Abstract
That predators attack and prey defend is an oversimplified view. When size changes during development, large prey may be invulnerable to predators, and small juvenile predators vulnerable to attack by prey. This in turn may trigger a defensive response in adult predators to protect their offspring. Indeed, when sizes overlap, one may wonder ‘who is the predator and who is the prey’! Experiments with ‘predatory’ mites and thrips ‘prey’ showed that young, vulnerable prey counterattack by killing young predators and adult predators respond by protective parental care, killing young prey that attack their offspring. Thus, young individuals form the Achilles' heel of prey and predators alike, creating a cascade of predator attack, prey counterattack and predator defence. Therefore, size structure and relatedness induce multiple ecological role reversals.
Keywords: role reversals, predator–prey interactions, stage structure, relatedness, antipredator behaviour
1. Introduction
Predators are usually larger than their prey. This difference in size has profound effects on the capture success of predators: the smaller the difference in size, the lower the probability of prey capture (Hespenheide 1973; Aljetlawi et al. 2004). Apart from variability in size due to genetic differences, size also changes dramatically during development. The consequences of such size changes have been well studied for prey (Murdoch et al. 1987; Persson & Eklov 1995; Chase 1999; Borer 2002; Claessen et al. 2002; Roos & Persson 2002; Cohen et al. 2003; Savage et al. 2004), but much less so for predators. Size overlap of predators and prey due to ontogeny may drive role reversals (Saito 1986; Barkai & McQuaid 1988; Polis et al. 1989; Dorn & Mittelbach 1999; Palomares & Caro 1999), but even young and vulnerable prey may kill younger and smaller predators. This in turn may trigger a defensive response in predators to protect their offspring, leading to yet another role reversal, but this possibility has never been addressed. We investigated whether multiple role reversals occurred in a system consisting of the predatory mite Iphiseius degenerans (Berlese) and its prey, the thrips Frankliniella occidentalis (Pergande). The predatory mite attacks young and small thrips larvae, which usually feed on plant tissue. Iphiseius degenerans are used as biocontrol agents of thrips (van Houten & van Stratum 1995). Hence, the system has been viewed as a classical predator–prey system. It was recently found, however, that thrips adults, but also the small and vulnerable larvae, kill predatory-mite eggs (Faraji et al. 2002; Janssen et al. 2002). Thrips larvae benefit from killing predator eggs because it supplements their diet (Janssen et al. 2003). Moreover, it lowers the predation risk of thrips larvae by deterring adult female predatory mites, the most voracious predator stage (Janssen et al. 2002). Female predators are deterred because they avoid sites where their offspring will suffer from high mortality due to prey counterattack (Janssen et al. 2002). Because predatory mites require food to produce eggs and develop one egg at a time, this avoidance results in a conflict between two mutually exclusive activities: feeding on prey or ovipositing in safe sites without counterattacking ‘prey’. As an alternative to avoiding counterattack, we hypothesize that female predators lay eggs where they forage and reduce counterattack by defending their offspring, but not that of other, unrelated (foreign) females (Hamilton 1964), i.e. they perform protective parental care in response to counterattacking prey.
2. Material and methods
(a) Predation experiments
The prey, first-instar thrips larvae (F. occidentalis), and the predator, phytoseiid mites (females and eggs; I. degenerans), were cultured as in Faraji et al. (2000). We studied their interactions on barbell-shaped arenas cut from a single cucumber leaf: two leaf discs (∅36 mm) connected by their shared midrib (6–7 cm long, 3 mm wide) floating on water-soaked cotton wool inside a Petri dish. Each disc contained ca 0.1 mg of Typha pollen as food for both prey and predator (Janssen et al. 2002). A single adult female predator was placed on one disc and was precluded from moving to the other disc by a thin (5 mm) strip of wet tissue paper, halfway the midrib. One day later, the female was removed and the position of her eggs (2–3) was recorded on a map. Subsequently, the tissue barrier was taken away and five prey were added to each disc. Prey allocated to the same disc were marked with either blue or red fluorescent powder (colour on each disc randomized among replicates), to enable recording the final position of prey relative to the initial position (Janssen et al. 2002). This allowed an accurate estimate of prey mortality on each disc. Subsequently, we placed a single female predator halfway the midrib. One day later, the number of prey larvae and predator eggs killed on each disc was scored.
To manipulate relatedness between predator females and eggs, we used two cultures, originating from one culture batch, yet separated for ca 180 generations. Female predators that had been ovipositing on a disc during 1 day prior to the experiment were placed on the midrib of either the same arena or of an arena previously occupied by a female predator from the other culture (‘foreign female’). Thus, female predators were exposed to cues (eggs, faeces and other cues) left on the substrate by themselves or by a foreign female. To investigate whether cues signalling high risk of egg predation by counterattacking prey affect the behaviour of predators, we added three foreign predator eggs to the disc with cues and pierced them with a thin needle (Janssen et al. 2002). Thus, four treatments were carried out: own disc and foreign disc with or without destroyed eggs.
Data on prey mortality are presented as average numbers of prey killed during 24 h on each disc of the arenas. Under H0, equal prey mortality on the two discs is expected; deviations were tested using the Wilcoxon matched-pairs signed-ranks test per treatment (Siegel & Castellan 1988; Field 2000). To assess how the interaction between relatedness and the threat of counterattack affected the killing of prey by female predators, a Kruskall–Wallis ANOVA was carried out on the number of prey killed on the discs with cues, followed by post hoc comparisons corrected for multiple comparisons (Siegel & Castellan 1988). We tested whether predators exposed to their own cues protected their offspring by comparing egg mortality in treatments where cues were own versus foreign using a Mann–Whitney U-test. During the experiment, eggs may be produced by predators and subsequently be killed by prey. Because killed eggs cannot be detected, only the eggs produced prior to prey introduction were included in the analysis; they were discriminated from eggs laid later in the experiment by their position and by their colour (colour changes with embryonic development).
The cues used by female predators to assess their relatedness to the eggs present could come from the eggs, from other cues that females left on the disc, from the destroyed eggs added, or from a combination of these cues. To assess which cues trigger parental care behaviour in predators, we systematically varied them on the treated disc: predators were either exposed to (i) their own eggs on a foreign disc (i.e. a disc containing cues other than eggs produced by an unrelated predator during a previous visit) with foreign eggs added and killed; (ii) eggs produced by an unrelated predator (foreign eggs) on a disc previously visited by the predator under test (own disc) with artificially killed foreign eggs; (iii) foreign eggs on a foreign disc with own eggs killed. Differences in prey mortality within treatments were assessed using the Wilcoxon matched-pairs signed-ranks test per treatment, as in the previous set of treatments.
(b) Behavioural observations
Using a time-lapse-video-equipped stereoscope (30× magnification), behaviour of predator and prey was observed on a small part of the treated leaf disc (1 cm2), which included the connection to the midrib. Ten additional replicates were carried out of two of the above treatments that yielded contrasting results in the predation experiments: foreign cues and foreign killed eggs versus own cues and destroyed eggs absent. The experimental procedure was identical to the predation experiment, except that female predators were placed on the disc with cues instead of on the midrib, and predator eggs were placed within the observation area. We recorded the total time spent by predators away from the disc under observation, the time spent by predators and by prey near predator eggs, and the frequency of the following events: predators chasing away prey (i.e. moving towards prey and touching it, triggering prey escape), predators killing prey and prey touching eggs. In addition, the per capita prey migration rate (events/prey/time) was calculated from prey migration events and prey number on the disc with cues corrected for prey mortality due to predation. Because only a small area of one disc was filmed, we could not assess overall prey mortality in the arena. Hence, this mortality was measured in an independent, yet identical experiment (10 replicates per treatment), in which prey survival on each of the discs in presence of a female predator was assessed at hourly intervals during 12 h. The mortality rate was estimated as the exponent of a negative exponential function fitted to the prey survival data. Differences between treatments were analysed using MANOVA, followed by Student t-tests to compare each variable between treatments.
3. Results
(a) Predation experiments
When predators were returned to their original arena, they killed significantly more prey on the disc where they had previously oviposited than on the untreated disc (figure 1a, Wilcoxon matched-pair test: Z=−3.05, p=0.002). In contrast, when a foreign female predator had oviposited on the treated disc, the female under test killed fewer prey on this disc than on the untreated disc, but this difference was not significant (figure 1a, Z=−1.89, p=0.061). In the absence of a simulated counterattack (no destroyed eggs added), predators still killed more prey on the disc where they oviposited previously (figure 1b, Z=−2.79, p=0.006), but they killed equal numbers of prey on both discs when the treated disc contained cues of foreign females (figure 1b, Z=−0.285, p=0.775).
Figure 1.
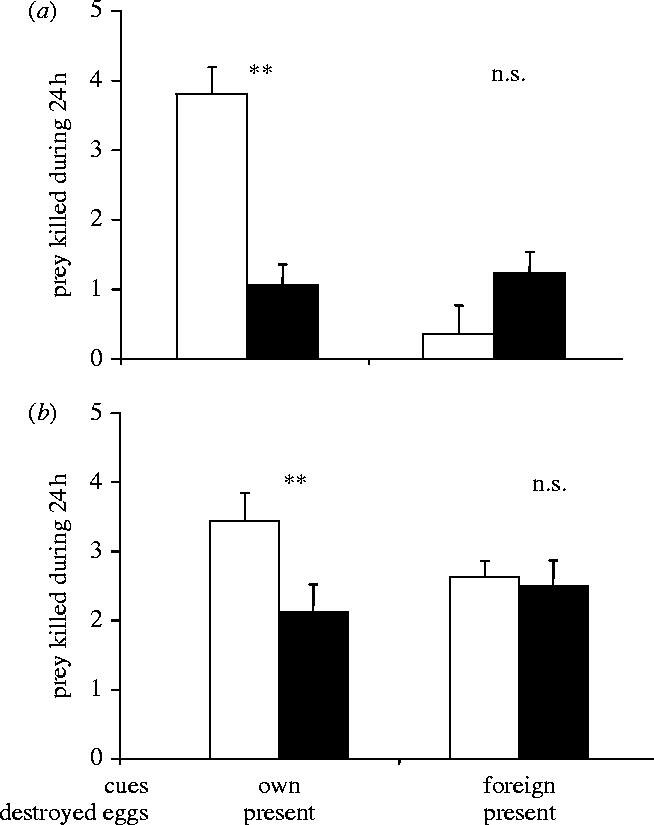
Average (+s.e.m.) number of prey killed by predator son treated patches (white bars) and on untreated patches (black bars). Treatments, indicated below the x-axis, consisted of combinations of cues (eggs, faeces, etc.) left by the predator under test (own) or by another predator (foreign), and presence (a) or absence (b) of artificially killed eggs, simulating an increased risk of counterattack by prey. Untreated patches received no eggs, no artificially killed eggs, nor other cues. 0.01<*p<0.05 (Wilcoxon signed-pairs test); 0.001<**p<0.01; n.s.: not significant, p>0.05. Sample size per treatment: 16–17.
Among treatments, the number of prey killed on treated discs differed significantly (Kruskall–Wallis ANOVA, KW=27.1, d.f.=3, p<0.0001). In presence of simulated counterattack (destroyed eggs), prey mortality was higher on discs with own cues than on discs with foreign cues (figure 1a, planned comparison, p<0.001). This difference was not found in absence of such a threat (figure 1b, n.s.). The addition of destroyed eggs did not affect the number of prey killed on treated discs with own cues (figure 1a,b, n.s.), whereas it resulted in decreased prey mortality on treated discs with foreign cues (figure 1a,b, p<0.005). The latter is consistent with earlier results showing that predators avoid sites with cues signalling a high risk of prey counterattack (Faraji et al. 2001; Janssen et al. 2002).
Counterattacking prey killed fewer predator eggs in treatments where eggs and other cues were of the predator under test than when eggs and other cues were of a foreign female (0 versus 0.18±0.08 predator eggs, killed Mann–Whitney U-test, U=2.27, p=0.023). This shows that the increased killing of prey by adult predators in response to own cues (figure 1) results in a lower risk of predator eggs being killed by prey.
In summary, predators killed higher numbers of counterattacking prey in response to own cues, but killed fewer prey on discs with foreign eggs and cues simulating the risk of counterattack on eggs (figure 1). The increased killing of prey near own eggs resulted in a lower risk of these eggs being attacked by prey. Hence, predators defended their offspring, but not that of foreign predators, from counterattacking prey. We interpret this as evidence for protective parental care.
In the next set of treatments, we exposed predators to different cues, to investigate which ones triggered the parental care observed. Compared to untreated discs, predators killed more prey on foreign discs with own eggs and with destroyed foreign eggs (figure 2, Z=−2.31, p=0.021). Female predators also killed more prey on discs with foreign eggs and foreign destroyed eggs than on untreated discs, but this difference was not significant (figure 2, Z=−1.892, p=0.063). In contrast, predators killed more prey on untreated discs than on foreign discs with foreign eggs but own destroyed eggs (figure 2, Z=−2.08, p=0.037), indicating that parental care of female predators is not triggered by the relatedness of destroyed eggs. These contrasting patterns of predation by predators affected the killing of predator eggs by prey: fewer eggs of predators were killed by prey when cues from the eggs or from the substrate were own versus foreign (0.33±0.1 versus 1.06±0.28, respectively; Mann–Whitney U-test, U=−2.92, p=0.004). Therefore, parental care is triggered by cues from own eggs as well as by cues that predators left on the disc during a previous visit, although to a lesser extent, and the relatedness of destroyed eggs does not affect parental care.
Figure 2.
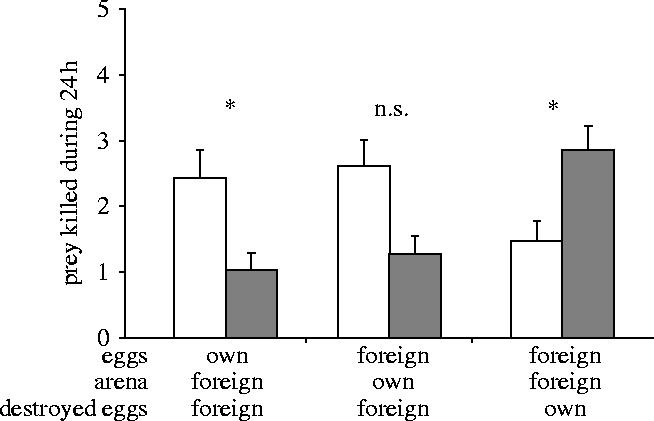
Average (+s.e.m.) number of prey killed by predators on treated patches (white bars) and on untreated patches (black bars). Treatments, indicated below the x-axis, consist of combinations of relatedness of eggs, origin of cues left on the disc, and of relatedness of destroyed eggs. Untreated patches received neither eggs, destroyed eggs, nor other cues. 0.01<*p<0.05 (Wilcoxon signed-pairs test); n.s.: not significant, p>0.05. Sample sizes 19, 23, 17.
(b) Behavioural observations
We observed the behaviour of predators and prey under two contrasting treatments (own versus foreign cues, presence versus absence of foreign destroyed eggs). The behaviour of predators differed between treatments (MANOVA, F7,12=4.17, Wilks' Lambda=0.29, p=0.015). Predators spent less time on untreated discs when treated discs contained own eggs instead of foreign eggs, but this difference bordered significance (table 1). Time spent within the observation area (nearby the eggs) did not differ between treatments (table 1). However, predators chased and killed more prey near own eggs than near foreign eggs (table 1). This behaviour did not result in fewer prey being close to the predator eggs, but led to a strong reduction in the number of prey contacts with predator eggs (table 1). Taken together, this behaviour is further evidence for protective parental care of predators. Parental care, however, did not result in a significant increase of the per capita emigration rate of prey from treated discs when cues were own versus foreign (table 1).
Table 1.
Behavioural observations (mean±s.e.) of predators and prey on arenas where the treated disc contained cues produced by a foreign predator (‘foreign’ column) or by the predator under test (‘own’ column).
| foreign | own | test statistic | p-value | |
|---|---|---|---|---|
| predators on untreated disc (h) | 11.5±1.99 | 5.7±1.98 | 2.06 | 0.053 |
| predators near eggs (h) | 1.9±0.56 | 3.1±0.85 | 1.6 | 0.26 |
| prey near eggs (h) | 11.4±3.58 | 9.7±2.48 | 0.39 | 0.7 |
| predators chasing prey (h−1) | 1.2±0.44 | 5.2±1.1 | 3.6 | 0.002 |
| predators killing prey (h−1) | 0.2±0.13 | 1.1±0.35 | 2.41 | 0.027 |
| prey touching predator eggs (h−1) | 8.8±2.89 | 1.9±0.67 | 2.32 | 0.032 |
| per capita prey emigration rate (h−1) | 0.19±0.02 | 0.07±0.08 | 1.66 | 0.116 |
The three first variables are measured as the time spent in each of them, the four last ones are frequencies. The test statistic given is the T of the t-test performed following a MANOVA on all variables (see main text). The statistic has 18 degrees of freedom. Sample size per treatment, 10.
4. Discussion
Our data demonstrate that predation serves not only to acquire food, but also as a defence mechanism. Indeed, predatory mites defend their eggs against counterattacking prey by killing more prey in the vicinity of their eggs. This does not occur when the eggs present are not related to the female under test, thus female predators perform protective parental care in response to prey counterattack. Parental care is triggered by cues emanating from the offspring, and to a smaller extent, by cues left on the substrate.
Because the parental care displayed by predators in response to prey counterattack results in more prey being killed, this raises the question of why prey would counterattack in the first place. One reason could be that larger, older prey are invulnerable to predator attacks (Bakker & Sabelis 1989), and therefore, do not run a higher risk when counterattacking. Yet, this does not explain counterattacks by small, vulnerable prey, which suffer increased predation when predators defend their eggs. Opportunities for counterattacks by vulnerable prey arise when predators leave their offspring unattended, for example to feed elsewhere. This is the case on sweet-pepper plants (Capiscum annum), where predatory mites feed on thrips as well as on pollen. Thrips, in turn, feed on leaf tissue and pollen. On sweet-pepper plants, interspecific aggregations of predators and prey arise in flowers (van Houten & van Stratum 1995). Predators prevent prey counterattacks on their eggs by ovipositing away from flowers (Faraji et al. 2001) and preferably lay their eggs in clusters inside small tufts of leaf hairs (domatia) at the underside of leaves (van Houten & van Stratum 1995). Hence, predatory mites can provide protective parental care for all their offspring while near the cluster of own eggs, which potentially reduces the cost of this behaviour (Tallamy 1999). However, female predators need to commute to the flowers to feed. Hence, they either dump their eggs on another females' cluster and rely on other females to protect their eggs (Tallamy & Horton 1990; Loeb 2003), or they are forced to leave their eggs unattended (Faraji et al. 2001). This opens the opportunity for thrips to counterattack, thereby deterring future visits by predatory mites, resulting in trophic role reversals, such as reported here as well as for other systems (Saito 1986; Barkai & McQuaid 1988; Polis et al. 1989; Dorn & Mittelbach 1999; Palomares & Caro 1999). However, when predators are close to their offspring and defend it from counterattacks by killing prey, the system reverts to a classical predator–prey interaction, but with predation serving to obtain food as well as to defend offspring.
Classical predator–prey models are based on the assumptions that (i) time scales of growth processes of predators and prey are similar; (ii) each species is composed of identical individuals; and (iii) contact rates obey the law of mass action (Begon et al. 1990). Assumption (i) holds best when predators and prey are of similar size (Savage et al. 2004), but predators are usually larger than their prey. Our predators and prey are of similar size, but nevertheless size matters: not only do adult predators consume young, small prey, but also young predators are vulnerable to counterattack by prey. Thus, overlap in the inherent size structure of these predator and prey populations violates (ii) to the extent that it is not clear anymore who the ‘predator’ is and who is the ‘prey’! We show that predators respond to prey counterattack by protective parental care. Because predatory mites feed to breed and need to protect their brood, their mobility is restricted and mass action (iii) does not hold, not even at a very small spatial scale. Our results show that the interplay among relatedness, size structure and space is a crucial feature of food-web dynamics, as it adds a new dimension to predator–prey interactions.
Acknowledgments
We are grateful to Maria Nomikou, Belén Belliure, Brechtje Eshuis, Erik van Gool, Christian Tudorache, Tessa van der Hammen en Paulien de Bruijn for discussions. Maria Nomikou and Belén Belliure are also thanked for practical help, and Iza-bela Lesna for support. Comments by Maarten Boerlijst and by J. A. J. Breeuwer considerably improved this manuscript. S.M. was funded by the Portuguese Foundation for Science and Technology (FCT—Praxis XXI, scholarship reference SFRH/BD/818/2000), A.J. and M.M. were employed by the University of Amsterdam, within the framework of a NWO Pioneer project granted to A. M. de Roos.
References
- Aljetlawi A.A, Sparrevik E, Leonardsson K. Prey–predator size-dependent functional response: derivation and rescaling to the real world. J. Anim. Ecol. 2004;73:239–252. [Google Scholar]
- Bakker F.M, Sabelis M.W. How larvae of Thrips tabaci reduce the attack success of phytoseiid predators. Entomol. Exp. Appl. 1989;50:47–51. [Google Scholar]
- Barkai A, McQuaid C. Predator–prey role reversal in marine benthic ecosystems. Science. 1988;242:62–64. doi: 10.1126/science.242.4875.62. [DOI] [PubMed] [Google Scholar]
- Begon M, Harper J.L, Townsend C.L. Blackwell Scientific Publications; Cambridge: 1990. Ecology—individuals, populations and communities. [Google Scholar]
- Borer E.T. Intraguild predation in larval parasitoids: implications for coexistence. J. Anim. Ecol. 2002;71:951–965. [Google Scholar]
- Chase J.M. Food web effects of prey size refugia: variable interactions and alternative stable equilibria. Am. Nat. 1999;154:559–570. doi: 10.1086/303260. [DOI] [PubMed] [Google Scholar]
- Claessen D, Oss C, Roos A.M, Persson L. The impact of size-dependent predation on population dynamics and individual life history. Ecology. 2002;83:1660–1675. [Google Scholar]
- Cohen J.E, Jonsson T, Carpenter S.R. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA. 2003;100:1781–1786. doi: 10.1073/pnas.232715699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn N.J, Mittelbach G.G. More than predator and prey: a review of interactions between fish and crayfish. Vie Milieu. 1999;49:229–237. [Google Scholar]
- Faraji F, Janssen A, Sabelis M.W. Predatory mites avoid ovipositing near counterattacking prey. Exp. Appl. Acarol. 2001;25:613–623. doi: 10.1023/a:1016100212909. [DOI] [PubMed] [Google Scholar]
- Faraji F, Janssen A, van Rijn P.C.J, Sabelis M.W. Kin recognition by the predatory mite Iphiseius degenerans: discrimination among own, conspecific and heterospecific eggs. Ecol. Entomol. 2000;25:147–155. [Google Scholar]
- Faraji F, Janssen A, Sabelis M.W. Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol. Entomol. 2002;27:660–664. [Google Scholar]
- Field A. SAGE Publications; London: 2000. Discovering statistics using SPSS for Windows. [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour I. J. Theor. Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hespenheide H.A. Ecological inferences from morphological data. Annu. Rev. Ecol. Syst. 1973;4:213–229. [Google Scholar]
- Janssen A, Faraji F, van der Hammen T, Magalhães S, Sabelis M.W. Interspecific infanticide deters predators. Ecol. Lett. 2002;5:490–494. [Google Scholar]
- Janssen A, Willemse E, Hammen T. Poor host plant quality causes omnivore to consume more predator eggs. J. Anim. Ecol. 2003;72:478–483. [Google Scholar]
- Loeb M.L.G. Evolution of egg dumping in a subsocial insect. Am. Nat. 2003;161:129–142. doi: 10.1086/344918. [DOI] [PubMed] [Google Scholar]
- Murdoch W.W, Nisbet R.M, Blythe S.P, Gurney W.S.C, Reeve J.D. An invulnerable age class and stability in delay-differential parasitoid–hosts models. Am. Nat. 1987;128:263–282. [Google Scholar]
- Palomares F, Caro T.M. Interspecific killing among mammalian carnivores. Am. Nat. 1999;153:492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- Persson L, Eklov J. Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology. 1995;76:70–81. [Google Scholar]
- Polis G.A, Myers C.A, Holt R.D. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989;20:297–330. [Google Scholar]
- Roos A.M, Persson L. Size-dependent life-history traits promote catastrophic collapses of top predators. Proc. Natl Acad. Sci. USA. 2002;99:12 907–12 912. doi: 10.1073/pnas.192174199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. Prey kills predator: counter-attack success of a spider mite against its specific phytoseiid predator. Exp. Appl. Acarol. 1986;2:47–62. [Google Scholar]
- Savage V.M, Gillooly J.F, Brown J.H, West G.B, Charnov E.L. Effects of body size and temperature on population growth. Am. Nat. 2004;163:429–441. doi: 10.1086/381872. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan N.J. McGraw Hill; Singapore: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Tallamy D.W. Semelparity and the evolution of parental care in insects. Anim. Behav. 1999;57:727–730. doi: 10.1006/anbe.1998.1008. [DOI] [PubMed] [Google Scholar]
- Tallamy D.W, Horton L.A. Costs and benefits of the egg-dumping alternative in Gargaphia lace bugs (Hemiptera: Tingidae) Anim. Behav. 1990;39:352–359. [Google Scholar]
- van Houten Y.M, van Stratum P. Control of western flower thrips in winter with Amblyseius cucumeris (Oudemans) and A. degenerans (Berlese) In: Parker B.L, editor. Thrips biology and management. Plenum Press; New York: 1995. pp. 245–248. [Google Scholar]


