Abstract
We present the results of the first quantitative, whole-lifespan study of the relationship between age-specific neurolipofuscin concentration and natural mortality rate in any organism. In a convenient laboratory animal, the African migratory locust, Locusta migratoria, we find an unusual delayed-onset neurolipofuscin accumulation pattern that is highly correlated with exponentially accelerating age-specific Gompertz–Makeham death rates in both males (r=0.93, p=0.0064) and females (r=0.97, p=0.0052). We then test the conservation of this association by aggregating the locust results with available population-specific data for a range of other terrestrial, freshwater, marine, tropical and temperate arthropods whose longevities span three orders of magnitude. This synthesis shows that the strong association between neurolipofuscin deposition and natural mortality is a phylogenetically and environmentally widespread phenomenon (r=0.96, p<0.0001). These results highlight neurolipofuscin as a unique and outstanding integral biomarker of ageing. They also offer compelling evidence for the proposal that, in vital organs like the brain, either the accumulation of toxic garbage in the form of lipofuscin itself, or the particular molecular reactions underlying lipofuscinogenesis, including free-radical damage, are the primary events in senescence.
Keywords: ageing biomarker, evolutionary and stochastic ageing theories, free radicals, Gompertz–Makeham mortality, lifespan, lipofuscin deleteriousness
1. Introduction
Lipofuscin was first described in neurones by Hannover (1842). It is one of the longest known and phylogenetically most widespread cellular manifestations of ageing. It consists of characteristically autofluorescing lipid–protein aggregates. These arise by incomplete lysosomal turnover of oxidatively damaged or redundant cellular components including mitochondria and photoreceptor membranes. Lipofuscin often accumulates near-linearly with time in post-mitotic cells (Sohal 1981).
Traditionally, lipofuscin has been regarded as a harmless wear-and-tear pigment. However, recent evidence from in vitro studies on cell monocultures suggests that lipofuscin itself may produce multiple negative effects. It may interfere with normal autophagic recycling of cellular components. It may sensitize lysosomes to both oxidative stress and visible light, destroying lysosomal integrity and causing apoptosis by release of lysosomal contents (Brunk & Terman 2002). The relevance of in vitro studies to normal ageing processes has been questioned in this context (Porta 2002). However, recent evidence for neurolipofuscin exocytosis in vivo begs the question as to why such a mechanism would evolve unless the presence of intracellular lipofuscin itself is somehow detrimental to cell function and organism survival (Fonseca et al. 2005).
A general inverse association between lipofuscin deposition and longevity has been known for some time. For example, the greater the natural lifespan, the lower the cardiac lipofuscin accumulation rate among various species of mammals, including primates (Munnel & Getty 1968; Nakano & Gotoh 1992). Experimental manipulation of ageing by elevation of environmental temperature reduces lifespan while increasing neurolipofuscin accumulation rate in freshwater crayfish, Cherax quadricarinatus (Sheehy et al. 1995). Flight restriction by solitary confinement increases lifespan and reduces neurolipofuscin accumulation rate in houseflies, Musca domestica (Sohal & Donato 1979). Traditionally, this inverse association has been attributed to the coincidental underlying influences of metabolic rate on both variables (Sohal 1981). However, lifespan is not always strongly dependent on metabolic rate (Promislow & Haselkorn 2002). Recent invertebrate interspecific comparisons using a standardized measurement protocol within cerebral regions of highest neurolipofuscin density indicate that the inverse association between lifespan and lipofuscin is considerably stronger than that between lifespan and environmental temperature or, presumably, metabolic rate. This finding suggests that the link between lifespan and lipofuscin may be more than mere coincidence (Sheehy 2002a).
The brain plays a pivotal role in the ageing process (Mattson et al. 2002). It is possible that potentially deleterious lipofuscin accumulation in the brain is a key factor responsible for senescent decline and death. However, despite 163 years of observation and research on lipofuscin, there appear to be no quantitative, whole-lifespan studies specifically of the relationship between age-specific neurolipofuscin concentration and natural mortality rate in any organism. Here, we present the first such study, using a convenient laboratory animal, the African migratory locust, Locusta migratoria. We then test the conservation of our results for locusts by incorporating them into a synthesis of available data on population-specific neurolipofuscin accumulation rates and natural mortality rates in a range of other arthropods. We discuss our findings, both in terms of the utility of neurolipofuscin as a biomarker of ageing and in terms of the insights they may offer on the fundamental mechanisms of senescence.
2. Material and methods
(a) Locust culture
Gregarious phase L. migratoria were established in three 40×40×40 cm cages from the egg pods of laboratory stock, at an initial density of approximately 200 hatchlings per cage. Cultures were maintained under a controlled light and temperature regime (12 h L at 32 °C : 12 h D at 25 °C). Locusts were fed daily with wheat seedlings and bran, and sand-filled containers were provided for oviposition. Containers with newly laid egg pods were removed for hatching elsewhere. Locust development, reproductive activity and adult mortality were monitored daily. To obtain sufficient sample sizes while avoiding error associated with asynchronous survivorship between the cages, we treated the three cages as a single experimental population. Daily observations from each cage were pooled and individuals for neurolipofuscin analysis were randomly sampled across the cages. Throughout the paper, ‘age’ refers to time since hatching.
(b) Demographic analysis
Initial adult locust cohort sizes (males, 132; females, 156) were determined by summing deaths over all ages and excluding individuals sampled for neurolipofuscin analysis. Age-specific mortality rates, μt, were estimated as
where Nt is the number alive at age t, and Δt is the time interval over which deaths are observed. The relationship between age and mortality rate was fitted with a three-parameter single exponential increase model, the Gompertz–Makeham curve
where a defines the initial or baseline intrinsic mortality rate, b is the rate at which mortality rate accelerates with age (the demographic rate of ageing), and M is the age-independent Makeham mortality rate. An unbiased least-squares fit of this model was achieved by censoring null mortality rates, followed by natural log transformation of both sides of the equation. Fitted curves and their confidence intervals were obtained using TableCurve 2D(Systat Software UK Limited) and detransformed for graphical depiction.
Population-specific average natural mortality rates, μp, for the various arthropods used in the comparative analyses were estimated from available longevity information (Sheehy 2002a; Sheehy & Prior 2005) using an empirically derived relationship (r=0.91) obtained from 134 minimally exploited populations of 79 invertebrate and vertebrate species (Hoenig 1983)
where tmax is population-specific maximum lifespan in years.
(c) Neurolipofuscin measurement
Six adult males and 6 females were sampled for neurolipofuscin analysis at monthly intervals throughout the cohort lifespan. Although neurolipofuscin deposits were widespread throughout the brain, they were quantified in the pars intercerebralis, a major neurosecretory centre (figure 1a), where they were most conspicuous (figure 1b). Validated and standardized neurolipofuscin quantification was achieved by analysis of confocal laser scanning images of histological sections as detailed in Sheehy (2002b) and references cited therein. Only sections containing both the central body and the pars intercerebralis were used. Only the area within the pars intercerebralis that contained the densest aggregations of neurolipofuscin granules was imaged. Damaged specimens or sections were discarded. A total of 553 images (avg. 10 per individual) was analysed.
Figure 1.
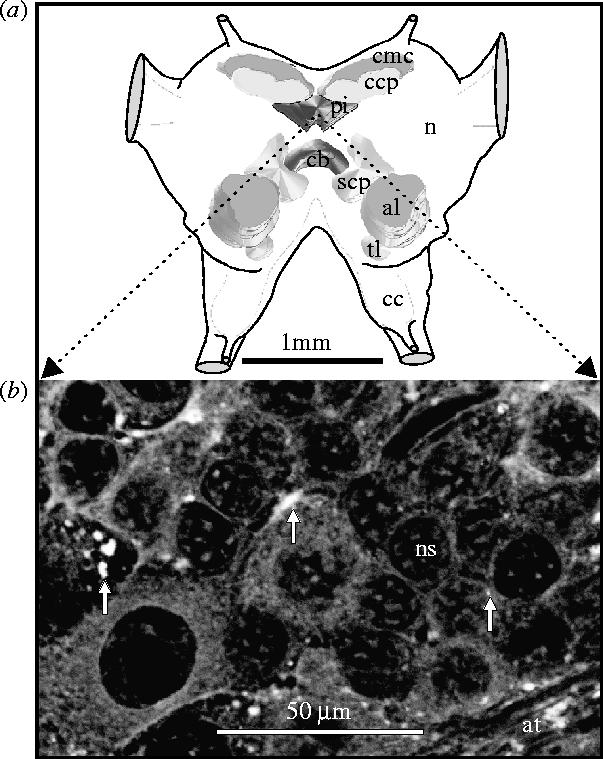
(a) Diagrammatic representation of the organization of the locust brain in dorsal view with partial three-dimensional serial-section reconstruction of prominent internal structures. al, antennal lobe; cb, central body; cc, circumoesophageal commissure; ccp, calyx of corpora pedunculata; cmc, mass of cells filling the calyx; n, neuropile; pi, pars intercerebralis; scp, stalk of corpora pedunculata; tl, tritocerebral lobe. (b) Autofluorescent neurolipofuscin granules (some arrowed) in the pars intercerebralis of a 25 week old male locust (unstained 6 μm wax section); at, axon tracts; ns, neurosomata.
(d) Statistics
The effects of age and sex on neurolipofuscin concentration were assessed by two-way ANOVA after natural-log transformation of the dependent variable, and confirmation of normality, homoscedasticity and independence of factors. The strengths of associations between mortality rate, longevity and neurolipofuscin concentration were assessed with Pearson correlation.
3. Results
(a) Demographics
Survivorship declined sharply after 12 weeks in female and then in male locusts (figure 2a) as mortality rates increased exponentially (figure 2b). Maximum observed lifespans for females and males were 24.3 and 28.3 weeks, respectively. From onset, reproductive activity continued throughout adult life in both sexes. Coefficients a, b and M of the Gompertz–Makeham mortality model were 0.0005, 0.050 and 0.0066, respectively, for males and 0.0036, 0.045 and 0.0018, respectively, for females. There were no statistically significant sex differences in these coefficients (p>0.05) despite the observed lifespan differences between males and females.
Figure 2.
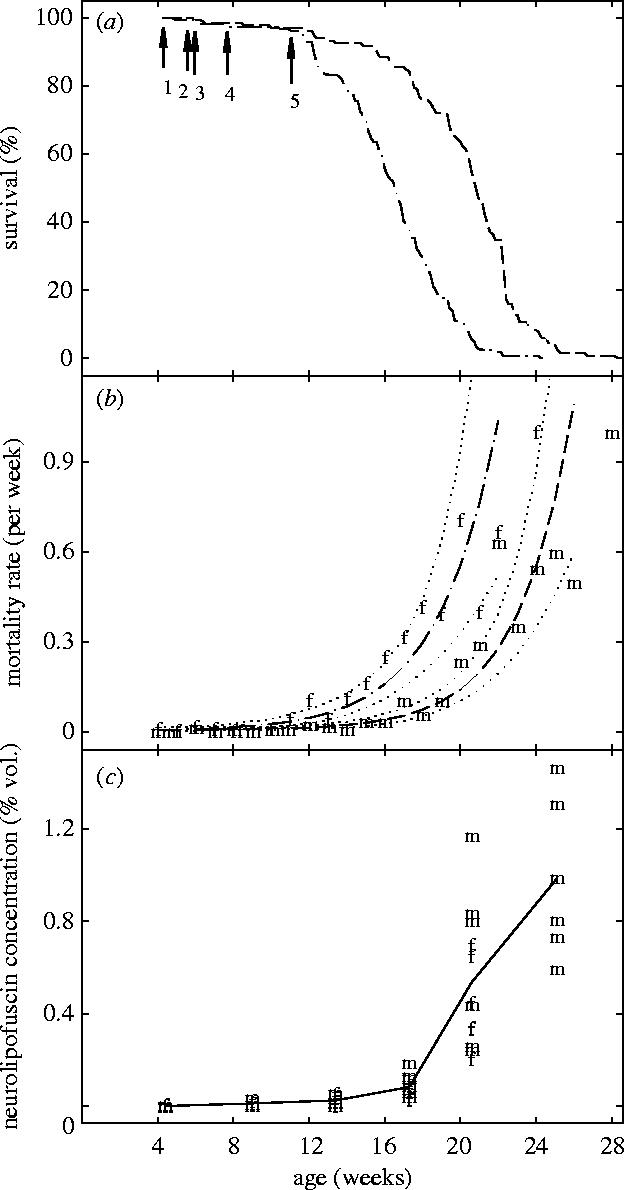
(a) Survivorship curves. (b) Age-specific mortality rates with fitted Gompertz–Makeham curves. (c) Age versus neurolipofuscin concentration in the pars intercerebralis. In (a–c), dashed lines, males, m; dashed–dotted lines, females, f; solid line, males and females combined; dotted lines, 95% confidence limits; (1) first individual moulting to adult; (2) last individual moulting to adult; (3) first copulation; (4) first oviposition; (5) first hatch. ‘Age’ is time since hatching.
(b) Neurolipofuscin accumulation
There was no resolvable neurolipofuscin in the pars intercerebralis of new adult locusts (figure 2c). Up to approximately 17 weeks of age, the concentration of neurolipofuscin remained relatively low, with female age-specific averages at 9 and 13.4 weeks (0.013 and 0.027% vol., respectively) being marginally higher than those for males at the same ages (0.012 and 0.024% vol., respectively). Thereafter, at 17.3 and 20.6 weeks, average neurolipofuscin concentrations in males (0.11 and 0.63% vol., respectively) were higher than those for females (0.06 and 0.44% vol., respectively). None of these sex differences was statistically significant (p>0.05). Neurolipofuscin began to accumulate relatively rapidly, at an average rate of 0.13% vol week−1 (6.6% vol yr−1) between 17 and 25 weeks of age. Age-specific individual variation in neurolipofuscin concentration also increased with age. Two-way ANOVA confirmed a very highly significant association between age and natural-log transformed neurolipofuscin concentration (p<0.0001) with no significant sex difference after accounting for age (p=0.31).
(c) Neurolipofuscin and mortality
There were highly significant correlations between average age-specific neurolipofuscin concentrations in the pars intercerebralis and average age-specific death rates in both male (r=0.93, p=0.0064) and female (r=0.97, p=0.0052) locusts. Aggregation of the locust results with previous population-specific neurolipofuscin and demographic data for other arthropods (Sheehy 2002a; Sheehy & Prior 2005) yielded a very highly significant positive correlation between average neurolipofuscin deposition rates and average natural mortality rates (r=0.96, p<0.0001; figure 3a). There was a comparably strong inverse relationship between neurolipofuscin accumulation rate and maximum lifespan (r=−0.96, p<0.0001; figure 3b).
Figure 3.
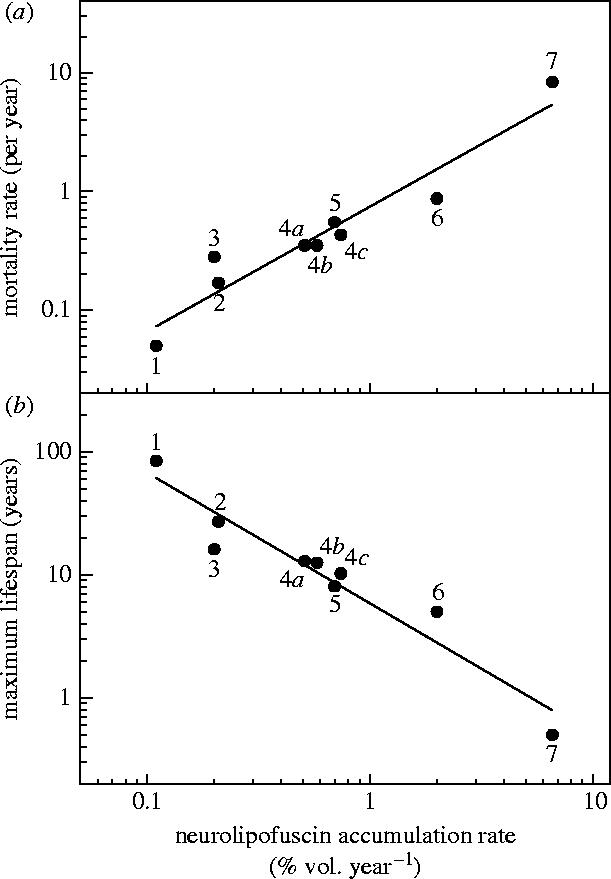
Synthesis of data (Sheehy 2002a; Sheehy & Prior 2005; this study) relating average neurolipofuscin accumulation rate in the central nervous system of various arthropods with (a) average natural mortality rate and (b) maximum lifespan. (1) European lobster, Homarus gammarus (temperate marine); (2) western rock lobster, Panulirus cygnus (temperate marine); (3) signal crayfish, Pacifastacus leniusculus (temperate freshwater); (4a–c) edible crab, Cancer pagurus (temperate marine), North Sea population, eastern English Channel population, western English Channel population, respectively; (5) Australian yabby, Cherax cuspidatus (temperate freshwater); (6) red-claw crayfish, Cherax quadricarinatus (tropical freshwater); (7) African migratory locust, Locusta migratoria (tropical and temperate terrestrial).
4. Discussion
(a) Strong correlation of neurolipofuscin concentrations and mortality rates
(i) Delayed onset
There is a considerable delay in onset, relative to lifespan, followed by a steep, near linear increase in neurolipofuscin concentration in the brain of adult locusts. We cannot explain this delay in terms of neurolipofuscin turnover or dilution due to cell growth or division. We observed no obvious age-specific changes in the size or number of cells in the pars intercerebralis. Neurogenesis in the adult brain of locusts and other insects, though extensively studied, appears to be restricted to the mushroom bodies (Cayre et al. 1996; Schmidt 2001).
The pattern of neurolipofuscin accumulation in adult locusts strongly correlates with age-specific changes in mortality rate in both males and females. Adult locusts exhibit Type I survivorship curves with highest mortality rates in old individuals (Slobodkin 1962). The locust neurolipofuscin accumulation pattern is unusual compared with those of crustacean arthropods that we have examined previously (Sheehy 2002a). In these, neurolipofuscin accumulation is rapid and near linear from an early age, but appears to decline slightly in old individuals. The fact that these crustaceans exhibit Type IV survivorship curves, with highest mortality rates in young individuals (Slobodkin 1962), alludes to the potentially far-reaching significance of neurolipofuscin deposition for animal population dynamics.
In locusts, the rapidly accelerating neurolipofuscin accumulation trajectory that we observed after 17 weeks may be associated directly or indirectly with reproduction, either by increased lipofuscinogenesis or reduced turnover. For example, physical activity associated with sexual conflict in competing male houseflies is correlated with shorter lifespans, increased oxidative damage and greater neurolipofuscin accumulation rates (Ragland & Sohal 1973; Sohal & Donato 1979; Yan & Sohal 2000). A fundamental assumption underlying many key studies of ageing in arthropod models is that mortality trajectories reflect the underlying process of senescence rather than merely increased risk of accidental death from trivial causes. However, proof has often been lacking (Partridge 1986). Our observations of an association between post-maturational neurolipofuscin accumulation and mortality are important because they confirm the link between senescence and adult death.
(ii) Sex differences and similarities
Gender comparisons are widespread in ageing research; the differing costs of reproduction for males and females and the role of sexual conflict in ageing are important current issues. The significance of our mortality results for male and female locusts is, first, that they provide a novel example of recent, somewhat surprising findings for several species of Drosophila (Promislow & Haselkorn 2002) that substantial differences in longevity within species are often not attributable to differences in the rate of demographic ageing (slope b in the Gompertz equation). Second, our results provide deeper explanation of this finding in that they show that the underlying rate of physiological ageing, as measured by neurolipofuscin accumulation, also does not appear to differ between the sexes. This is also true for every other arthropod so far examined (Sheehy 2002a). On the face of it, this seems to suggest that lifespan differences between the sexes may not be due to ageing, but to differences in immediate risk of death associated with reproduction or some other factor. For example, female mortality may be increased by toxicity of seminal fluid (Wolfner et al. 1997) or internal injury from male genitalia during copulation (Blanckenhorn et al. 2002). Alternatively, gender differences in lifespan are also explicable in terms of the onset, rather than rate, of demographic ageing (baseline mortality rate a in the Gompertz mortality curve; Promislow & Haselkorn 2002). In this regard, the marginally higher, but statistically non-significantly differing neurolipofuscin concentrations that we found in young adult female locusts warrant further investigation in future. Sex differences in age at maturation (sexual bimaturism) are common in animals (Andersson 1994) and the onset of cardiac lipofuscin accumulation has been reported previously to correlate with sexual maturation in various mammals (Nakano et al. 1990).
(iii) Late-life variability
Considerable variability in neurolipofuscin concentrations in the older chronological age groups of locusts suggests individuality in the rate of physiological ageing. In cross-sectionally sampled experimental populations, it is possible that such variability, in combination with selective mortality of the physiologically oldest individuals, affects the apparent trajectory of neurolipofuscin accumulation (Donato et al. 1979a,b). For example, the lower average neurolipofuscin concentrations in older female locusts, relative to males, and the slight reduction in the slope of the accumulation trend line generally, may reflect selective mortality of the females with highest neurolipofuscin concentrations, rather than changes in neurolipofuscin accumulation rates in individuals. For this reason, precise comparison and interpretation of the form of lipofuscin accumulation trajectories relative to mortality curves is complicated, save to say that they are strongly correlated in both sexes.
(iv) Intra- and interspecific patterns
Our synthesis of arthropod data includes information from three latitudinally varying natural populations of a single species, the edible crab, Cancer pagurus. More broadly, it includes various insect and crustacean species from different temperate and tropical, terrestrial, freshwater and marine environments. It also includes, along with short-lived locusts, species of intermediate lifespan and one of the longest-lived members of the phylum, the European lobster, Homarus gammarus. This synthesis shows that the strong association between neurolipofuscin deposition and mortality that we observed within a single population of locusts is a phylogenetically and environmentally widespread phenomenon.
Nevertheless, there do appear be some notable exceptions to this relationship. For example, the Antarctic krill, Euphausia superba, is reported to accumulate negligible quantities of neurolipofuscin (Sheehy 1990; Bluhm et al. 2001), yet it is currently estimated to live for only 11 years or less (Nicol 2000). Is there something special about the way this organism ages, perhaps associated with extremely cold environmental temperatures, a diet rich in antioxidant algal carotenoids, or periodic caloric restriction (starvation over winter)? Or is it simply that we may have significantly underestimated what its natural lifespan would be in the absence of extrinsic sources of mortality such as predation and human exploitation? Time will tell.
(b) Lipofuscin and metabolic, genetic and evolutionary theories of ageing
We observed a dramatic upturn in neurolipofuscin concentrations and mortality rates after maturation in locusts. This appears to be consistent with a trade-off between the energy invested in reproduction and that invested in homeostasis or survival as postulated under the ‘disposable soma’ theory of ageing (Kirkwood 1977).
In terms of the proximate causes of ageing, many of the results of recent research on antioxidants, caloric restriction and genetic modulation of ageing and longevity, particularly through endocrine regulation by insulin-like signals (Finkel & Holbrook 2000; Mattson et al. 2001; Hekimi & Gaurente 2003; Longo & Finch 2003; Tatar et al. 2003) point toward a common mechanism: the interplay between cellular damage production and damage repair. A hallmark of this interplay is lipofuscin, formed through incomplete proteolytic turnover of disabled cellular components (Szweda et al. 2003).
Recent immunochemical and ultrastructural evidence has demonstrated the occurrence of fluorescent post-translationally modified proteins derived from radical-mediated reactions. These include lipid peroxidation- or glucoxidation-induced damage, in particular, by malondialdehyde, 4-hydroxynonenal, and advanced glycation end products within lipofuscin granules of rat cerebral cortex neurones and human retinal pigment epithelium (RPE) (Schutt et al. 2003; Szweda et al. 2003). Lipofuscin concentration is positively correlated with mitochondrial damage (evaluated by decreased inner membrane potential) suggesting imperfect autophagy and ensuing lysosomal degradation of oxidatively damaged mitochondria (Terman et al. 2004). Lipofuscin itself contributes to further increases in damage accumulation during ageing. This occurs both by inhibition of the proteasome (Sitte et al. 2000) and by generation of further free radicals. Human RPE lipofuscin granules contain retinyl palmitate. Photochemical oxidation of retinyl palmitate generates anhydroretinol, an intracellular signalling retinoid in the signal transduction cascade that causes apoptosis by generating reactive oxygen intermediates (Lamb et al. 2001). The interaction of blue light and the pyridinium bisretinoid A2E, a molecular component of RPE lipofuscin, can result in accumulating genome damage including oxidized purine and pyrimidine bases (Sparrow et al. 2003).
Lipofuscin accumulation can be manipulated by caloric restriction (Idrobo et al. 1987; Katz et al. 1993; Moore et al. 1995), which may not only lower metabolic rate and therefore radical production, but can also lead to major hormonal changes. These stimulate gene transcription, protein synthesis and protein degradation, which also retard the overall accumulation of lipofuscin (Moore et al. 1995). Metabolic rate and free radical production are also sensitive to environmental temperature in poikilotherms. A recent review of results for numerous laboratory-manipulated and natural populations (Sheehy 2002a) found that neurolipofuscin accumulation rate was positively correlated with environmental temperature in all cases.
These findings suggest that lipofuscin either results from, or plays a key role in, most of the major proximate mechanisms currently thought to be responsible for ageing. These include long-standing theories such as ‘free radical’ (Harman 1956), ‘altered protein’ (Gershon & Gershon 1970), ‘rate of living’ (Pearl 1928) as reformulated by Sohal (1986), ‘mitochondrial damage’ (Miquel & Fleming 1984) and ‘DNA damage and repair’ (Jacobs & Court Brown 1966). They also include more recent ‘protein synthesis/degradation’, ‘signal transduction’, ‘phagocytosis’, ‘apoptosis’ and ‘neuroendocrine’ concepts of ageing (see Arking 1998). Previous studies have dismissed purely mechanistic or stochastic explanations of ageing and longevity in eusocial insects in favour of evolutionary ones (Keller & Genoud 1997). These studies apparently overlook the metabolic implications of potentially large differentials in physical activity level between relatively sedentary queens and working castes involved in foraging, guarding and nest maintenance (Fahrenholz et al. 1992; Yan & Sohal 2000). On the other hand, our results for locusts suggest that both evolutionary and stochastic mechanisms are applicable to insect ageing and longevity.
(c) Is neurolipofuscin accumulation causally responsible for mortality?
In order to assess the relationship between cellular lipofuscin accumulation and senescent functional decline, several requirements must be met: (i) functionality must be quantifiable (ii) the cell type used must accumulate lipofuscin during senescence and (iii) lipofuscin accumulation must be correlated with impairments to functionality (Katz 2002). Our experimental system meets all of these requirements. We use senescence-associated death as the ultimate marker of functional deterioration. Our results are not proof of cause. They do, however, offer compelling evidence for some previous proposals: In vital organs like the brain, either the accumulation of toxic garbage in the form of lipofuscin itself (Terman 2001), or the particular molecular events underlying the accumulation of lipofuscin, particularly free radical damage (Harman 1956), are the ‘primary events in senescence’ (Arking 1998).
(d) Lipofuscin as a biomarker of ageing
Arthropod systems such as Drosophila continue to provide significant insights for human ageing (Yeoman & Faragher 2001). Arthropods are the largest, most diverse group of animals on the planet and the present study extends the use of such systems to species with larger more experimentally accessible brains, whose lifespans are, in some cases, comparable with that of humans. Modern biogerontology frequently seeks to construct panels of individual ageing biomarkers to test the effects of particular interventions on the physiological rate of ageing of the system under investigation. Our results, along with those from previous studies on mammals, support the view that, in lipofuscin, biogerontology has a single, phylogenetically widespread and easily measured morphological entity that, as well as representing a net outcome of the balance between multiple harmful underlying biochemical reactions centrally responsible for ageing and the various systems that have evolved to protect against such harm, is also potentially deleterious in its own right. It follows that, at the very least, lipofuscin represents an outstanding and unique integral biomarker of ageing. At most, further research of the kind presented here may confirm that its toxic accumulations in key parts of key organs such as the brain are the principal cause of intrinsic natural mortality.
Acknowledgments
This research has been supported by the National Council for Scientific and Technological Development, Brazil, through a PhD studentship to D.B.F; by funding to P.M.J.S. and M.R.J.S. from the UK Department of Environment, Food and Rural Affairs and the Swedish Council for Forestry and Agriculture Research; and by funding to M.R.J.S. from the Australian Fisheries Research and Development Corporation, the Australian Research Council, the University of Queensland and the Royal Zoological Society of New South Wales. The authors wish to thank C. D'Lacey for assistance with confocal microscopy and J. Liggins, L. Barnett and M. Andrews for help with locust rearing.
Footnotes
All editorial/publisher correspondence and proofs to M.R.J. Sheehy at Leicester please.
Present address: Departamento de Oceanografia, Laboratório de Crustáceos Decápodos, Fundação Universidade Federal do Rio Grande, CP: 474, RS, CEP: 96201-900, Brazil.
Present address: Smith & Nephew Research Centre, York Science Park, Heslington, York YO1 5DF, UK.
References
- Andersson M.B. Princeton University Press; 1994. Sexual selection. [Google Scholar]
- Arking R. 2nd edn. Sinauer Associates; Sunderland, MA: 1998. Biology of aging: observations and principles. [Google Scholar]
- Blanckenhorn W.U, Hosken D.J, Martin O.Y, Reim C, Teuschl Y, Ward P.I. The costs of copulating in the dung fly Sepsis cynipsea. Behav. Ecol. 2002;13:353–358. 10.1093/beheco/13.3.353 [Google Scholar]
- Bluhm B.A, Brey T, Klages M, Arntz W.E. Occurrence of the autofluorescent pigment, lipofuscin, in polar crustaceans and its potential as an age marker. Polar Biol. 2001;24:642–649. 10.1007/s003000100258 [Google Scholar]
- Brunk U.T, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. 10.1016/S0891-5849(02)00959-0 [DOI] [PubMed] [Google Scholar]
- Cayre M, Strambi C, Charpin P, Augier R, Meyer M.R, Edwards J.S, Strambi A. Neurogenesis in adult insect mushroom bodies. J. Comp. Neurol. 1996;371:300–310. doi: 10.1002/(SICI)1096-9861(19960722)371:2<300::AID-CNE9>3.0.CO;2-6. 10.1002/(SICI)1096-9861(19960722)371:2%3C300::AID-CNE9%3E3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- Donato H, Hoselton M.A, Sohal R.S. An analysis of the effects of individual variation and selective mortality on population averages in ageing populations. Exp. Gerontol. 1979a;14:133–140. doi: 10.1016/0531-5565(79)90028-7. 10.1016/0531-5565(79)90028-7 [DOI] [PubMed] [Google Scholar]
- Donato H, Hoselton M.A, Sohal R.S. Lipofuscin accumulation: effects of individual variation and selective mortality on population averages. Exp. Gerontol. 1979b;14:141–147. doi: 10.1016/0531-5565(79)90029-9. 10.1016/0531-5565(79)90029-9 [DOI] [PubMed] [Google Scholar]
- Fahrenholz L, Lamprecht I, Schricker B. Calorimetric investigations of different castes of honey bees, Apis mellifera carnica. J. Comp. Physiol. B. 1992;162:119–130. 10.1007/BF00398337 [Google Scholar]
- Finkel T, Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Fonseca D.B, Sheehy M.R.J, Blackman N, Shelton P.M.J, Prior A.E. Reversal of a hallmark of brain ageing: lipofuscin accumulation. Neurobiol. Aging. 2005;26:69–76. doi: 10.1016/j.neurobiolaging.2004.02.013. 10.1016/j.neurobiolaging.2004.02.013 [DOI] [PubMed] [Google Scholar]
- Gershon H, Gershon D. Detection of inactive enzyme molecules in ageing organisms. Nature. 1970;227:1214–1217. doi: 10.1038/2271214a0. [DOI] [PubMed] [Google Scholar]
- Hannover A. Mikroskopiske undersögelser af nervesystemet. Kgl. Danbske Vidensk. Kabernes Selkobs Naturv. Math. Afh. Copenhagen. 1842;10:1–112. [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Gaurente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. 10.1126/science.1082358 [DOI] [PubMed] [Google Scholar]
- Hoenig J.M. Empirical use of longevity data to estimate mortality rates. Fish. Bull. 1983;81:898–903. [Google Scholar]
- Idrobo F, Nandy K, Mostofsky D.I, Blatt L, Nandy L. Dietary restriction—effects on radial maze-learning and lipofuscin pigment deposition in the hippocampus and frontal-cortex. Arch. Gerontol. Geriatr. 1987;6:355–362. doi: 10.1016/0167-4943(87)90014-8. 10.1016/0167-4943(87)90014-8 [DOI] [PubMed] [Google Scholar]
- Jacobs P.A, Court Brown W.M. Age and chromosomes. Nature. 1966;212:823–824. doi: 10.1038/212823a0. [DOI] [PubMed] [Google Scholar]
- Katz M.L. Potential role of retinal pigment epithelial lipofuscin accumulation in age-related macular degeneration. Arch. Gerontol. Geriatr. 2002;34:359–370. doi: 10.1016/s0167-4943(02)00012-2. 10.1016/S0167-4943(02)00012-2 [DOI] [PubMed] [Google Scholar]
- Katz M.L, White H.A, Gao C.L, Roth G.S, Knapka J.J, Ingram D.K. Dietary restriction slows age pigment accumulation in the retinal-pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1993;34:3297–3302. [PubMed] [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. 10.1038/40130 [Google Scholar]
- Kirkwood T.B.L. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Lamb L.E, Zareba M, Plakoudas S.N, Sarna T, Simon J.D. Retinyl palmitate and the blue-light-induced phototoxicity of human ocular lipofuscin. Arch. Biochem. Biophys. 2001;393:316–320. doi: 10.1006/abbi.2001.2492. 10.1006/abbi.2001.2492 [DOI] [PubMed] [Google Scholar]
- Longo V.D, Finch C.E. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. 10.1126/science.1077991 [DOI] [PubMed] [Google Scholar]
- Mattson M, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech. Ageing Dev. 2001;122:757–778. doi: 10.1016/s0047-6374(01)00226-3. 10.1016/S0047-6374(01)00226-3 [DOI] [PubMed] [Google Scholar]
- Mattson M, Duan W, Maswood N. How does the brain control lifespan? Ageing Res. Rev. 2002;1:155–165. doi: 10.1016/s1568-1637(01)00003-4. 10.1016/S1568-1637(01)00003-4 [DOI] [PubMed] [Google Scholar]
- Miquel J, Fleming J.E. A two-step hypothesis on the mechanisms of in vitro cell aging: cell differentiation followed by intrinsic mitochondrial mutagenesis. Exp. Gerontol. 1984;19:31–36. doi: 10.1016/0531-5565(84)90029-9. 10.1016/0531-5565(84)90029-9 [DOI] [PubMed] [Google Scholar]
- Moore W.A.L, Davey V.A, Weindruch R, Walford R, Ivy G.O. The effect of caloric restriction on lipofuscin accumulation in mouse brain with age. Gerontology. 1995;41(Suppl. 2):173–183. doi: 10.1159/000213741. [DOI] [PubMed] [Google Scholar]
- Munnel J.F, Getty R. Rate of accumulation of cardiac lipofuscin in the aging canine. J. Gerontol. 1968;23:154–158. doi: 10.1093/geronj/23.2.154. [DOI] [PubMed] [Google Scholar]
- Nakano M, Gotoh S. Accumulation of cardiac lipofuscin depends on metabolic rate of mammals. J. Gerontol. A Biol. 1992;47:B126–B129. doi: 10.1093/geronj/47.4.b126. [DOI] [PubMed] [Google Scholar]
- Nakano M, Mizuno T, Gotoh S. Accumulation of cardiac lipofuscin in mammals—correlation between sexual-maturation and the first appearance of lipofuscin. Mech. Ageing Dev. 1990;52:93–106. doi: 10.1016/0047-6374(90)90148-9. 10.1016/0047-6374(90)90148-9 [DOI] [PubMed] [Google Scholar]
- Nicol S. Understanding krill growth and aging: the contribution of experimental studies. Can. J. Fish. Aquat. Sci. 2000;57(Suppl. 3):168–177. 10.1139/cjfas-57-S3-168 [Google Scholar]
- Partridge L. Sexual activity and life span. In: Collatz K.G, Sohal R.S, editors. Insect aging: strategies and mechanisms. Springer; Berlin: 1986. pp. 45–54. [Google Scholar]
- Pearl R. Experiments on longevity. Q. Rev. Biol. 1928;3:391–407. 10.1086/394311 [Google Scholar]
- Porta E.A. Pigments in aging: an overview. Ann. NY Acad. Sci. 2002;959:57–65. doi: 10.1111/j.1749-6632.2002.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Promislow D.E.L, Haselkorn T.S. Age-specific metabolic rates and mortality rates in the genus Drosophila. Ageing Cell. 2002;1:66–74. doi: 10.1046/j.1474-9728.2002.00009.x. 10.1046/j.1474-9728.2002.00009.x [DOI] [PubMed] [Google Scholar]
- Ragland S.S, Sohal R.S. Mating behaviour, physical activity and aging in the housefly, Musca domestica. Exp. Gerontol. 1973;8:135–145. doi: 10.1016/0531-5565(73)90003-x. 10.1016/0531-5565(73)90003-X [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. J. Neurobiol. 2001;48:181–203. doi: 10.1002/neu.1050. 10.1002/neu.1050 [DOI] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz F.G, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. 10.1167/iovs.03-0172 [DOI] [PubMed] [Google Scholar]
- Sheehy M.R.J. Widespread occurrence of fluorescent morphological lipofuscin in the crustacean brain. J. Crust. Biol. 1990;10:613–622. [Google Scholar]
- Sheehy M.R.J. Role of environmental temperature in ageing and longevity: insights from neurolipofuscin. Arch. Gerontol. Geriatr. 2002a;34:287–310. doi: 10.1016/s0167-4943(01)00216-3. 10.1016/S0167-4943(01)00216-3 [DOI] [PubMed] [Google Scholar]
- Sheehy M.R.J. A flow-cytometric method for neurolipofuscin quantification and comparison with existing histological and biochemical approaches. Arch. Gerontol. Geriatr. 2002b;34:233–248. doi: 10.1016/s0167-4943(01)00217-5. 10.1016/S0167-4943(01)00217-5 [DOI] [PubMed] [Google Scholar]
- Sheehy M.R.J, Prior A.E. Marine Fisheries R & D Final Report MF0225. Department of Environment, Food and Rural Affairs; London: 2005. Analysis of stock age structure and population parameters in edible crab, Cancer pagurus, using lipofuscin age pigments: data for resource management. [Google Scholar]
- Sheehy M.R.J, Greenwood J.G, Fielder D.R. Lipofuscin as a record of rate of living in an aquatic poikilotherm. J. Gerontol. A Biol. 1995;50:B327–B336. doi: 10.1093/gerona/50a.6.b327. [DOI] [PubMed] [Google Scholar]
- Sitte N, Huber M, Grune T, Ladhoff A, Doecke W.D, von Zglinicki T, Davies K.J.A. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. 10.1096/fj.14.11.1490 [DOI] [PubMed] [Google Scholar]
- Slobodkin L.B. Holt, Rinehart & Winston; New York: 1962. Growth and regulation of animal populations. [Google Scholar]
- Sohal R.S. Metabolic rates, ageing, and lipofuscin accumulation. In: Sohal R.S, editor. Age pigments. Elsevier/North Holland Biomedical Press; Amsterdam: 1981. pp. 303–316. [Google Scholar]
- Sohal R.S. The rate of living theory: a contemporary interpretation. In: Collatz K.G, Sohal R.S, editors. Insect aging: strategies and mechanisms. Springer; Berlin: 1986. pp. 23–44. [Google Scholar]
- Sohal R.S, Donato H. Effect of experimental prolongation of life span on lipofuscin content and lysosomal enzyme activity in the brain of the housefly, Musca domestica. J. Gerontol. 1979;34:489–496. doi: 10.1093/geronj/34.4.489. [DOI] [PubMed] [Google Scholar]
- Sparrow J.R, Zhou J.L, Cai B.L. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue-light illumination. Invest. Ophthalmol. Vis. Sci. 2003;44:2245–2251. doi: 10.1167/iovs.02-0746. 10.1167/iovs.02-0746 [DOI] [PubMed] [Google Scholar]
- Szweda P.A, Camouse M, Lundberg K.C, Oberley T.D, Szweda L.I. Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Ageing Res. Rev. 2003;2:383–405. doi: 10.1016/s1568-1637(03)00028-x. 10.1016/S1568-1637(03)00028-X [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. 10.1126/science.1081447 [DOI] [PubMed] [Google Scholar]
- Terman A. Garbage catastrophe theory of ageing: imperfect removal of oxidative damage? Redox Rep. 2001;6:15–26. doi: 10.1179/135100001101535996. 10.1179/135100001101535996 [DOI] [PubMed] [Google Scholar]
- Terman A, Dalen H, Eaton J.W, Neuzil J, Brunk U.T. Aging of cardiac myocytes in culture—oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann. NY Acad. Sci. 2004;1019:70–77. doi: 10.1196/annals.1297.015. 10.1196/annals.1297.015 [DOI] [PubMed] [Google Scholar]
- Wolfner M.F, et al. New genes for male accessory gland proteins in Drosophila melanogaster. Insect Biochem. Mol. Biol. 1997;27:825–834. doi: 10.1016/s0965-1748(97)00056-8. 10.1016/S0965-1748(97)00056-8 [DOI] [PubMed] [Google Scholar]
- Yan L.J, Sohal R.S. Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associated oxidative damage to specific mitochondrial proteins. Free Radic. Biol. Med. 2000;29:1143–1150. doi: 10.1016/s0891-5849(00)00423-8. 10.1016/S0891-5849(00)00423-8 [DOI] [PubMed] [Google Scholar]
- Yeoman M.S, Faragher R.G.A. Ageing and the nervous system: insights from studies on invertebrates. Biogerontology. 2001;2:85–97. doi: 10.1023/a:1011597420036. 10.1023/A:1011597420036 [DOI] [PubMed] [Google Scholar]


