Abstract
Many orb-weaving spiders decorate their webs with extra, bright white, ultraviolet light reflecting silk. Previous studies suggest that these decorations increase a spider's foraging efficiency by improving web attractiveness, which is known as the prey-attraction hypothesis. One assumption of this hypothesis is that individuals which decorate their webs at a higher frequency are expected to have a higher growth rate. Using a decoration-building orb-weaving spider, Argiope versicolor, I show a strong positive relationship between the growth rate in terms of weight gain and the frequency of decoration-building, as well as the rate of insect interception. This is the first study to reveal a fitness consequence of decorating behaviour in spiders.
Keywords: foraging, decorating behaviour, spider web, Argiope versicolor, prey-attraction, growth rate
1. Introduction
A predator's ability to encounter prey is fundamental to its foraging success and may determine its growth rate. The orb-web is a well known device that spiders use to forage and defend against predators (Tolbert 1975; Edmunds & Edmunds 1986; Eberhard 1990; Blackledge & Wenzel 1999). An orb-web spider's foraging performance is affected by the attractiveness or repellence of the web and the resident spider's ability to capture its prey (Bjorkman-Chiswell et al. 2004). By improving the foraging success, web-weaving spiders can locate suitable web sites (Heiling 1999), live in groups (Uetz 1989) or adjust the architecture or orientation of their webs (Rypstra 1982; Sandoval 1994; Herberstein et al. 2000a). However, a certain species of orb-weaving spiders alter the attractiveness or repellence of their webs by adding an extra silk structure, termed a decoration or stabilimentum, to the central portion after the typical orb-web construction. This silk device has stimulated intensive studies and also invited intensive debates since Simon (1895) first described it and proposed its function of stabilizing the web. Many functional hypotheses have been proposed for silk decorations, including mechanical, physiological and visual signalling functions (Herberstein et al. 2000b). However, fewer hypotheses have been experimentally tested. Nevertheless, the prey-attraction hypothesis (Craig & Bernard 1990; Elgar et al. 1996; Tso 1996, 1998; Watanabe 1999; Li et al. 2004 but see Blackledge & Wenzel 1999) and the predator-defence hypothesis (Horton 1980; Eisner & Nowicki 1983; Schoener & Spiller 1992; Kerr 1993; Blackledge 1998a; Blackledge & Wenzel 1999) are the two most recent and plausible, but also contentious, hypotheses.
The prey-attraction hypothesis argues that decoration-building spiders may improve their foraging success by improving web attractiveness. Researchers suggest that flying insects may be attracted to the silk decorations that reflect ultraviolet (UV) light (Craig & Bernard 1990; Watanabe 1999; Li et al. 2004 but see Zschokke 2002), which increases the prey-interception rate of the web (Craig & Bernard 1990). The evidence from many experimental and field correlative studies supports this hypothesis (Herberstein et al. 2000b). In the laboratory, when given a choice between a decorated web and an undecorated web, insects approach the decorated web more often than the undecorated web when the silk decorations are illuminated by UV+ white light (Craig & Bernard 1990; Watanabe 1999; Li et al. 2004). In the field, the decorated webs intercept more insects than undecorated ones (Craig & Bernard 1990; Tso 1996, 1998; Watanabe 1999; Herberstein 2000; Li et al. 2004), even when the decorated webs are smaller (Tso 1996). According to the prey-attraction hypothesis, the presence of the silk decorations may attract more prey to decorated webs and, thus, increase the prey interception rate of spiders. A possible underlying assumption of the prey-attraction hypothesis is that individuals that decorate their webs at a high frequency in their juvenile stage will grow fast (Craig et al. 2001). I experimentally tested whether spider growth rate is related to the frequency of decoration-building and the rate of insect interception using Argiope versicolor (Doleschall), a decoration-building orb spider (Araneae: Araneidae) from tropical southeast Asia (Seah & Li 2002).
2. Material and methods
(a) Study subjects
Argiope versicolor is common in tropical lowland forests in Singapore. Like all other Argiope species, A. versicolor sometimes builds orb webs without decorations. It also spins webs with different forms of silk decorations, including discoid decorations built by juveniles only and cruciform decorations spun by adults (Seah & Li 2002). An individual spider can switch between a decorated and an undecorated web on a daily basis (Seah & Li 2002). The decorated webs spun by A. versicolor juveniles have been demonstrated to attract more prey both in the laboratory and in the field (Li et al. 2004).
(b) Field study
I used A. versicolor juveniles in all experiments. A. versicolor juveniles sometimes spin undecorated webs and when a decoration is present it is only in the discoid form (see figure 1; Seah & Li 2002). The field site, the Bukit Timah Nature Reserve, is one of four remaining patches of the lush forest that once covered Singapore. The reserve is the only genuine patch of primary rainforest linked by secondary forests. A. versicolor is common and distributed along the trails and paths (Seah & Li 2002). Although spiders built their webs daily and changed their web sites during the study, they travelled only short distances of a few metres at a time. Thus, it was relatively easy to relocate the spiders. If a spider could not be found, then I searched in a 5 m circle for at least 2 days after it disappeared.
Figure 1.

A spider-eating jumping spider P. labiata feeding on a juvenile A. versicolor on a web with a discoid decoration. Scale bar, 1 cm.
Between August 2002 and March 2003, 90 juvenile A. versicolor were individually marked with different colours or colour combinations using permanent marker pens. They were then collected and taken to the laboratory. The initial weight (IWi) of each spider was measured by a digital balance (Genius, Fisher Scientific Ltd). After measurement, each spider was taken back to its own web in the field. I designated the date 1 day after measuring the initial weight of spiders as the first day. The spiders were then individually monitored for 6 days. Webs and spiders were surveyed once each hour for, on average, 6 h each day between 09.00 and 18.00. Data on web diameters, the presence or absence of a decoration, whether the spider relocated its web or not and captured prey were recorded. Some of the individuals disappeared from their habitats during this period. On the seventh day, the webs and spiders were not monitored for insect interception but I collected the spiders that could be located between 17.00 and 19.00, brought them back to the laboratory and used the same digital balance (Genius, Fisher Scientific Ltd) to measure their final weights (FWi).
(c) Data analysis
The frequency of decoration-building was calculated as the number of days in which a spider built a web with a discoid form in the 6 day period. The rate of insect interception was calculated as the mean number of insects trapped by each web per 6 h trial. The growth rate was calculated as a weight gain in the 6 day period: GWi=FWi−IWi, where i=the ith individual. Data were checked for normal distribution (Kolmogorov–Smirnov test). Data that were not normally distributed were log 10 or square root transformed. I performed linear statistical modelling (LSM) regression to examine whether the weight gain (i.e. the growth rate) was affected by the initial spider weight, the frequency of decoration-building and the rate of insect interception. As the prey-attraction hypothesis predicts that the decorated webs attract more insects than the undecorated webs (Craig & Bernard 1990), I performed a repeated measures ANOVA (with the type of web as the main factor, the spider as a random factor and the date as blocks) to determine the effects of the presence of a decoration and the date on the insect interception rate of webs. It is known that web area affects the insect interception rate (Sandoval 1994) so I calculated the average web area of the six webs and the average rate of insect interception for each spider in the 6 day period. I then performed a Spearman correlation to determine the relationship between the web area and the rate of insect interception. The individual web area was calculated using the formula π×1/2(vertical diameter)×1/2(horizontal diameter) (Blackledge 1998b). A repeated measures ANOVA with the type of web as the main factor, the spider as a random factor and the date as blocks was also performed to examine the effects of web type (presence or absence of a decoration) and the date on web area. The data were analysed with SPSS 12.0 for Windows. Two-tailed tests were used (Zar 1996).
3. Results
Ninety A. versicolor were marked and surveyed. Three of them died in the laboratory when weighed. Among the remaining 87 spiders that were returned to their own webs in the field, only 65 (75%) were recaptured at the end of the experiment. In five webs, a web-invading, spider-eating jumping spider, Portia labiata (Thorell), was observed feeding on A. versicolor (figure 1). In addition, 10 (11%) of the spiders could not be relocated after heavy rainfall during the night. Thus, 12 out of the 22 spiders that had disappeared were most likely preyed on, giving 13% (12 out of 90 spiders) predation. Ten out of 12 spiders were those that spun the decorating webs. Spiders that decorated their webs had a higher predation risk than those that did not decorate their webs (χ12=5.333, p=0.021).
Out of 65 recaptured spiders, 44 (69%) still carried the markers on the day that they were recaptured, which indicated that they had not moulted during this period. Twenty-one spiders had moulted before being recaptured. Thirteen (62%) out of 21 moulted spiders had final weights that were even lighter than their initial weight; this may be because they might have recently moulted (2 days before being recaptured for weighing). Since these newly moulted spiders and moulting spiders did not feed, these spiders were excluded from data analysis and the data from the remaining 52 spiders were used.
LSM regression results showed that A. versicolor's weight gain varied significantly with the frequency of decoration-building (number of days in which a spider built a decorated web in a 6 day period) and the average rate of insect interception. However, the initial spider weight had no significant effect on the weight gain (table 1). The spiders that decorated their webs at a higher frequency intercepted more insects and thus gained more weight (figure 2). The results from the repeated measures ANOVA showed that the decorated webs attracted significantly more insects than the undecorated ones (F1,173.049=49.351, p<0.0001; figure 3). However, the insect interception rate did not change with the date (F5,206=1.678, p=0.141) and the individual spider (F94,206=1.016, p=0.455).
Table 1.
LSM (General Linear Model) regression model of weight gain (square root transformed) against initial spider weight (square root transformed), frequency of decoration-building and insect interception rate (IICR; log 10 transformed).
| effect | coefficient | s.e. | t | p |
|---|---|---|---|---|
| constant | 0.616 | 0.052 | 11.890 | <0.0001 |
| initial weight | 0.126 | 0.073 | 1.722 | 0.091 |
| frequency of decoration-building | 0.001 | 0.001 | 2.090 | 0.042 |
| IICR | 0.002 | 0.001 | 2.211 | 0.032 |
Figure 2.

Scatterplot matrix of the weight gain (Y) of A. versicolor juveniles (n=52) in a 6 day period by the initial weight (X1), the frequency (X2) of decoration-building (number of days in which A. versicolor built a web with a stabilimentum in a 6 day period) and the rate (X3) of insect interception (IICR; average number of insects trapped per web per 6 h trial) of webs built by A. versicolor juveniles (n=52; Y=0.616+0.126X1+0.001X2+0.002X3; p<0.001, r2=0.414).
Figure 3.
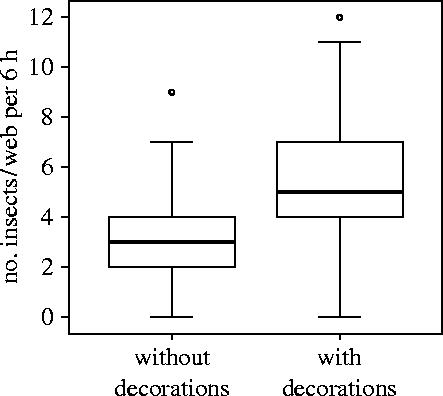
Number of insects intercepted by webs with or without discoid decorations built by A. versicolor juveniles. Medians (line within the box), quartiles (box), 90th percentiles (whiskers) and extreme points (circles) are shown.
The decorated webs (49.96±1.77 cm2) were significantly smaller than those without decorations (63.08±1.85 cm2; Repeated measures ANOVA: F1,177.854=23.705, p<0.0001). The rate of insect interception was negatively correlated with the web area (Spearman correlation: rs=−0.367, n=52, p<0.007). However, the date had no significant effect on the web area (F5,206=0.935, p=0.459).
4. Discussion
This study clearly demonstrates a strong positive relationship between the frequency of decoration-building and the growth rate in the decoration-building orb spider A. versicolor, thereby providing support for the prey-attraction explanation for decorating webs. While many studies reveal that webs with decorations intercept more prey (Craig & Bernard 1990; Tso 1996, 1998; Hauber 1998; Watanabe 1999; Herberstein 2000; Bruce et al. 2001; Li et al. 2004 but see Blackledge & Wenzel 1999), no behaviour studies have confirmed the underlying assumption (see Craig et al. 2001) that this behaviour results in a greater growth rate and, thus, a fitness advantage. The observed higher growth rate of A. versicolor juveniles that decorate their webs is due to the higher interception rate with insects and is consistent with the foraging behaviour described in an earlier study (Li et al. 2004), and thus supports the prey-attraction explanation (reviewed in Herberstein et al. 2000b).
Although the predation on A. versicolor juveniles was not very high (13%), the results of this study show that the juveniles had a significantly higher predation rate if they decorated their webs at higher frequency. Thus, my predation data support the predator-attraction hypothesis that conspicuous decorations attract unintended predators, thus increasing predation risk (Robinson & Robinson 1970; Bruce et al. 2001; Craig et al. 2001; Seah & Li 2001). This study is inconsistent with the anti-predator defence hypothesis (Horton 1980; Eisner & Nowicki 1983; Schoener & Spiller 1992; Kerr 1993; Blackledge 1998a; Blackledge & Wenzel 1999). However, the prey-attraction explanation and predator-defence explanation are not mutually exclusive. The decorations may primarily function as prey attractants and, at the same time, they may also serve to defend the resident spider against particular predators instead of specialized spider-eating predator salticids such as P. labiata (Seah & Li 2001) or praying mantid Pseudomantis albofimbriata (Bruce et al. 2001). Nevertheless, my data suggest a conflict in decoration-building behaviour between foraging success and reduced survivorship in A. versicolor. To increase their foraging success by decorating webs to attract prey and reduce the risk of predation, spiders must have strategies to balance the trade-off. Variation in decorating behaviour seems to be a strategy that has evolved in these spiders. Unpredictable and inconsistent decorating behaviour is favoured by natural selection because this inhibits the learning of both prey and predators (Craig 1994; Seah & Li 2001; Li & Lee 2004). This is because their learning is constrained by the anatomical and physiological limits of their perceptual capabilities and the complexity of their neural processing systems (Bélise & Cresswell 1997; Endler & Basolo 1998; Dukas 1999).
In conclusion, this study does not support the predator-defence explanation. However, the data do support the prey and predator-attraction explanation. Most importantly, this study supports the hypothesis that spiders which decorate their webs at higher frequencies will grow faster than those which decorated their webs at lower frequencies. The conflicting effects of foraging success and reduced predation risk may favour the retention of decorating behaviour within populations and variability in individual decorating behavioural responses. However, long-term experimental work is needed to investigate the correlation between decorating behaviour and growth and reproduction.
Acknowledgments
I am grateful to Ang Li for helping with the field experiments. I thank the Singapore National Parks Board for the research permit (NP/RP121). This work was supported by generous grants (R-154-000-072-112 and R-154-000-140-112) from the National University of Singapore Academic Research Fund.
References
- Bélisle C, Cresswell J. The effects of limited memory capacity on foraging behavior. Theor. Popul. Biol. 1997;52:78–90. doi: 10.1006/tpbi.1997.1319. [DOI] [PubMed] [Google Scholar]
- Bjorkman-Chiswell B.T, Kulinski M.M, Muscat R.L, Nguyen K.A, Norton B.A, Symonds M.R, Westhorpe G.E, Elgar M.A. Web-building spiders attract prey by storing decaying matter. Naturwissenschaften. 2004;91:245–248. doi: 10.1007/s00114-004-0524-x. [DOI] [PubMed] [Google Scholar]
- Blackledge T.A. Signal conflict in spider webs driven by predators and prey. Proc. R. Soc. B. 1998a;265:1991–1996. 10.1098/rspb.1998.0530 [Google Scholar]
- Blackledge T.A. Stabilimentum variation and foraging success in Argiope aurantia and Argiope trifasciata (Araneae: Araneidae) J. Zool. Lond. 1998b;246:21–27. [Google Scholar]
- Blackledge T.A, Wenzel J.W. Do stabilimenta in orb webs attract prey or defend spiders? Behav. Ecol. 1999;10:372–376. [Google Scholar]
- Bruce M.J, Herberstein M.E, Elgar M.A. Signalling conflict between prey and predator attraction. J. Evol. Biol. 2001;14:786–794. [Google Scholar]
- Craig C.L. Limits to learning: effects of predator pattern and colour on perception and avoidance-learning by prey. Anim. Behav. 1994;47:1087–1099. [Google Scholar]
- Craig C.L, Bernard G.D. Insect attraction to ultraviolet-reflecting spider webs and web decorations. Ecology. 1990;71:616–623. [Google Scholar]
- Craig C.L, Wolf S.G, Davis J.L.D, Hauber M.E, Maas J.L. Signal polymorphism in the web-decorating spider Argiope argentata is correlated with reduced survivorship and the presence of stingless bees, its primary prey. Evolution. 2001;55:986–993. doi: 10.1554/0014-3820(2001)055[0986:spitwd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dukas R. Costs of memory: ideas and predictions. J. Theor. Biol. 1999;197:41–50. doi: 10.1006/jtbi.1998.0856. [DOI] [PubMed] [Google Scholar]
- Eberhand W.G. Function and phylogeny of spider webs. Ann. Rev. Ecol. Syst. 1990;21:341–372. [Google Scholar]
- Edmunds J, Edmunds M. The defensive mechanisms of orb weavers (Araneae: Araneidae) in Ghana, West Africa. In: Eberhard W.G, Lubin Y.D, Robinson B.C, editors. Proceedings of the ninth international congress of arachnology, Panama 1983. Smithsonian Institution Press; Washington, DC: 1986. pp. 73–89. [Google Scholar]
- Eisner T, Nowicki S. Spider web protection through visual advertisement: role of the stabilimentum. Science. 1983;219:185–187. doi: 10.1126/science.219.4581.185. [DOI] [PubMed] [Google Scholar]
- Elgar M.A, Allan R.A, Evans T.A. Foraging strategies in orb-spinning spiders: ambient light and silk decorations in Argiope aetherea Walckenaer (Araneae: Araneoidea) Aust. J. Ecol. 1996;21:464–467. [Google Scholar]
- Endler J.A, Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Hauber M. Web decorations and alternative foraging tactics of the spider Argiope appensa. Ethol. Ecol. Evol. 1998;24:47–57. [Google Scholar]
- Heiling A.M. Why do nocturnal orb-web spiders (Araneidae) search for light? Behav. Ecol. Sociebiol. 1999;46:43–49. [Google Scholar]
- Herberstein M.E. Foraging behavior in orb-web spiders (Araneidae): do web decorations increase prey capture success in Argiope keyserlingi Karsch, 1878? Aust. J. Zool. 2000;48:217–223. [Google Scholar]
- Herberstein M.E, Craig C.L, Elgar M.A. Foraging strategies and feeding regimes: web and decoration investment in Argiope keyserlingi Karsch (Araneae: Araneidae) Evol. Ecol. Res. 2000a;2:69–80. [Google Scholar]
- Herberstein M.E, Craig C.L, Coddington J.A, Elgar M.A. The functional significance of silk decorations of orb-web spiders: a critical review of the empirical evidence. Biol. Rev. 2000b;75:649–669. doi: 10.1111/j.1469-185x.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Horton C.C. A defensive function for the stabilimentum of two orb-weaving spiders (Araneae Araneidae) Psyche. 1980;87:13–20. [Google Scholar]
- Kerr A.M. Low frequency of stabilimenta in orb webs of Argiope appensa (Araneae: Araneidae) from Guam: an indirect effect of an introduced avian predator? Pacif. Sci. 1993;47:328–337. [Google Scholar]
- Li D, Lee W.S. Predator-induced plasticity in web-building behaviour. Anim. Behav. 2004;67:309–318. [Google Scholar]
- Li D, Lim M.L.M, Seah W.K, Tay S.L. Prey attraction as a possible function of discoid stabilimenta of juvenile orb-spinning spiders. Anim. Behav. 2004;68:629–635. [Google Scholar]
- Robinson M.H, Robinson B. The stabilimentum of the orb-web spider Argiope argentata: an improbable defense against predators. Can. Entomol. 1970;102:641–655. [Google Scholar]
- Rypstra A.L. Building a better insect trap: an experimental investigation of prey capture in a variety of spider webs. Oecologia. 1982;52:31–36. doi: 10.1007/BF00349008. [DOI] [PubMed] [Google Scholar]
- Sandoval C.P. Plasticity in web design in the spider Parawixia bistriata: a response to variable prey type. Funct. Ecol. 1994;8:701–707. [Google Scholar]
- Schoener T.W, Spiller D.A. Stabilimenta characteristics of the spider Argiope argentata on small islands: support of the predator-defense hypothesis. Behav. Ecol. Sociobiol. 1992;31:309–318. [Google Scholar]
- Seah W.K, Li D. Stabilimenta attract unwelcome predators to orb-webs. Proc. R. Soc. B. 2001;268:1553–1558. doi: 10.1098/rspb.2001.1709. 10.1098/rspb.2001.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah W.K, Li D. Stabilimentum variations in Argiope versicolor (Araneae: Araneidae) from Singapore. J. Zool. Lond. 2002;258:531–540. [Google Scholar]
- Simon E. Roset; Paris: 1895. Historie naturelle des Araignées. [Google Scholar]
- Tolbert W.W. Predator avoidance behaviors and web defensive structures in orb-weavers Argiope aurantia and Argiope trifasciata (Aranae Araneidae) Psyche. 1975;82:29–52. [Google Scholar]
- Tso I.M. Stabilimentum of the garden spider Argiope trifasciata: a possible prey attractant. Anim. Behav. 1996;52:183–191. [Google Scholar]
- Tso I.M. Isolated spider web stabilimentum attracts insects. Behaviour. 1998;135:311–319. [Google Scholar]
- Uetz G.W. The ricochet effect and prey capture in colonial spider. Oecologia. 1989;81:154–159. doi: 10.1007/BF00379799. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Prey attraction as a possible function of the silk decoration of the uloborid spider Octonoba sybotides. Behav. Ecol. 1999;10:607–611. [Google Scholar]
- Zar J.H. 3rd edn. Prentice Hall; London: 1996. Biostatistical analysis. International Editions. [Google Scholar]
- Zschokke S. Ultraviolet reflectance of spiders and their webs. J. Arachnol. 2002;30:246–254. [Google Scholar]


