Abstract
When the reproductive value of sons and daughters differ, parents are expected to adjust the sex ratio of their offspring to produce more of the sex that provides greater fitness returns. The body condition of females or environmental factors, such as food abundance and mate quality, may influence these expected fitness returns. In a previous study of tree swallows (Tachycineta bicolor), we found that females produced more sons in their broods when they were in better body condition (mass corrected for size). We tested this relationship by experimentally clipping some flight feathers to reduce female body condition. As predicted, we found that females with clipped feathers had a lower proportion of sons in their broods and poorer body condition. However, female body condition alone was not a significant predictor of brood sex ratio in our experiment. We suggest that brood sex ratio is causally related to some other factor that covaries with body condition, most likely the foraging ability of females. The hypothesis that brood sex ratios are influenced by individual differences in female foraging ability is supported by a high repeatability of brood sex ratio for individual females. Thus, maternal effects may have a strong influence on the sex ratios of offspring.
Keywords: brood sex ratio, CHD gene, condition, maternal effects, repeatability, Tachycineta bicolor
1. Introduction
If the fitness benefits of sons and daughters differ, then individuals are expected to produce more of the sex that will provide greater fitness returns (Trivers & Willard 1973). In many organisms, high quality sons provide greater fitness returns than high quality daughters because males have greater variance in reproductive success than females (Arnold 1994), which may arise through polygyny or, in the case of monogamous birds, as a consequence of extra-pair paternity (reviewed in Whittingham & Dunn 2005). If extra-pair paternity is common, a successful son may have the potential to produce many more offspring than a successful daughter, regardless of the social mating system. Under these circumstances, Trivers & Willard (1973) predicted that mothers in good condition could achieve greater fitness if they produced high quality sons and that mothers in poor condition would improve their fitness by producing daughters rather than poor quality sons.
Recent studies suggest that individuals adaptively adjust offspring sex ratios in relation to particular factors associated with the breeding environment of the female (reviewed in Hasselquist & Kempenaers 2002; Komdeur & Pen 2002; Sheldon & West 2004). These factors can include food availability, female body condition and mate quality. In some situations, environmental factors that cause differences in the reproductive value of sons and daughters may be predictable (West & Sheldon 2002). For example, the benefits of producing high quality sons could be predicted if the female were mated to a high quality male (and male traits were heritable and related to reproductive success) or if maternal condition were related positively to her son's condition and subsequent reproductive success. There is mounting evidence in birds and mammals that certain factors, such as these, are associated with individual biases in offspring sex ratios (reviewed in Hasselquist & Kempenaers 2002; Sheldon & West 2004).
Some authors have questioned whether brood sex ratio adjustment is an adaptive response to environmental conditions because evidence for a sex ratio bias varies among years or populations (Radford & Blakey 2000). However, most studies of brood sex ratios are correlative and there may be confounding factors involved, or it may be that the key environmental factor varies between years or populations. Recent experimental studies, especially in birds, provide evidence that females are able to adjust the sex of their offspring in relation to specific factors which are expected to influence their expected fitness returns. Experimental studies of captive birds have shown that both female condition and food abundance influence brood sex ratios (Bradbury & Blakey 1998; Kilner 1998; Arnold et al. 2003). In natural populations, adjustments in brood sex ratio in relation to laying order (Badyaev et al. 2002), male quality or attractiveness (Sheldon et al. 1999), the reproductive value of helpers (Komdeur et al. 1997) as well as food abundance and maternal condition (Nager et al. 1999; Kalmbach et al. 2001) have been demonstrated experimentally. In addition to the factors mentioned above, two recent studies also suggest that there may be individual female effects on brood sex ratio (Oddie & Reim 2002; Griffith et al. 2003). Individual female effects, such as genotypic differences, body condition and foraging ability, are potentially important influences on sex ratio that have not been considered in most studies. Our study combines an experimental approach and an investigation of individual female effects to examine variation in brood sex ratios in tree swallows (Tachycineta bicolor).
In tree swallows, sons have the potential for much greater fitness returns than daughters. Although socially monogamous, tree swallows have very high levels of extra-pair paternity (Dunn et al. 1994) that results in high variance in male reproductive success (Kempenaers et al. 2001). Maternal condition has a strong positive influence on the condition of sons (Whittingham & Dunn 2000) and young that are in better condition are more likely to survive and recruit into the breeding population (McCarty 2001). Thus, female tree swallows in good condition may be able to realize greater fitness benefits by producing sons rather than daughters. In a previous study of tree swallows, we found that females in better condition produced more sons that were also in better condition (Whittingham & Dunn 2000). In this study we report on an experimental test of this correlation. To examine the impact of female condition on brood sex ratio, we attempted to reduce female condition before egg laying by clipping some flight feathers. We predicted that experimental females would be in poorer condition and, thus, produce broods that were more female biased than control females. We also examined the repeatability of brood sex ratio for individual females producing two broods within and between breeding seasons. If females show significant repeatability of brood sex ratio after controlling for their condition, then it indicates that some females are consistently producing more of one sex or the other, and, thus, maternal influences need to be considered in addition to female condition.
2. Material and Methods
(a) Study area and species
We studied tree swallows from 1997 to 2002 at the University of Wisconsin-Milwaukee Field Station near Saukville, Wisconsin, USA. (43°23′ N, 88°01′ W). The study area (6 ha) contained 80–92 nest boxes with predator guards, located in two grids 600 m apart (Whittingham & Dunn 2000). In our population, tree swallows are socially monogamous and single brooded, although they will renest if their clutch is lost early in the season. Females build a grass cup nest inside the nest box during late April and early May and begin egg laying when the nest cup is 4–6 cm deep. Females lay one egg per day and begin incubation (14–15 days) with the penultimate egg. Young remain in the nest after hatching for 18–22 days (Robertson et al. 1992). Females were classified as second-year (SY) or after second-year (ASY) on the basis of plumage coloration (Hussell 1983).
We checked nest boxes every other day during the nest building period from late April through the second week of May. To determine laying date, clutch size and hatching success, we checked nest boxes daily, starting when the height of the nest cup reached 3 cm until clutch completion and, again, from day 13 of incubation until the nestlings were 12 days old. Nests were checked during days 18–22 to determine fledging success. All adults were caught inside nest boxes; they were measured (wing cord and tarsus length), weighed and marked with a US Fish and Wildlife Service band on the right leg and a coloured plastic band on the left leg. Adults were marked on their breast or wings with non-toxic felt tip markers or acrylic paint in order to identify individuals in the field. On day 12 we weighed and measured nestlings and collected a small (50 μl) blood sample for molecular analysis of sex (see below). Blood samples were stored in a lysis buffer at 4 °C. All unhatched eggs and dead nestlings were collected and tissues were frozen at −20 °C.
(b) Female condition experiment
In 2001 and 2002 we conducted an experimental study of the effect of female condition and foraging ability on reproductive performance (Nooker et al. in press). ASY females were assigned randomly to the control or experimental group, with the exception that 10 females from 2001 that returned in 2002 were assigned to the alternative group. Experimental birds had three primaries on each wing (numbers three, five and seven counting from the innermost feather outward) and four central tail feathers clipped at the base of each feather, similar to our previous experiment on males (Whittingham et al. 1994). Clipped feathers are replaced during moult in July and August, so the effects are short-term. Ten SY females included in this study were monitored as controls. Females were caught in the nest box when nests had at least 3 cm of nest material prior to egg laying. To monitor the effects of the feather clipping treatment on body condition, females were recaptured and weighed on the second day of incubation and again when nestlings were four days old. Body condition was estimated as the residuals of body mass regressed on tarsus length (a measure of structural size; Jakob et al. 1996). This regression was performed using only control females. The condition of experimental females was then estimated by the residual of each experimental female from the regression line for control females. We also re-examined the relationship between brood sex ratio and female condition (see Whittingham & Dunn 2000) with additional years of data. For this analysis, we used estimates of female condition collected from throughout the entire breeding season; to correct for seasonal changes we included capture date in the regression model used to estimate body condition.
(c) Repeatability experiment
From 1997 to 1999 we conducted an experimental study in which we induced females to produce two broods in the same breeding season. This allowed us to examine the repeatability of brood sex ratios for individual females. We induced females to produce two broods by removing the eggs from the female's first clutch two days after clutch completion and cross fostering the eggs to the nest of a foster female. Clutch loss at the experimental female's nest simulated predation and induced the female to lay a second clutch. At foster nests, eggs from the resident female's original clutch were removed on the day they were laid and replaced with an artificial egg as part of another study (Whittingham & Schwabl 2002). All foster females incubated a clutch of artificial eggs for 2–3 days when the experimental clutch was transferred to their nest. All foster females readily accepted and incubated the artificial eggs and the foster clutch of eggs. Assignment of nests to foster or experimental groups was made randomly from the nests in the population that were at the same stage of early incubation.
(d) Molecular sex determination
We determined the sex of tree swallow embryos and nestlings using primers (P8 and P2) that amplify an intron of the CHD1 gene on the avian sex chromosomes (Griffiths et al. 1998). Our procedures are described in detail in Whittingham & Dunn (2000). Briefly, DNA was extracted from blood samples using a 5 M salt solution (Miller et al. 1988) or from solid tissue samples using standard phenol and chloroform methods (Hillis et al. 1990). Polymerase chain reaction products were digested with HaeIII (Promega) and were separated by electrophoresis in a 2% NuSieve 3 : 1 agarose (FMC Corp.) gel stained with ethidium bromide. Gels were examined under ultraviolet light and individuals were scored as male if they had one band and as female if they had two bands. As controls, an adult male and female were amplified and examined alongside young of unknown sex on each gel.
(e) Data analysis
We tested whether the brood sex ratio was significantly different than 1 : 1 using a one-sample Wilcoxon signed-rank test, which was based on the difference between the proportion of males and females within each brood. The Wilcoxon test uses each brood as a data point and, thus, accounts for the lack of independence among individuals in a brood (Neuhauser 2004). A two-sample Wilcoxon signed-rank test was used to compare the brood sex ratio of experimental and control females. To examine the sex ratio in each brood in relation to experimental treatment (feather clipping) and other factors such as female condition and year, we used generalized linear models (GLMs) with binomial errors and logit links (McCullagh & Nelder 1983) as implemented in the Macintosh computer package GLMstat (Beath 1997). This analysis used the number of males in each brood as the dependent (response) variable and the number sampled in each brood as the binomial denominator. The significance of predictor variables was tested by the change in chi-square values of the model with and without each variable. These chi-square values are biased by the lack of independence of young within a brood. Thus, we converted them to F statistics by dividing by the effect degrees of freedom. These F-values were compared with an F-distribution with error degrees of freedom of n−b−1, where n is the number of broods and b is the number of parameters in the model (Krackow & Tkadlec 2001). For the analysis of the repeatability of brood sex ratio we used a generalized linear mixed model (GLMM) as implemented in Sas v.8.02 (SAS Institute 2001) that accounts for the repeated nature of the data. Repeatability (Lessels & Boag 1987) was determined by calculating the intraclass correlation coefficient (rI; Zar 1999). Means are presented with their standard errors (±s.e.) and all tests were two-tailed unless noted otherwise. Sample sizes vary because it was not possible to collect data on all variables for all individuals.
3. Results
(a) Female condition
Our sample contained 77 broods of 38 control and 39 experimental female tree swallows in 2001 (19 control and 16 experimental females) and 2002 (19 control and 23 experimental females). We obtained DNA from 340 of 349 eggs that included 213 fledglings and 127 embryos from eggs that did not hatch or young that died in the nest. We did not obtain DNA from nine eggs (five experimental and four controls); six eggs or nestlings disappeared from their nest and three eggs were collected but showed no embryonic development. Thus, our sample of 97.4% of eggs is close to the primary sex ratio.
Experimental feather clipping had a significant impact on the proportion of male young produced. Females with clipped feathers produced fewer sons (42.4% males, 39 broods) than control females (59.4% males, 38 broods; Wilcoxon test, p=0.0002). Experimental and control females were similar in body condition before laying (t=0.30, d.f.=51, p=0.77) but by day two of incubation females with clipped feathers had lost an average of 1.1 g (±0.2), while control females had lost 0.45 g (±0.4; t=4.0, d.f.=55, p=0.0002). Females were captured and experimental females were clipped an average of 18 days (±0.9) before the start of egg laying (range for clipped females: 11–37 days). In terms of body condition, clipped females showed a greater reduction than control females (F1,47=8.1, p=0.007) but the change in condition did not differ between years (F1,47=0.1, p=0.78) in a multiple regression.
In this study clipped females started laying 2.0 days later than control females but the difference was not significant (t=1.3, d.f.=69, p=0.17). In a larger sample (including birds whose young we were not able to sex), females with clipped feathers laid, on average, seven days later than control females (t=5.0, d.f.=92, p<0.001; Nooker et al. in press). Over both years, the sex ratio of young produced in the population (51%, 77 broods) did not deviate significantly from 1 : 1 (Wilcoxon test, p=0.86), nor was there any significant deviation when we analysed each year separately (2001: 53% males, 35 broods; Wilcoxon test, p=0.51; 2002: 49% males, 42 broods; Wilcoxon test, p=0.69).
We used a GLM to examine the sex ratio of broods in relation to experimental feather clipping, year and change in body condition from pre-laying to day 2 of incubation (n=14 control, 36 experimental broods with data for all variables). Experimental females produced fewer sons than control females (F1,46=4.2, p=0.046) but there was no effect of year (F1,46=0.85, p=0.36) or the change in female condition (F1,46=0.0.3, d.f.=1, p=0.87; figure 1). Removing year from the model or replacing change in condition with pre-laying condition made no qualitative change to the results. By itself, change in female condition was not a significant predictor of the proportion of males in a brood (GLM; F1,48=0.78, p=0.38). Thus, it appears that although the treatment (feather clipping) had a strong effect on female condition and brood sex ratio, female condition alone was not a significant predictor of brood sex ratio.
Figure 1.
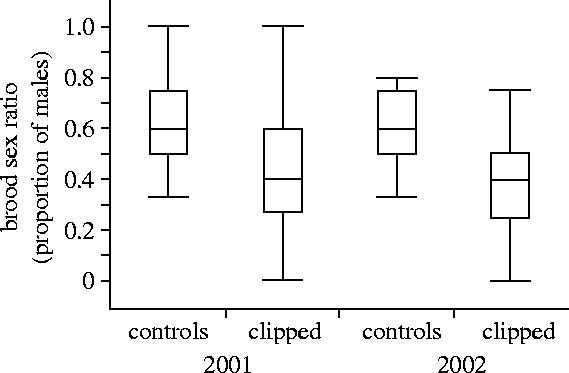
Broods of control females had a greater proportion of sons than females with experimentally clipped flight feathers (see text for statistical results from a generalized linear model). Each box plot displays the median (horizontal line inside box), 25th and 75th percentiles (ends of the box) and 10th and 90th percentiles (ends of lines outside each box) of the proportion of males in a brood.
Given the lack of an effect of female condition on brood sex ratio, we examined previous years to see if the correlation between brood sex ratio and female body condition in 1998 (reported by Whittingham & Dunn 2000) also occurred in other years. For this analysis we examined the brood sex ratio of females that were not manipulated in 1998 (n=31), 2000 (n=63), 2001 (n=19) and 2002 (n=19). Condition was estimated, as done by Whittingham & Dunn (2000), by regressing female body mass on tarsus length and date of capture. This analysis of four years of data revealed that females in better condition produced more sons (GLM; F1,129=6.6, p=0.011; figure 2). Condition was also related to brood sex ratio (GLM; F1,128=6.6, p=0.011) in a model that included year (F1,128=0.02, p=0.89).
Figure 2.
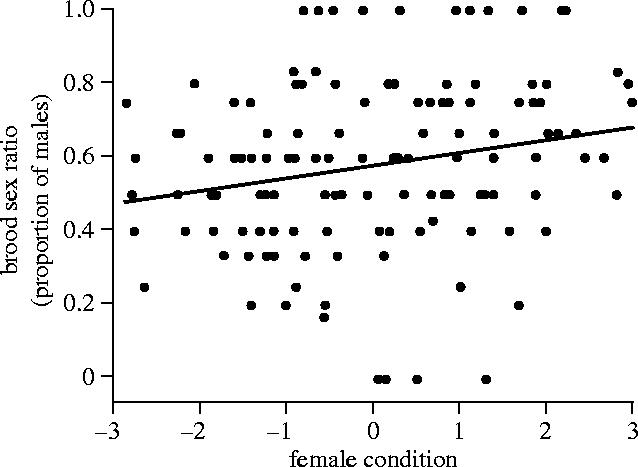
Proportion of sons in a brood in relation to the body condition of control females over four years (1998, 2000, 2001 and 2002). Line shown is from a linear regression but the relationship was tested with a generalized linear model (F1,129=6.6, p=0.011).
Overall, the feather clipping treatment significantly reduced female condition and experimental females produced fewer sons than control females. However, the response to treatment varied substantially among females with some experimental females producing broods of all sons or all daughters. Thus, in addition to our treatment, it appeared that maternal influences may also play a role. Next, we examined the repeatability of brood sex ratio for individual females producing two broods.
(b) Repeatability
We conducted a study of the repeatability of brood sex ratio from 1997 to 1999 by experimentally inducing females to produce two broods in one breeding season. We examined 237 eggs and young from 46 broods of 23 ASY females that produced two broods in one season. Ten unhatched eggs were not sampled because there was no embryonic development or they were missing from the nest. Female condition at the first nest was correlated positively with the proportion of sons in the first brood (GLMM; F1,14=7.3, p=0.017). We did not have measures of female condition at the time that the second broods were produced, so we do not know if female condition was similar for the first and second broods. The proportion of males in first broods was positively related to the proportion of males in second broods (GLM; F1,21=9.0, p=0.007) and, as a consequence, the repeatability of brood sex ratio for individual females was high (rI=0.657). First clutches (5.6±0.15 eggs) were larger than second clutches (5.0±0.15 eggs; paired t-test t22=3.44, p=0.002).
We also examined the repeatability of brood sex ratio between years for 16 different females for which we had two years of data and which were not included in previous experimental analyses. If females bred in more than two years we used data from their first two years (n=2 females). We sampled a total of 165 eggs and nestlings (two eggs were not sampled) in 32 broods. Clutches of females in year one (4.8±0.25) were smaller than their clutches in year two (5.6±0.20; paired t-test t15=3.10, p=0.007). Female condition was not related between the two years (r2=0.10, F1,12=1.4, p=0.26). However, the proportion of males in broods in year one was positively related to the proportion of males in broods in year two (GLM; F1,14=8.6, p=0.011) and, as a consequence, the repeatability of brood sex ratio between years was high for individual females (rI=0.688). In our feather clipping experiment we used 10 females in the experimental group in one year and in the control group in the other year. In this case, the repeatability of brood sex ratio for individual females could have reduced our ability to detect an effect of our experiment on brood sex ratio.
4. Discussion
Our experimental study found that feather clipping had a strong effect on brood sex ratio and female body condition. Females with clipped feathers were in poorer condition and produced fewer sons, as predicted. Furthermore, analysis of our long-term data suggested that condition was related to brood sex ratio, as was previously found in 1998 (Whittingham & Dunn 2000). However, it is interesting to note that female condition alone was not a significant predictor of brood sex ratio in our experiment. These results suggest that female condition may not be the primary causal factor influencing brood sex ratio, even though it was manipulated in our experiment. Instead, we suggest that females' food intake rate, which is probably lower for clipped feathers, might be more directly related to brood sex ratio. The effect of this type of experimental treatment may be particularly strong in aerial insectivores such as tree swallows. In a related study of clipped females (with a larger sample) we suggested that the lower reproductive performance of clipped females was primarily due to the lower intake rate of food, rather than lower body condition per se. This conclusion was based on the negative effect of treatment (clipping) on reproduction (both laying date and clutch size), even after controlling for body condition, food abundance and weather (Nooker et al. in press). A recent experimental study of the flying ability of tree swallows found that faster fliers bred earlier in the season, even after controlling for body mass and wing loading (essentially controlling for condition; Bowlin & Winkler 2004). This suggests that individual differences in flight performance and, thus, foraging efficiency can influence reproduction beyond any effects of condition. The strong influence of female effects is reinforced by the high repeatability of brood sex ratio for individual females. If the sex ratio of broods is influenced by the rate of food intake and some females are consistently better foragers than others, then we should expect to see a high repeatability of brood sex ratios for individual females.
Relatively few experimental studies have examined the effect of food or female body condition prior to egg-laying on brood sex ratio. In lesser black-backed gulls (Larus fuscus), females in poorer condition produced relatively more females in their broods than females in better condition, as predicted (condition was manipulated with egg removals and supplemental food; Nager et al. 1999). In contrast to most birds, females are the larger and presumably more expensive sex to produce in great skuas (Catharacta skua). When female skuas were provided with supplemental food the brood sex ratio was biased towards females, as predicted (Kalmbach et al. 2001). Four other experimental studies have examined the sex ratios of broods of captive zebra finches (Taeniopygia guttata) provided with different diets designed to manipulate the reproductive value of male and female offspring. These studies generally found that females produced broods with more males when they were given restricted or poorer quality diets (Bradbury & Blakey 1998; Kilner 1998; Rutkowska & Cichon 2002), although in one study the effect was restricted to large clutch sizes (Arnold et al. 2003). Male-biased broods were predicted under these conditions because daughters suffer disproportionately more from poor rearing conditions and, thus, a mother could increase her expected fitness returns if she produced more sons when food was restricted (Bradbury & Blakey 1998; Kilner 1998; Arnold et al. 2003). These experimental studies have shown that females breeding under more stressful conditions bias the sex of their broods in the direction expected to maximize fitness returns for the female.
We found that brood sex ratio was highly repeatable for individual females within and between seasons. At least between seasons, this was not due to similar body condition as there was no correlation between female condition in different years. Significant repeatability of individual sex ratios may constrain the ability to detect relationships between brood sex ratio and variables such as female condition or food availability, which are likely to have a strong environmental component. Few studies have examined repeatability of brood sex ratio but the results suggest variation among species. Brood sex ratio was significantly repeatable between years in blue tits (Parus caeruleus; Griffith et al. 2003), but not in great tits (Parus major; Oddie & Reim 2002) or tawny owls (Strix aluco; Appleby et al. 1997). These maternal effects should be considered in future studies of brood sex ratios.
The Trivers–Willard hypothesis (1973) predicts that mothers in good condition will achieve greater fitness when they produce more sons. This prediction depends on two assumptions: (i) maternal condition has a positive influence on offspring condition and (ii) biasing brood sex ratio positively influences the fitness of females. In tree swallows we have shown previously that maternal condition has a positive influence on the condition of sons (Whittingham & Dunn 2000); however, few other studies of maternal condition and brood sex ratio have examined this relationship (Nager et al. 2000; Velando 2002). The second assumption will be more difficult to address because many study species have low recruitment rates and, thus, it is difficult to estimate effects on female fitness. However, there is both correlative and experimental evidence that biased brood sex ratios positively influence the fitness of females via the reproductive success of her offspring (Appleby et al. 1997; Komdeur et al. 2002). The failure to fulfil these assumptions may explain why some studies do not find a relationship between maternal condition and brood sex ratio (e.g. Westneat et al. 2002; Ramsay et al. 2003). However, it is important to note that the few experimental studies to date have invariably shown that females in better condition bias their brood sex ratio in the direction expected for greater fitness returns. It is also important to realize that brood sex ratio may covary with female condition but may not be directly affected by condition. In the case of tree swallows, it is likely that brood sex ratio was influenced more by the rate of food intake (manipulated by feather clipping) than by our estimates of female body condition.
Acknowledgments
We thank E. Clotfelter, M. Stapleton and K. Thusius for help in the field. We are grateful to the National Science Foundation and the American Ornithologist's Union for financial support. This work was conducted under UWM Animal Care and Use Committee permits No. 35 (1997–1998), No. 26 (1998–1999), No. 19 (1999–2000), No. 90 (2000–2001) and No. 107 (2001–2002).
Footnotes
Present address: Division of Biology, Kansas State University, Manhattan, KS 66506, USA.
References
- Appleby B.M, Petty S.J, MacDonald D.W. Does variation of sex ratio enhance reproductive success of offspring in tawny owls (Strix aluco) Proc. R. Soc. B. 1997;264:1111–1116. 10.1098/rspb.1997.0153 [Google Scholar]
- Arnold K.E, Griffiths R, Stevens D.J, Orr K.J, Adam A, Houston D.C. Subtle manipulation of egg sex ratio in birds. Proc. R. Soc. B. 2003;270(Suppl.):S216–S219. doi: 10.1098/rsbl.2003.0068. 10.1098/rsbl.2003.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.J. Bateman's principles and the measurement of sexual selection in plants and animals. Am. Nat. 1994;144(Suppl.):S126–S149. [Google Scholar]
- Badyaev A.V, Hill G.E, Beck M.L, Dervan A.A, Duckworth R.A, McGraw K.J, Nolan P.M, Whittingham L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. [DOI] [PubMed] [Google Scholar]
- Beath K.J. K. J. Beath; Sydney: 1997. GLMstat user manual version 3.1. [Google Scholar]
- Bowlin M.S, Winkler D.W. Natural variation in flight performance is related to timing of breeding in the tree swallow Tachycineta bicolor. Auk. 2004;121:345–352. [Google Scholar]
- Bradbury R.B, Blakey J.K. Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc. R. Soc. B. 1998;265:895–899. 10.1098/rspb.1998.0375 [Google Scholar]
- Dunn P.O, Whittingham L.A, Lifjeld J.T, Robertson R.J, Boag P.T. Effects of breeding density, synchrony and experience on extra-pair paternity in tree swallows. Behav. Ecol. 1994;5:123–129. [Google Scholar]
- Griffith S, Ornborg J, Russell A, Andersson S, Sheldon B.C. Correlations between ultraviolet coloration, overwinter survival and offspring sex ratio in the blue tit. J. Evol. Biol. 2003;16:1045–1054. doi: 10.1046/j.1420-9101.2003.00550.x. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double M, Orr K, Dawson R. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1076. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Kempenaers B. Parental care and adaptive brood sex ratio manipulation in birds. Phil. Trans. R. Soc. B. 2002;357:363–372. doi: 10.1098/rstb.2001.0924. 10.1098/rstb.2001.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis D.M, Larson A, Davis S.K, Zimmer E.A. Nucleic acids III: sequencing. In: Hillis D.M, Moritz C, editors. Molecular systematics. Sinauer; Sunderland, MA: 1990. pp. 318–370. [Google Scholar]
- Hussell D. Age and plumage color in female tree swallows. J. Field Ornithol. 1983;54:312–318. [Google Scholar]
- Jakob E.M, Marshall S.D, Uetz G.W. Estimating fitness: a comparison of body condition indices. Oikos. 1996;77:61–67. [Google Scholar]
- Kalmbach E, Nager R.G, Griffiths R, Furness R.W. Increased reproductive effort results in male-biased offspring sex ratio: an experimental study in a species with reversed sexual size dimorphism. Proc. R. Soc. B. 2001;268:2175–2179. doi: 10.1098/rspb.2001.1793. 10.1098/rspb.2001.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempenaers B, Everding S, Bishop C, Boag P, Robertson R. Extra-pair paternity and the reproductive role of male floaters in the tree swallow (Tachycineta bicolor) Behav. Ecol. Sociobiol. 2001;49:251–259. [Google Scholar]
- Kilner R. Primary and secondary sex ratio manipulation by zebra finches. Anim. Behav. 1998;56:155–164. doi: 10.1006/anbe.1998.0775. [DOI] [PubMed] [Google Scholar]
- Komdeur J, Pen I. Adaptive sex allocation in birds: the complexities of linking theory and practice. Phil. Trans. R. Soc. B. 2002;357:373–380. doi: 10.1098/rstb.2001.0927. 10.1098/rstb.2001.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur J, Daan S, Tinbergen J, Mateman C. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs. Nature. 1997;385:522–487. [Google Scholar]
- Komdeur J, Magrath M.J.L, Krackow S. Pre-ovulation control of hatchling sex ratio in the Seychelles warbler. Proc. R. Soc. B. 2002;269:1067–1072. doi: 10.1098/rspb.2002.1965. 10.1098/rspb.2002.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krackow S, Tkadlec E. Analysis of brood sex ratios: implications of offspring clustering. Behav. Ecol. Sociobiol. 2001;50:293–301. [Google Scholar]
- Lessels C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- McCarty J.P. Variation in growth of nestling tree swallows across multiple temporal and spatial scales. Auk. 2001;118:176–190. [Google Scholar]
- McCullagh P, Nelder J.A. Chapman & Hall; London: 1983. Generalized linear models. [Google Scholar]
- Miller S.A, Dykes D.D, Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nager R.G, Monaghan P, Griffiths R, Houston D.C, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. Natl Acad. Sci. USA. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nager R.G, Monaghan P, Genovart M. Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus) Behav. Ecol. Sociobiol. 2000;48:452–457. [Google Scholar]
- Neuhauser M. Tests for a biased sex ratio when the data are clustered. Environ. Ecol. Stat. 2004;11:295–304. [Google Scholar]
- Nooker, J. K., Dunn, P. O. & Whittingham, L. A. In press. Effects of food abundance, weather and female condition on reproduction in tree swallows. Auk
- Oddie K.R, Reim C. Egg sex ratio and paternal traits: using within-individual comparisons. Behav. Ecol. 2002;13:503–510. [Google Scholar]
- Radford A.N, Blakey J.K. Is variation in brood sex ratios adaptive in the great tit (Parus major)? Behav. Ecol. 2000;11:294. [Google Scholar]
- Ramsay S, Mennill D, Otter K, Ratcliffe L, Boag P. Sex allocation in black-capped chickadees Poecile atricapill. J. Avian Biol. 2003;34:134–139. [Google Scholar]
- Robertson R.J, Stutchbury B.J, Cohen R.R. Tree swallow. In: Poole A, Gill F, editors. Birds of North America. vol. 11. The Academy of Natural Sciences/American Ornithologists' Union; Philadelphia/Washington, DC: 1992. [Google Scholar]
- Rutkowska J, Cichon M. Maternal investment during egg laying and offspring sex: an experimental study of zebra finches. Anim. Behav. 2002;64:817–822. [Google Scholar]
- SAS Institute. SAS Institute; North Carolina: 2001. SAS user's guide. [Google Scholar]
- Sheldon B.C, West S.A. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 2004;163:40–54. doi: 10.1086/381003. [DOI] [PubMed] [Google Scholar]
- Sheldon B, Andersson S, Griffith S, Ornborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. [Google Scholar]
- Trivers R, Willard D. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Velando A. Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav. Ecol. 2002;13:443–449. [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- Westneat D.F, Stewart I.R.K, Woeste E.H, Gipson J, Abdulkadir L, Poston J.P. Patterns of sex ratio variation in house sparrows. Condor. 2002;104:598–609. [Google Scholar]
- Whittingham L.A, Dunn P.O. Offspring sex ratios in tree swallows: females in better condition produce more sons. Mol. Ecol. 2000;9:1123–1129. doi: 10.1046/j.1365-294x.2000.00980.x. [DOI] [PubMed] [Google Scholar]
- Whittingham L.A, Dunn P.O. Effects of extra-pair and within-pair reproductive success on the opportunity for selection in birds. Behav. Ecol. 2005;16:138–144. [Google Scholar]
- Whittingham L.A, Schwabl H. Maternal testosterone in tree swallow eggs varies with female aggression. Anim. Behav. 2002;63:63–67. [Google Scholar]
- Whittingham L.A, Dunn P.O, Robertson R.J. Female response to reduced male parental care in birds: an experiment in tree swallows. Ethology. 1994;96:260–269. [Google Scholar]
- Zar J.H. 4th edn. Prentice Hall; New Jersey: 1999. Biostatistical analysis. [Google Scholar]


