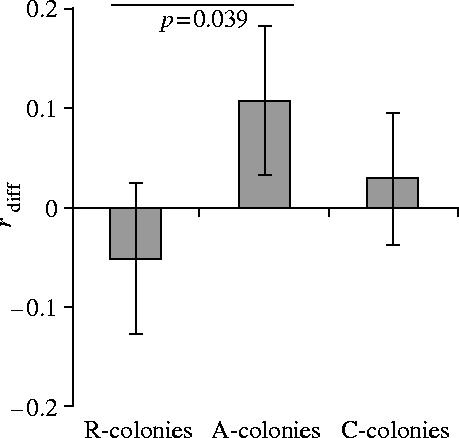
Differences in the relatedness estimate for the pre-experimental phase (r2001) and the post-experimental phase (r2004) for the three treatments (mean±s.e.m.), calculated as rdiff=r2001−r2004. R-colonies, colonies where queens were removed (N=30); A-colonies, colonies where queens were added (N=25); C-colonies, colonies where queen number was not manipulated (N=24).
(c) Colony sex ratio
The number of male- and female-producing colonies varied significantly across years (G-test of independence: G2=35.8, p<0.0001). Usually, female-producing colonies produced males and workers as well as gynes. Male-producing colonies produced males and workers. A small proportion of colonies produced only worker brood (2002: 0.10; 2003: 0.15; 2004: 0.04) or did not produce any offspring at all (2002: 0.07; 2003: 0.01; 2004: 0.04), and these nests were excluded from further analysis.
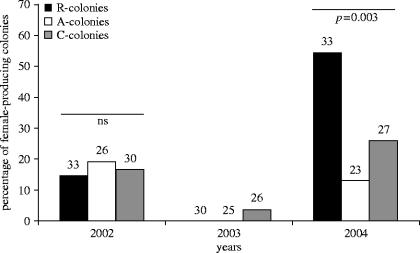
In the first breeding season after starting the experiment (2002), the percentage of nests producing gynes did not differ significantly (G-test of independence: G2=0.16, p=0.92) between the three treatments (figure 2). The second year (2003) was exceptionally hot and dry and only one of the colonies produced gynes. As a result, no meaningful comparison and statistical test could be carried out. In the final year (2004) the percentage of nests producing gynes differed significantly across treatments (figure 2, G2=11.6, p=0.003). As predicted by the queen-replenishment hypothesis, gynes were produced in a significantly greater proportion in colonies where queens were removed than in colonies where queens had been added (post hoc pairwise comparison: G1=10.5, p<0.01) or in control colonies, although the difference was marginally significant after applying the sequential Bonferroni method in the latter comparison (post hoc pairwise comparison: G1=4.99, p<0.025). There was no significant difference in the proportion of colonies that produced gynes between colonies where queens were added and the control colonies (post hoc pairwise comparison: G1=1.26, p=0.26).
Figure 2.
Percentage of colonies that produced gynes for the three treatments across the three breeding seasons. R-colonies, colonies where queens were removed; A-colonies, colonies where queens were added; C-colonies, colonies where queen number was not manipulated. Numbers above the columns indicate the total number of nests per treatment where sexuals were produced.
4. Discussion
The queen-replenishment hypothesis predicts that colonies subject to local resource competition should produce only male sexual brood when colony queen number is high. However, local resource competition would decrease with decreasing queen number, and below a certain threshold in queen number, colonies would benefit from recruiting gynes into their own colony to avoid brood limitation and to enhance colony survival and colony productivity (Brown & Keller 2000). Our experimental manipulation of queen number significantly affected colony sex ratios as predicted by the queen-replenishment hypothesis such that the proportion of gyne-producing colonies was higher for colonies where queens were removed than for colonies where queens were added and control colonies. Our study is the first to provide experimental evidence for the queen-replenishment hypothesis.
Another prediction of our experiment was that a higher proportion of nests where queens were added in comparison to the control colonies should specialize in male production. Although our results indicate the predicted trend, the observed difference was not significant. This suggests that queen removal had a greater effect on colony sex ratio than queen addition, which could indicate that not all of the queens transferred in our queen-addition treatment were accepted into their new colonies.
Our results showed that it took three breeding seasons before colonies changed sex ratio investment according to our treatments. There are at least three possible reasons for this result. First, we collected queens from only 25% of the mound to avoid excessive colony disturbance. As a consequence, the number of queens that could be removed in each sampling event was limited, and multiple samples over several periods may have been required to shift colony queen number below or above the critical threshold. Second, colony members might need a considerable amount of time until they detect a change in colony queen number. This would lead to a time lag between a change in queen number and change in the colony sex ratio investment strategy. Third, the queen-replenishment hypothesis predicts that colonies will fail to produce gynes while resource competition among queens is strong, while gynes will be produced when resource competition among queens is weak. One factor that determines the degree of competition among queens is their number within a nest, and manipulating this number is the focus of our experiment. However, competition among queens is also influenced by colony resource availability (Brown et al. 2002). For example, some given number of queens may compete when resources are scarce while they may not compete when resources are abundant. Thus, the number of queens at which colonies switch from producing males to producing gynes could increase with increasing resources. As a result, the important proximate cue for gyne production may not be queen number per se, but rather queen number relative to resource availability. If so, differences between years in environmental resource availability may partially explain our results. Queen transfer early in our study may have had no effect on the sex ratio because environmental resources were limited to the degree that queen removal did not reduce competition among queens. Indeed, the weather conditions in the Swiss Jura in 2003 were very exceptional. A hot, dry period lasted for more than three months during spring and summer, and may have significantly reduced food availability. The experimental results consistent with the queen-replenishment hypothesis may have occurred in the following year because resources were more abundant relative to queen number.
As predicted by the queen-replenishment hypothesis, our results show that queen number appears to be an important determinate of colony sex ratio investment. However, the proximate mechanisms by which queen number affects colony sex ratio remains so far unknown. For example, it is unknown how colony members (queens and/or workers) determine when to change the colony sex ratio or how they do it. It is possible that colony members assess queen number within the nest, either directly by counting the number of queens, or indirectly by estimating relatedness among brood, which changes with effective queen number. However, local resource competition among queens depends not only upon queen number, but also upon environmental resource availability. When resources are abundant, a given number of queens may experience no competition; however, when resources are scarce, the same number of queens may experience strong competition. Thus, the point at which gyne production is initiated to replenish queen number may be determined by the interaction between queen number and resource availability. Determining how queen number and resource conditions interact to determine colony sex allocation will be an important challenge for further research.
Acknowledgments
We thank C. Liautard and W. D. Brown for their help during the initiation of the experiment. We are grateful to N. Bruyndonckx, M. Gillard, M. A. Gonzalez and C. Vogler for their help in the laboratory. Many thanks to S. Helms Cahan who provided valuable advice on the genotyping. This study was supported by several grants from the Swiss National Science Foundations.
References
- Boomsma J.J. Sex ratio variation in polygynous ants. In: Keller L, editor. Queen number and sociality in insects. Oxford University Press; 1993. pp. 86–109. [Google Scholar]
- Boomsma J.J, Grafen A. Intraspecific variation in ant sex ratios and the Trivers–Hare hypothesis. Evolution. 1990;44:1026–1034. doi: 10.1111/j.1558-5646.1990.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Boomsma J.J, Grafen A. Colony-level sex ratio selection in the eusocial Hymenoptera. J. Evol. Biol. 1991;4:383–407. [Google Scholar]
- Bourke A.F.G, Chan G.L. Split sex ratios in ants with multiple mating. Trends Ecol. Evol. 1994;9:120–122. doi: 10.1016/0169-5347(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Bourke A.F.G, Franks N.R. Princeton University Press; 1995. Social evolution in ants. [Google Scholar]
- Brown W, Keller L. Colony sex ratios vary with queen number but not relatedness asymmetry in the ant Formica exsecta. Proc. R. Soc. B. 2000;267:1751–1757. doi: 10.1098/rspb.2000.1206. 10.1098/rspb.2000.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.D, Keller L. Queen recruitment and split sex ratios in polygynous colonies of the wood ant Formica exsecta. Ecol. Lett. 2002;5:102–109. [Google Scholar]
- Brown W.D, Keller L, Sundström L. Sex allocation in mound-building ants: the roles of resources and queen replenishment. Ecology. 2002;83:1945–1952. [Google Scholar]
- Brown W.D, Liautard C, Keller L. Sex-ratio dependent execution of queens in polygynous colonies of the ant Formica exsecta. Oecologia. 2003;134:12–17. doi: 10.1007/s00442-002-1072-8. 10.1007/s00442-002-1072-8 [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Keller L. Testing kin selection with sex allocation data in eusocial Hymenoptera. Heredity. 1999;82:473–478. doi: 10.1038/sj.hdy.6885340. [DOI] [PubMed] [Google Scholar]
- Cherix D, Werner P, Catzeflis F. Organisation spatiale d'un système polycalique chez Formica (Coptoformica) exsecta Nyl. (Hymenoptera: Formicidae) Mitt. Schweiz. Entomol. Ges. 1980;53:163–171. [Google Scholar]
- Clark A.B. Sex ratio and local resource competition in a prosimian primate. Science. 1978;201:163–165. doi: 10.1126/science.201.4351.163. [DOI] [PubMed] [Google Scholar]
- Crozier R. Heterozygosity and sex determination in haplo-diploidy. Am. Nat. 1971;105:399–412. [Google Scholar]
- Crozier R, Pamilo P. Oxford University Press; 1996. Evolution of social insect colonies: sex allocation and kin selection. [Google Scholar]
- Evans J.D, Shearman D.C.A, Oldroyd B.P. Molecular basis of sex determination in haplodiploids. Trends Ecol. Evol. 2004;19:1–3. doi: 10.1016/j.tree.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Fjerdingstad E.J, Gertsch P.J, Keller L. Why do some social insect queens mate with several males? Testing the sex ratio manipulation hypothesis in Lasius niger. Evolution. 2002;56:553–562. doi: 10.1111/j.0014-3820.2002.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Fournier D, Keller L, Passera L, Aron S. Colony sex ratios vary with breeding system but not relatedness asymmetry in the facultatively polygynous ant Pheidole pallidula. Evolution. 2003;57:1336–1342. doi: 10.1111/j.0014-3820.2003.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Goodnight K.F, Queller D.C. Goodnight Software; Houston, TX: 1994. Relatedness. [Google Scholar]
- Gyllenstrand N, Gertsch P.J, Pamilo P. Polymorphic microsatellite DNA markers in the ant Formica exsecta. Mol. Ecol. Notes. 2002;2:67–69. [Google Scholar]
- Hammond R.L, Bruford M.W, Bourke A.F.G. Ant workers selfishly bias sex ratios by manipulating female development. Proc. R. Soc. B. 2002;269:173–178. doi: 10.1098/rspb.2001.1860. 10.1098/rspb.2001.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms K.R. Colony sex ratios, conflict between queens and workers, and apparent queen control in the ant Pheidole desertorum. Evolution. 1999;53:1470–1478. doi: 10.1111/j.1558-5646.1999.tb05411.x. [DOI] [PubMed] [Google Scholar]
- Helms K.R, Fournier D, Keller L, Passera L, Aron S. Colony sex ratios in the facultatively polygynous ant Pheidole pallidula: a reanalysis with new data. Evolution. 2004;58:1141–1142. doi: 10.1111/j.0014-3820.2004.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Keller L. Social life: the paradox of multiple-queen colonies. Trends Ecol. Evol. 1995;10:355–360. doi: 10.1016/s0169-5347(00)89133-8. [DOI] [PubMed] [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Keller L, Reeve H.K. Encyclopedia of evolution. Oxford University Press; 2002. Kin selection. pp. 595–600. [Google Scholar]
- Liautard C, Keller L. Restricted effective queen dispersal at a microgeographic scale in polygynous populations of the ant Formica exsecta. Evolution. 2001;55:2484–2492. doi: 10.1111/j.0014-3820.2001.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Liautard C, Brown W.D, Helms K.R, Keller L. Temporal and spatial variations of gyne production in the ant Formica exsecta. Oecologia. 2003;136:558–564. doi: 10.1007/s00442-003-1300-x. 10.1007/s00442-003-1300-x [DOI] [PubMed] [Google Scholar]
- Mehdiabadi N.J, Reeve H.K, Mueller U.G. Queens versus workers: sex-ratio conflict in eusocial Hymenopera. Trends Ecol. Evol. 2003;18:88–93. [Google Scholar]
- Nonacs P. Ant reproductive strategies and sex allocation theory. Q. Rev. Biol. 1986;61:1–21. [Google Scholar]
- Pamilo P, Rosengren R. Sex ratio strategies in Formica ants. Oikos. 1983;40:24–35. [Google Scholar]
- Passera L, Aron S, Vargo E.L, Keller L. Queen control of sex ratio in fire ants. Science. 2001;293:1308–1310. doi: 10.1126/science.1062076. [DOI] [PubMed] [Google Scholar]
- Pearson B, Raybould A.F, Clarke R.T. Temporal changes in the relationship between observed and expected sex-investment frequencies, social structure and intraspecific parasitism in Leptothorax tuberum (Formicidae) Biol. J. Linn. Soc. 1997;61:515–536. [Google Scholar]
- Queller D.C, Strassmann J.E. Kin selection and social insects. Bioscience. 1998;48:165–175. [Google Scholar]
- Ratnieks F.L.W, Boomsma J.J. On the robustness of split sex ratio predictions in social Hymenoptera. J. Theor. Biol. 1997;185:423–439. [Google Scholar]
- Rice W.R. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Ross K.G. The breeding system of the fire ant Solenopsis invicta, and its effects on colony genetic structure. Am. Nat. 1993;141:554–576. doi: 10.1086/285491. [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. Freeman; New York: 1995. Biometry. [Google Scholar]
- Sundström L, Chapuisat M, Keller L. Conditional manipulation of sex ratios by ant workers: a test of kin selection theory. Science. 1996;274:993–995. doi: 10.1126/science.274.5289.993. [DOI] [PubMed] [Google Scholar]