Abstract
Several avian species show a bright carotenoid-based coloration during spring and following a period of duller coloration during the previous winter, despite carotenoids presumably being fully deposited in feathers during the autumn moult. Carotenoid-based breast feathers of male linnets (Carduelis cannabina) increased in hue (redness), saturation and brightness after exposing them to outdoor conditions from winter to spring. This represents the first experimental evidence showing that carotenoid-based plumage coloration may increase towards a colourful expression due to biotic or abiotic environmental factors acting directly on full-grown feathers when carotenoids may be fully functional. Sunlight ultraviolet (UV) irradiation was hypothesized to denature keratin and other proteins that might protect pigments from degradation by this and other environmental factors, suggesting that sunlight UV irradiation is a major factor in the colour increase from winter to spring. Feather proteins and other binding molecules, if existing in the follicles, may be linked to carotenoids since their deposition into feathers to protect colourful features of associated carotenoids during the non-breeding season when its main signalling function may be relaxed. Progress towards uncovering the significance of concealment and subsequent display of colour expression should consider the potential binding and protecting nature of feather proteins associated with carotenoids.
Keywords: carotenoids, Carduelis cannabina, feathers, high-performance liquid chromatography, keratin
1. Introduction
Birds are frequently coloured with carotenoid pigments acquired from diet and generally structurally modified before being deposited in the integument (McGraw 2004a). The expression of carotenoid-based plumage coloration may function as a condition-dependant trait, reliably indicating the nutritional status and foraging ability of the bearer and then honestly revealing information on individual quality that is used in mate choice (reviewed in Hill 1999). Knowledge about the proximate mechanisms that govern the expression of carotenoid-based feather ornaments (Hill 1999), both during incorporation in developing feathers and afterwards, is much less conclusive. The expression of feather colour may be modulated by controlling the concentration and structural modification of pigments before and during their deposition (Hill 1999; McGraw et al. 2003; McGraw 2004a) and may be due to feather properties other than the presence and concentration of pigments (Troy & Brush 1983).
Among the mechanisms influencing colour expression, those operating after carotenoid deposition in feathers remain poorly understood. Despite feathers being metabolically inactive after moulting, colour expression derived from the presence and concentration of pigments may be significantly modified after feather growth by a variety of factors, including feather preen waxes (Uchida 1970; Piersma et al. 1999), alteration of the feather structure by biotic and abiotic factors (Troy & Brush 1983; Willoughby et al. 2002; McGraw & Hill 2004) and soil bathing (Negro et al. 1999; Montgomerie et al. 2001). Thus, more diverse aspects of composite plumage ornaments than previously thought may be targets of specific forms of selection in the evolution of colour expression. Advances towards revealing the composite nature of ornaments have focused on developmental and functional aspects of ornament components known to have a role in mating when signal selection might be strongest (Hill 1999; Badyaev et al. 2001; Badyaev & Young 2004). The same or different components of ornaments may be the selection target in the evolution of strategies to shift colour expression, depending on the fitness consequences derived of either displaying or concealing such expression. Progress in the knowledge of these strategies may be enabled by identifying the proximate mechanisms and components of ornaments involved in colour shifting, which deserves further research.
Several studies have documented seasonal fading of carotenoid-based plumage colour due to oxidation and abrasion in old feathers (Test 1940; McGraw & Hill 2004), especially before being moulted when colour intensity may have a lesser value in signalling status (Hill 1999; but see McGraw & Hill 2004). Much less information is available on mechanisms potentially shifting coloration during the period between carotenoid incorporation and the time when carotenoids may be fully functional (Negro et al. 1998; McGraw 2004b). These processes may in fact occur, as indicated by the fact that some species show a bright coloration during spring and a duller coloration during the previous winter (Troy & Brush 1983; Willoughby et al. 2002), despite carotenoids presumably being fully deposited on feathers during the autumn moult (Hill 1999; McGraw 2004b). Several explanations about the possible ultimate causes of this delayed bright coloration have been argued, including reduction of predation and conflict with conspecifics during the restrictive environmental conditions in winter (Promislow et al. 1992; Senar 1999) and individual recognition in flocks (Whitfield 1987). Less information is available on the mechanisms by which carotenoid-based feather coloration is concealed and subsequently expressed, despite their major importance as a means for understanding selective pressures in the evolution of feather morphology and the consequences for sexual selection. Here, we assessed the potential mechanisms by which colour is concealed during winter and subsequently expressed during spring in male linnets Carduelis cannabina.
Several hypotheses were considered to attempt to understand the mechanisms involved in the coloration change from dull to reddish in male linnets from winter to spring. First, we considered the hypothesis that carotenoids are progressively incorporated into full-grown feathers from late winter, causing an increasing red-colour acquisition as spring advances. This ‘carotenoid incorporation hypothesis’ predicts an increase in the concentration of carotenoids in red feathers in spring as compared with the concentration in brown feathers during the previous winter. This hypothesis is based on the possibility that carotenoids may be incorporated into feathers by being dissolved in lipoid secretions from the epidermis or the uropygial gland (Piersma et al. 1999; Menon & Menon 2000). Because carotenoids dissolved in epidermal lipids and potentially deposited in feathers will have been taken up from the blood, this hypothesis also predicts an increase of circulating levels of carotenoids just before or during red colour acquisition (early spring). Second, the ‘moult hypothesis’ states that the change from brown (in winter) to red (in spring) breast feathers is due to feather replacing in a prenuptial moult. Third, the structure of carotenoids present in brown breast feathers during winter may change (i.e. by oxidation), causing a chromatic shift towards red in spring. This ‘carotenoid transformation hypothesis’ predicts that carotenoids present in winter and spring are structurally different. Fourth, abrasion of light feather tips of breast feathers may promote the increase in redness from winter to spring simply because red colour already exists in its definitive expression in winter but remains concealed by the light fringes of feather tips (Newton 1972; Jenni & Winkler 1994). This ‘tip abrasion hypothesis’ predicts that colour of protected breast feathers during winter is similar to that of exposed feathers during spring. Fifth, colour changes from winter to spring may be promoted by structural modifications of feathers, especially the loss of barbules, due to wear (Troy & Brush 1983; Willoughby et al. 2002). An alternative derived from this hypothesis suggests that biotic or abiotic factors may primarily degrade or modify the physical structure of feather protein, in which matrix carotenoid pigments lie (Brush 1990), rather than the structure of feathers. As a consequence, carotenoids contained in the feather-protein matrix may be progressively exposed to show the bright nuptial coloration. These ‘barbules loss’ and ‘protein protection’ hypotheses predict that the change of colour from winter to spring is due to wear with, or without, structural modification of feathers, respectively.
2. Material and methods
(a) Study species and field procedures
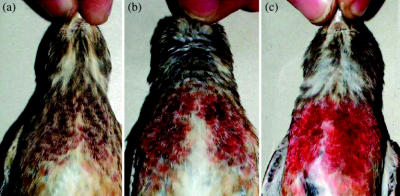
The linnet is a sexually dichromatic cardueline finch in which adult males conspicuously show a crimson red colour in the breast and crown during spring and summer (figure 1c), while females and juveniles are duller. Carotenoid-based coloration of freshly moulted feathers is expressed as a red area on the feather centre, which is partially covered with pale bands at feather tips that are superimposed on each other and give males a duller appearance during autumn and winter (figure 1a). It has been assumed that bright red feather patches of the same colour as that exposed in spring remain partially concealed by the light tips of breast feathers during winter, which are subsequently worn away to disclose the bright red coloration in spring (Newton 1972; Jenni & Winkler 1994). However, whether the bright red spring colour is present in its definitive expression during autumn and winter remains controversial as it is contrary to the evidence of the duller brown appearance of the concealed breast and crown feathers of male linnets (figure 1a).
Figure 1.
Examples of colour shifting from (a) early winter: november to (b) mid-winter: February and (c) early spring: April in breast carotenoid-based coloration of different adult male linnets. Note that the reddish coloration of breast feathers partially covered by pale tips increased as tips progressively abraded from winter to spring.
Linnets were caught in mist-nets from about 1 h before sunset, when they arrived at a communal roost situated in a large reed bed located at Laguna de San Juan, Madrid, Spain. We distinguished birds in their first year of life from adults using the criteria described in Jenni & Winkler (1994). We counted the number of moulting feathers in different parts of the body to assess the role of moult in red colour acquisition from winter to spring. Several breast feathers were collected from a sample of male and female linnets captured over several consecutive days of winter and spring. Before releasing the birds, blood (greater than or equal to 30 μl) was extracted from the brachial vein in heparinized microcapillary tubes and then transferred to vials. Blood samples were transported in a cooler to our laboratory within a day of collection. Blood samples were centrifuged at 3000g for 10 min to separate plasma from cells. The plasma was frozen at −20 °C until analysis.
(b) Colour measurements
Male breast feathers were fixed dorsally by their distal (light tips and a coloured portion of feathers) and proximal (coloured) extremes with transparent adhesive cellophane on a single sheet of white paper, leaving the central coloured part of the feather without any protection from cellophane. Feathers were scanned using a HP Scanjet 4300C scanner within 4 days of collection. Images of scanned feathers were analysed using Adobe Photoshop software to objectively compare the colour of male linnets' winter and spring breast feathers. Hue, saturation and brightness were measured in each pixel in an area of 5×5 pixels selected at random within the central coloured area of breast feathers and the average of these values was computed and compared between ages (adult, n=23; juveniles, n=17) and seasons (winter: December, n=19; spring: April, n=21) by using two-way ANOVAs. Given that pigment elaboration in male linnets varies from dull brown to bright red, plumage hue was measured in the red spectra from the RGB colour system with values varying from 0° (black) to 255° (red) and intermediate values corresponding to different degrees of brown. Plumage saturation was considered as a measure of colour intensity, measured as percentages relative to grey (0%) and full colour (100%). Brightness was measured as the intensity of light reflected in percentages relative to black (0%) and white (100%).
(c) Extraction, identification and quantification of feather and plasma carotenoids
A known weight of breast feather sample equivalent to 0.005–0.010 g (ca 10 feathers) was placed within a folded filter paper and introduced into a 10 ml round-capped test tube for subsequent extraction. Ten millilitres of N, N-dimethylformamide were added and the tube was placed at 60 °C for 60 min, including sonication for 5 min every 30 min. The extraction procedure was repeated three times with fresh extraction solvent. All fractions were pooled in a separator funnel and treated with 50 ml of diethyl ether to collect the pigments. A sufficient amount of 10% NaCl was added to allow the separation of the phases. The ether phase was filtrated through anhydrous sodium sulphate, evaporated in a rotary evaporator and taken up to 0.2 ml with acetone. In the case of plasma, the sample was extracted with 5 volumes of acetone, shaken for 1 min, sonicated for 1 min and left to settle in ice for 5 min to allow proteins to precipitate. Resulting extracts were subsequently centrifuged at 12 000g for 5 min and the upper layer stored at −30 °C until analysed by high-performance liquid chromatography (HPLC).
HPLC was carried out using a HP1100 separation module controlled by the HPChem Station System Manager. Separation was performed on a Waters Spherisorb ODS2 column (250×4.6 mm I.D., particle size 5 μm) by using the chromatographic method described by Mínguez-Mosquera & Hornero-Méndez (1993), which consists of a binary solvent gradient acetone–water at a flow rate of 1.5 ml min−1. The diode array detector wavelength was set to 450 nm and the UV-visible spectra of each peak were recorded and stored online in the 350–600 nm wavelength range. Samples were cleaned prior to injection by centrifugation at 12 000g.
Identification of carotenoids present in plasma and feathers was conducted by separation and isolation of the pigments by thin layer chromatography (TLC) and co-chromatography with standards, acquisition of UV-visible spectra with a PhotoDiode Array Spectrophotometer model HP 8452A in different solvents as well as chemical derivatization microscale tests for the examination of 5,6-epoxide, hydroxyl carbonyl groups (Eugster 1995). The chromatographic, spectroscopic and chemical properties of the pigments were compared with several authentic carotenoid samples as well as with the data in the literature (Foppen 1971; Davies & Köst 1988; Britton 1995). Standards for lutein, β-carotene and β-cryptoxanthin were isolated from natural sources by means of TLC, following the procedure described by Mínguez-Mosquera & Hornero-Méndez (1993).
Quantification of plasma and feathers carotenoids (in μg g−1) was performed using external standard calibration curves except for those present in trace amounts, which were not quantified. Concentration of plasma carotenoid was used as the dependent variable in an ANOVA with age, sex and date of capture (days from 1 November) as independent variables. To determine the possibility of seasonal changes in the concentration of feather carotenoids in male linnets, we conducted an ANOVA with age and season as factors. We considered season as a factor with two levels (winter and spring), rather than the date of capture as a continuous variable, because feathers used to analyse feather carotenoid concentration were collected in few consecutive days in winter (December) and spring (April) rather than throughout the study period.
(d) Experimental change of colour in breast feathers
We experimentally assessed (i) whether the change of breast feather colour from winter to spring was due to external environmental factors and (ii) whether this change was related to the structural modification of feathers (the barbules loss hypothesis) or not (the protein protection hypothesis).
To assess whether the change of breast feather colour from winter to spring was environmentally induced, breast feathers of each individual male (n=19) were mounted separately on two single white papers corresponding to the two different experimental groups. These groups of feathers were considered as exposed to (outdoor) or protected from (indoor) environmental factors and they were scanned prior to any treatment (December). Mounted feathers in the indoor group were preserved from light at a constant room temperature (20–22 °C) and constant humidity (40–60%) from December to May, while feathers in the outdoor group were exposed to ambient air, temperature (2–25 °C), humidity (30–80%) and sunlight but preserved from rain during the same period. After the experiment (May), we scanned the feathers again to determine the colour change in breast feathers from both the outdoor and indoor groups. Images of scanned feathers were analysed as described above. Overall, we obtained average values from the exposed coloured central part of feathers in two replicates (outdoor and indoor) before (December) and after the experimental period (May). The change in colour from winter to spring was analysed by comparing hue, saturation and brightness values from breast feathers of male linnets using repeated-measures ANOVAs (n=19 pairs), with age (adult or juvenile) as an additional factor.
To assess whether the change of colour from winter to spring was related to the structural modification of feathers or not, the distal coloured parts of the feathers were taped with cellophane to avoid feather abrasion (especially the loss of barbules), while the central coloured part of the feather remained exposed without any protection. By using repeated-measures ANOVAs, the average hue, saturation and brightness values (5×5 pixels) of the coloured distal (protected from abrasion with cellophane) and central (without any protection with cellophane) portions of each feather in the outdoor group were compared, both before (December) and after the experimental period (May). All variables were tested for normality (Kolmogorov–Smirnov test).
3. Results
(a) Identification of plasma and feather carotenoids
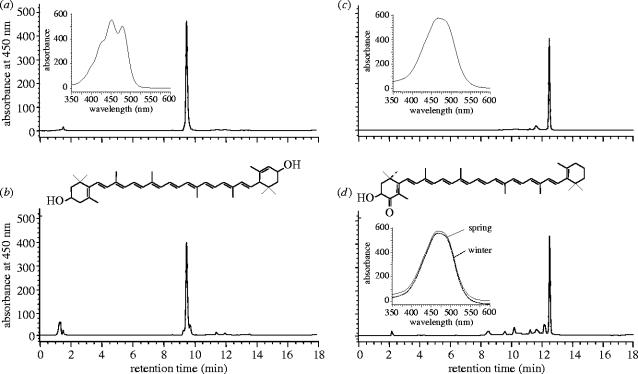
A preliminary screening performed by means of Thin Layer Chromatography TLC indicated that carotenoid composition was represented by only one major pigment in both types of samples, although it was of a different nature for plasma and feathers, as deduced by their different mobility (Rf value) and colour in plate. This was confirmed by HPLC, which showed a single main peak in the chromatograms from both types of sample, representing in each case more than 90% of the pigment composition. As shown in figure 2b, the main carotenoid found in the plasma of male and female birds eluted at 9.58 min and showed a UV-visible spectrum with three maxima (426, 450, 478 nm) which is consistent with a chromophore containing nine conjugated double bonds and one β-ring. This pigment was preliminarily identified as lutein, which was confirmed by comparing retention time, ultraviolet (UV) visibility and other properties with an authentic sample of lutein (figure 2a). This pigment was present in a major proportion (greater than 94%) of all plasma samples throughout the study period.
Figure 2.
Chromatograms and UV–visible spectra corresponding to (a) lutein standard, (b) carotenoids present in plasma, (c) 3-OH-echinenone standard and (d) the carotenoids present in male linnet feathers in winter and spring.
In the case of feathers, a main carotenoid eluted at 12.50 min and the UV-visible spectrum showed only one maximum at 466 nm and no fine structure in either feather from winter and spring (figure 2d). This was consistent with a chromophore of nine conjugated double bonds plus two β-rings and the possibility of end-groups containing ketonic functions. These chromatographic, spectroscopic and chemical properties indicated a structure consistent with 3-hydroxy-echinenone (figure 2c), which was found in males but not in females. The only major pigment present in feathers in both winter and spring was 3-hydroxy-echinenone (greater than 90%).
Two additional carotenoid pigments, β-carotene and β-cryptoxanthin, were also found in trace amounts. Owing to their low concentration, these pigments were only identified by comparing their UV-visible spectra, retention times and co-chromatography with authentic standard samples of pure pigments. β-Carotene was present in all plasma samples and absent in feathers (ca 5%), whereas β-cryptoxanthin was only present in small proportions (2–5%) in some plasma and feathers samples.
(b) Seasonal changes in the colour and concentration of plasma and feather carotenoids and moult
The coloured portion of male linnet breast feathers collected in winter (n=19) showed lower values of both hue in the red scale (166.89±5.12; brightness: 70.21±2.65; saturation: 39.16±1.11) than those from spring (n=21: hue: 196.00±4.43; F1,39=23.054, p<0.0001; brightness: 77.43±1.60; F1,39=5.805, p=0.021; saturation: 41.90±0.70; F1,39=5.887, p=0.020; age effect was only significant for hue: F1,39=7.997, p=0.008; two-way interactions were not significant in all cases).
We found no age or sex differences in plasma carotenoid concentration (both p>0.05). A significant interaction between sex and squared date of capture (F1,63=4.535, p=0.037) indicated that carotenoid plasma concentration was highest in winter (November: 42.78±7.08 μg g−1, n=20; December: 54.71±8.44 μg g−1, n=6) and then decreased during late winter (February: 31.99±4.80 μg g−1, n=12; March: 24.78±3.47 μg g−1, n=6) and early spring (April: 20.21±4.96 μg g−1; n=6) in males, while no seasonal differences were found for females (overall mean=30.25±2.67 μg g−1; n=40). We did not find significant differences in the concentration of 3-hydroxy-echinenone in breast feathers of male linnets between winter (319.86±39.05 μg g−1; n=19) and spring (352.29±47.96 μg g−1; n=17; F1,36=0.19, p=0.66), nor a significant interaction of season with age (F1,36=0.50, p=0.48). There was a trend showing a higher concentration of 3-hydroxy-echinenone in the breast feathers of adults (383.52±47.65 μg g−1; n=18) than in yearling males (286.83±35.13 μg g−1; n=18), albeit not reaching significance (F1,36=2.67, p=0.11) which was probably due to the small sample size.
Overall, only 10.1% of birds (n=705) captured from October to April were moulting some body feathers. There was no significant effect of age, sex or month of capture on the presence or absence of moult (log-linear analysis, all p>0.05). The number of moulting feathers in different parts of the body (2.67±0.59; n=58) did not differ according to age, sex or month of capture (GLM with Poisson error and logarithmic link, all p>0.05). We recorded the place where body feathers were growing in a sample of 45 birds, four (8.9%) of which (three females, one male) were moulting some breast feathers (a single feather in all cases).
(c) Experimental change of feather colour
The results of the experiment show a significant increase from winter to spring in hue in the red scale (repeated-measures ANOVA: F1,18=103.114, p<0.0001), saturation (F1,18=9.295, p=0.007) and brightness (F1,18=5.516, p=0.031) of feathers exposed to outdoor conditions (figure 3), while no seasonal difference was found in the indoor group (hue: F1,18=0.001, p=0.979; saturation: F1,18=0.130, p=0.723; brightness: F1,18=0.297, p=0.593; figure 3). In all cases age effect was not significant (all p>0.05). The increase in colour values from winter to spring in exposed feathers cannot be explained by differences in the initial colour of feathers between the outdoor and indoor groups (as demonstrated by the lack of differences between both groups in winter; all p>0.05; figure 3) because they belong to the same individuals.
Figure 3.
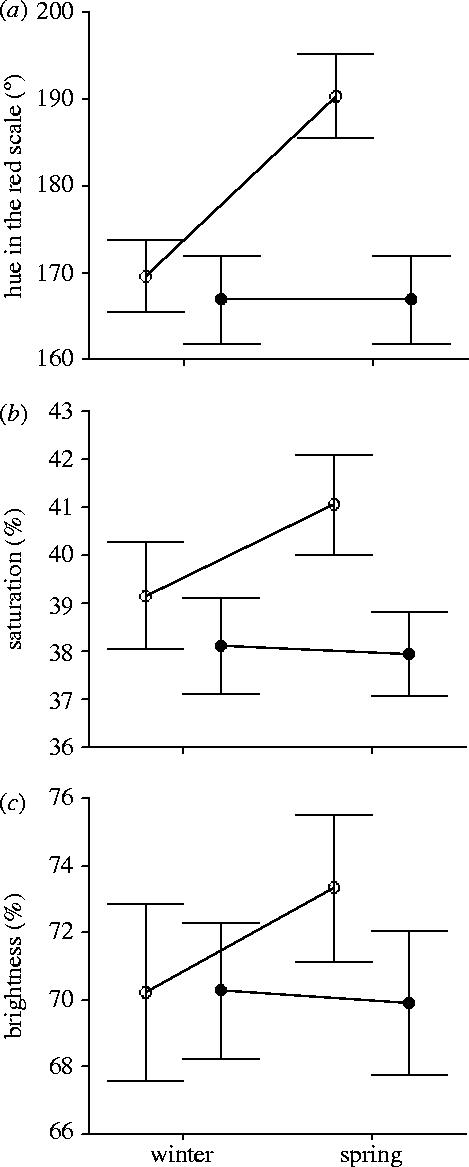
Mean±s.e. values for (a) hue (redness), (b) saturation and (c) brightness in relation to season and experimental treatment of male linnets' breast feathers (n=19 in all groups). Open symbols represent feathers in the outdoor group and closed symbols represent feathers in the indoor group.
The increase in colour from winter to spring also occurred in the proximal coloured portion of feathers in the outdoor group when protected from abrasion with cellophane (repeated-measures ANOVA: hue: F1,18=23.149, p<0.0001; saturation: F1,18=15.206, p=0.001; brightness: F1,18=5.545, p=0.031; age effect was not significant in all cases). However, no difference was found in hue, saturation and brightness between feather portions protected and unprotected with cellophane in either winter or spring (all p>0.05).
4. Discussion
Carotenoid-based breast feathers of male linnets increased in hue (redness), saturation and brightness simply by exposing them to outdoor conditions from winter to spring. These striking results represent, to our knowledge, the first experimental evidence showing that carotenoid-based plumage coloration may increase towards colourful expression due to biotic or abiotic environmental factors acting directly on full-grown feathers. The increase in coloration from the non-breeding to the breeding season resulted in the colourful nuptial plumage of male linnets when its main function may operate (Hill 1999), which suggests that this process is a product of sexual selection. This contrasts with the view that feather coloration is a static trait because feathers are metabolically inactive after moult and, thus, opens a wide range of possibilities for the influence of unknown selective pressures on the evolution of feather morphology and the consequences for sexual selection.
The change from duller to reddish coloration of male linnets from winter to spring was not related to the incorporation or transformation of additional or new carotenoids to feathers after moult. We found that the red coloration of breast feathers was carotenoid-based, with a major concentration of a particular pigment (3-hydroxy-echinenone; see also Stradi et al. 1997) that did not differ between winter and spring being found in all samples, which rejects the carotenoid incorporation hypothesis. This also indicates that a particular pigment is not structurally transformed into a different pigment once deposited in feathers (the carotenoid transformation hypothesis). We did not find an increase in the circulating levels of carotenoids just before or during red colour acquisition (early spring), as the carotenoid incorporation hypothesis predicts, but the opposite. The reason why carotenoid plasma concentration was higher in winter than in early spring in males, while no seasonal differences were found for females, remains unknown. Yet, it suggests sexual and seasonal differences in the use of ingested carotenoids during the non-breeding season that may be associated with a health function (Bortolotti et al. 1996; Negro et al. 1998; McGraw et al. 2002). In addition, we found that circulating levels of plasma carotenoids were mostly composed of lutein while the major carotenoid component of feathers was 3-hydroxy-echinenone, which probably originated from the metabolic oxidation of β-cryptoxanthin (Stradi et al. 1997). The proportions of different carotenoids in plasma and feathers suggest that a selective incorporation of β-cryptoxanthin into feather follicles from blood (see McGraw et al. 2003), with a subsequent transformation in 3-hydroxy-echinenone (Stradi et al. 1997; McGraw 2004a), may occur. This may be accompanied by an active accumulation of the pigment during the period preceding moult, which needs to be confirmed during the process of incorporating pigments in growing feathers. The moult hypothesis was examined despite previous published evidence of the lack (or reduced extent) of prebasic moult in this species (Jenni & Winkler 1994), which was confirmed in this study.
Our results were contrary to the predictions derived from the tip abrasion hypothesis, as the coloured portion of breast feathers covered by tips during winter was less red than during spring. This does not invalidate a major function of feather tips in protecting, rather than simply hiding, the breast-coloured patch. Still, this indicates that other factors acting on the feather are implicated in the increase of colourful plumage expression that coincides with mating. To our knowledge, only two previous studies have documented that the carotenoid-based colour of fresh feathers partially protected by feather tips during winter may increase in intensity to reach the colourful breeding plumage typical in several cardueline finch species (Troy & Brush 1983; Willoughby et al. 2002). These authors argued that the more intense colour as the season progressed was associated with the loss of barbules and its consequences in exposing the coloured rami to wear. However, we found that the increase in colour from winter to spring also occurred in the proximal coloured portion of feathers in the outdoor group when protected from abrasion. This allowed us to reject the possibility that the increase in colour from winter to spring was exclusively due to structural modifications of feathers, especially the loss of barbules, due to wear in linnets. The alternative hypothesis stating that biotic or abiotic factors may primarily degrade the physical structure of proteins in which matrix carotenoid pigments lie (Brush 1990) seems to fulfil our experimental results. These results showed a significant increase from winter to spring in redness, saturation and brightness of feathers exposed to outdoor conditions, while no seasonal difference was found in the indoor group. The degree of colour change was similar to that found in free-living birds, which suggests that a common environmental factor may be behind these changes. This raises two main related questions. That is, ‘what are the environmental factors responsible for the change of colour expression?’ and ‘what components of the feather structure are being specifically modified by these factors?’.
Environmental factors probably implicated in the colour increase may include abiotic, rather than biotic, factors such as bacteria degrading feathers (Burtt & Ichida 1999), which probably cannot be completely avoided by simply maintaining feathers in indoor conditions, although this needs further examination. Among abiotic factors known to degrade the vertebrate integument by denaturing a wide range of proteins, oxygen and UV light, for example, are good candidates to consider because of their ubiquity and potential active action on feathers both in outdoor conditions and on free-living birds. Feathers in the outdoor group were exposed to sunlight UV irradiation and oxygen while feathers in the indoor group were not exposed to sunlight, which indicated that oxygen might not be solely responsible for the colour change. Sunlight UV irradiation is known to impair feather structure by denaturing keratin and other proteins that constitute feathers (Brush 1980; Burtt 1986), but it may also degrade carotenoids contained in the feather-protein matrix (Burtt 1986; Mortensen & Skibsted 1999) that might then act by protecting pigments from degradation by these factors (McGraw & Hill 2004). That UV irradiation has a major role in the change towards colourful expression makes further sense, considering that it may operate mainly when pale tips become worn early in the breeding season, overlapping with longer day length and consequently, a longer and stronger exposure to sunlight. Whatever the factor implicated, the evidence reported here that plumage coloration may change towards a colourful expression due to modifications occurring in full-grown feathers may provide fruitful research avenues for understanding the evolution of feather morphology and its consequences for sexual selection.
Among the components of the feather structure that would be altered by UV radiation and other environmental factors, proteins are presumably involved because feathers are about 90% protein (Stettenheim 2000). Among them, β-keratin and other less abundant keratins differing in strength due to differences in amino acid composition and molecular structure may be related to physical properties required at each body part (Brush 1980). Recently, the existence of carotenoid-binding proteins (generically termed as carotenoproteins) has been suggested in feathers of several passerine species (McGraw et al. 2003). Carotenoproteins have long been known to exist in plants, invertebrates and the human retina (Zagalsky 1985). They are, for instance, involved in the change from browinsh-blue to purple-red colour after their denaturation by several factors in crustaceans (Zagalsky 1985). In birds, carotenoproteins have been argued as being potentially implicated in the control of pigment incorporation into feathers by preferentially sequestering or excluding a particular pigment over others in the maturing follicle (McGraw et al. 2003). This suggests that carotenoproteins and other binding molecules, if existing in the follicles, may be linked to carotenoids because of their deposition into feathers (McGraw et al. 2003). This also lead us to hypothesize that, in addition to their potential regulatory feat in binding carotenoids (McGraw et al. 2003), potential feather proteins may temporally protect the colourful features of associated carotenoids during the non-breeding season when its main signalling function may be relaxed (Hill 1999; McGraw 2004b).
In the case of linnets and other passerines, a single or few major carotenoids incorporated into feathers (McGraw et al. 2003) may indicate direct developmental pathways in the evolution of strategies for differential sequestration of preferred carotenoids associated with linking proteins (that might also protect the pigment). The increase in colour expression from winter to spring due to environmental factors has been also recorded in other cardueline finches (Troy & Brush 1983; authors' unpublished data) that incorporate 3-hydroxy-echinenone and other carotenoids into their feathers (Stradi et al. 1997). However, this change is not apparent or has been not recorded when carotenoid-based ornaments included other single major carotenoids or a combination of carotenoids in other bird species where the proximate control of plumage coloration has been investigated (Inouye et al. 2001; McGraw & Hill 2001; McGraw et al. 2001, 2002, 2003; Sasks et al. 2003). This may indicate that a particular feather protein associated with particular pigments or a specific link with particular pigments may be behind the colour change in breast and crown feathers of linnets and other close related finches. Alternatively, potential binding-proteins may not protect the pigment but they simply may become denatured due to environmental factors (e.g. UV irradiation) when feather tips became worn due to abrasion. In this case, potential carotenoproteins might only actively function in the selective incorporation of pigment into feathers (McGraw et al. 2003) while passively operating, as a consequence of its degradation, by showing the bright underlying colour resulting exclusively from the spectral features of the carotenoid. Another possibility states that different binding-proteins may be involved in the selective incorporation and protection of pigments into feathers. Progress towards uncovering the significance of concealment and subsequent display of colour expression should consider the potential binding and protecting nature of feather proteins associated with carotenoids. For instance, in the case of colour expression shifting towards a colourful elaboration (e.g. colour hue), selection would be expected to act both on the amount of particular pigments and on the amount and strength of potential substances (carotenoid-binding proteins and feather tip keratins) and structures (feather tips) protecting and sequestering them. It may be further modulated by behaviours potentially adopted to actively modify colour expression, for example, sunbathing and soil bathing. Examining whether these potential targets of selection are involved in the development and function of carotenoid-based plumage ornaments is worthy of further investigation.
Acknowledgments
We thank C. Lakunza for improving the English of the manuscript. The standard for 3-hydroxy-echinenone was a generous gift from Dr George Britton (School of Biological Sciences, University of Liverpool, UK). Two anonymous reviewers greatly improved the manuscript.
References
- Badyaev A.V, Young R.L. Complexity and integration in sexual ornamentation: an example with carotenoid and melanin plumage pigmentation. J. Evol. Biol. 2004;17:1317–1327. doi: 10.1111/j.1420-9101.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Hill G.E, Dunn P.O, Glen J.C. Plumage color as a composite trait: developmental and functional integration of sexual ornamentation. Am. Nat. 2001;158:221–235. doi: 10.1086/321325. [DOI] [PubMed] [Google Scholar]
- Bortolotti G.R, Negro J.J, Tella J.L, Marchant T.A, Bird D.M. Sexual dichromatism in birds independent of diet, parasites and androgens. Proc. R. Soc. B. 1996;263:1171–1176. [Google Scholar]
- Britton G. UV/visible spectroscopy. In: Britton G, Liaan-Jensen S, Pfander H, editors. Carotenoids. Spectroscopy. vol. 1B. Birkhäuser; Basel: 1995. pp. 13–63. [Google Scholar]
- Brush A.H. Pattern in the amino acid composition of avian epidermal proteins. Auk. 1980;97:742–753. [Google Scholar]
- Brush A.H. Metabolism of carotenoid pigments in birds. FASEB J. 1990;4:2969–2977. doi: 10.1096/fasebj.4.12.2394316. [DOI] [PubMed] [Google Scholar]
- Burtt E.H. An analysis of physical, physiological, and optical aspects of avian coloration with emphasis on wood-warblers. Ornithol. Monogr. 1986;38:1–126. [Google Scholar]
- Burtt E.H, Ichida J.M. Occurrence of feather-degrading bacilli in the plumage of birds. Auk. 1999;116:364–372. [Google Scholar]
- Davies B.H, Köst H.P. Carotenoids. In: Köst H.P, editor. Handbook of chromatography. vol. 1. CRC Press; Boca Ratón, FL: 1988. [Google Scholar]
- Eugster C.H. Chemical derivatization: microscale tests for the presence of common functional groups in carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Isolation and analysis. vol. 1A. Birkhäuser; Basel: 1995. pp. 71–80. [Google Scholar]
- Foppen F.H. Tables for identification of carotenoid pigments. Chromatogr. Rev. 1971;14:133–298. doi: 10.1016/0009-5907(71)80012-1. [DOI] [PubMed] [Google Scholar]
- Hill G.E. Mate choice, male quality, and carotenoid-based plumage coloration. In: Adams N, Slowtow R, editors. Proceedings of the 22nd International Ornithological Congress. University of Natal; Durban: 1999. pp. 1654–1668. [Google Scholar]
- Inouye C.Y, Hill G.E, Stradi R.D, Montgomerie R. Carotenoid pigments in male house finch plumage in relation to age, subspecies, and ornamental coloration. Auk. 2001;118:900–915. [Google Scholar]
- Jenni L, Winkler R. Academic Press; London: 1994. Moult and ageing in European passerines. [Google Scholar]
- McGraw K.J. Colour songbirds metabolize carotenoids at the integument. J. Avian Biol. 2004a;35:1–6. [Google Scholar]
- McGraw K.J. Winter plumage coloration in male goldfinches: do reduced ornaments serve signalling functions in the non-breeding season? Ethology. 2004b;110:707–715. [Google Scholar]
- McGraw K.J, Hill G.E. Carotenoid access and intraspecific variation in plumage pigmentation in male American goldfinches (Carduelis tristis) and northern cardinals (Cardinalis cardinalis) Funct. Ecol. 2001;15:732–739. doi: 10.1086/323797. [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Hill G.E. Plumage color as a dynamic trait: carotenoid pigmentation of male house finches (Carpodacus mexicanus) fades during the breeding season. Can. J. Zool. 2004;82:734–738. [Google Scholar]
- McGraw K.J, Hill G.E, Stradi R, Parker R.S. The influence of carotenoid acquisition and utilization on the maintenance of special-typical plumage pigmentation in male American goldfinches (Carduelis tristis) and northern cardinals (Cardinalis cardinalis) Physiol. Biochem. Zool. 2001;74:843–852. doi: 10.1086/323797. [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Hill G.E, Stradi R, Parker R.S. The effect of dietary carotenoid access on sexual dichromatism and plumage pigment composition in the American goldfinch. Comp. Biochem. Physiol. B. 2002;131:261–269. doi: 10.1016/s1096-4959(01)00500-0. [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Beebee M.D, Hill G.E, Parker R.S. Lutein-based plumage coloration in songbirds is a consequence of selective pigment incorporation into feathers. Comp. Biochem. Physiol. B. 2003;135:689–696. doi: 10.1016/s1096-4959(03)00164-7. [DOI] [PubMed] [Google Scholar]
- Menon G.K, Menon J. Avian epidermal lipids: functional considerations and relationship to feathering. Am. Zool. 2000;40:540–552. [Google Scholar]
- Mínguez-Mosquera M.I, Hornero-Méndez D. Separation and quantification of the carotenoid pigments in red peppers (Caspicun annuum L), papikra and oleoresin by reversed-phase HPLC. J. Agric. Food Chem. 1993;41:1616–1620. [Google Scholar]
- Montgomerie R, Lyon B, Holder K. Dirty ptarmigan: behavioral modification of conspicuous male plumage. Behav. Ecol. 2001;12:429–438. [Google Scholar]
- Mortensen A, Skibsted L.H. Carotenoid photobleaching. Methods Enzymol. 1999;299:408–421. [Google Scholar]
- Negro J.J, Bortolotti G.R, Tella J.L, Fernie K.J, Bird D.M. Regulation of integumentary colour and plasma carotenoids in American kestrels consistent with sexual selection theory. Funct. Ecol. 1998;12:307–312. [Google Scholar]
- Negro J.J, Margalida A, Hiraldo F, Heredia R. The function of the cosmetic coloration of the bearded vultures: when art imitates life. Anim. Behav. 1999;58:F14–F17. doi: 10.1006/anbe.1999.1251. [DOI] [PubMed] [Google Scholar]
- Newton I. Taplinger Publishing; New York: 1972. Finches. [Google Scholar]
- Piersma T, Decker M, Damste J.S.S. An avian equivalent of make-up? Ecol. Lett. 1999;2:201–203. [Google Scholar]
- Promislow D.E, Montgomerie R, Martin T.E. Mortality costs of sexual dimorphism in birds. Proc. R. Soc. B. 1992;250:143–150. [Google Scholar]
- Sasks L, McGraw K, Hörak P. How feather colour reflects its carotenoid content. Funct. Ecol. 2003;17:555–561. [Google Scholar]
- Senar J.C. Plumage coloration as a signal of social status. In: Adams N, Slowtow R, editors. Proceedings of the 22nd International Ornithological Congress. University of Natal; Durban: 1999. pp. 1669–1686. [Google Scholar]
- Stettenheim P.R. The integumentary morphology of modern birds—an overview. Am. Zool. 2000;40:461–477. [Google Scholar]
- Stradi R, Celentano G, Boles M, Mercato F. Carotenoids in bird plumage: the pattern in a series of red-pigmented Carduelinae. Comp. Biochem. Physiol. B. 1997;117:85–91. [Google Scholar]
- Test F.H. Effects of natural abrasion and oxidation on the coloration of flickers. Condor. 1940;67:76–80. [Google Scholar]
- Troy D.M, Brush A.H. Pigments and feather structure of the redpolls Carduelis flammea and C. hornemanni. Condor. 1983;85:443–446. [Google Scholar]
- Uchida Y. On the color change in Japanese Crested Ibis. A new type of cosmetic coloration in birds. Misc. Rep. Yamashina Inst. Ornithol. 1970;6:56–72. [Google Scholar]
- Whitfield D.P. Plumage variability, status signalling and individual recognition in avian flocks. Tree. 1987;2:13–18. doi: 10.1016/0169-5347(87)90194-7. [DOI] [PubMed] [Google Scholar]
- Willoughby E.J, Murphy M, Gorton H.L. Molt, plumage abrasion and color change in Lawrence's goldfinch. Wilson Bull. 2002;114:380–392. [Google Scholar]
- Zagalsky P.F. Invertebrate carotenoproteins. Methods Enzymol. 1985;111:216–247. doi: 10.1016/s0076-6879(85)11011-6. [DOI] [PubMed] [Google Scholar]