Abstract
Avian eggs contain considerable amounts of maternal yolk androgens, which have been shown to beneficially influence the physiology and behaviour of the chick. As androgens may suppress immune functions, they may also entail costs for the chick. This is particularly relevant for colonial species, such as the black-headed gull (Larus ridibundus), in which the aggregation of large numbers of birds during the breeding season enhances the risk of infectious diseases for the hatching chick.
To test the effect of maternal yolk androgens on the chick's immune function, we experimentally manipulated, in a field study, yolk androgen levels within the physiological range by in ovo injection of either androgens (testosterone and androstenedione) or sesame oil (control) into freshly laid eggs. We determined cell-mediated immunity (CMI) and humoral immunity of the chicks at the beginning of the nestling period to evaluate early modulatory effects of yolk androgens on immune function.
Embryonic exposure to elevated levels of androgens negatively affected both CMI and humoral immunity in nestling gull chicks. Consequently, maternal yolk androgens not only entail benefits of enhanced competitiveness and growth as previously shown, but also costs in terms of immunosuppression. The outcome of embryonic yolk androgen exposure thus likely depends on the post-hatching circumstances for the developing offspring such as parasite exposure and degree of sibling competition.
Keywords: testosterone, lipopolysaccharides, antibody, phytohemagglutinin, humoral immunity
1. Introduction
Bird eggs contain maternally derived steroid hormones (Prati et al. 1992; Schwabl 1993). The systematic variation in maternal yolk hormone concentrations, both within and between clutches, suggests that differential hormone transmission may represent an adaptive maternal strategy (e.g. Gil et al. 1999; Reed & Vleck 2001; Groothuis & Schwabl 2002; Verboven et al. 2003; Pilz & Smith 2004; see also Groothuis et al. (2005a) for a recent review). The effects of prenatal hormone exposure on the development and phenotype of the chick have now been examined in a number of experimental studies, in which fresh eggs were injected with androgens. Manipulation of the androgen environment of an embryo induced a wide range of effects on physiology and behaviour of the offspring (e.g. Schwabl 1996; Lipar & Ketterson 2000). In short, maternal yolk androgens may accelerate embryonic development, enhance post-natal growth rate and affect competitiveness in both the nestling and the juvenile stage (e.g. Schwabl 1993, 1996; Lipar & Ketterson 2000; Eising et al. 2001; Pilz et al. 2004; see also Groothuis et al. 2005a). These findings suggest that maternal yolk androgens are beneficial to offspring. The large variation in yolk hormones within clutches related to the position of the egg in the laying sequence has consequently been interpreted as a possibility for the mother to individually increase the survival probabilities of her offspring (Schwabl 1993; Eising et al. 2001).
Prenatal exposure to androgens, however, can also be detrimental for survival of the offspring (Sockman & Schwabl 2000). This may relate to the fact that although accelerated growth may be beneficial in sibling competition, it may carry costs as well. It may increase the vulnerability to starvation (Blanckenhorn 2000) and has been suggested to increase oxidative stress, which in the long-term might reduce lifespan (Rollo 2002; reviewed by Metcalfe & Monaghan 2003).
Furthermore, accelerated growth, such as that induced by maternal yolk androgens (Schwabl 1996; Eising et al. 2001), may be at the cost of the immune system because of the trade-off between body mass gain and immune function, which are both energetically costly (reviewed by Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Demas 2004). There is indeed recent evidence that maternal yolk hormones mediate the allocation of resources between growth and cell-mediated immunity (CMI) (Andersson et al. 2004; Groothuis et al. 2005a).
Prenatal androgen exposure may also have a direct suppressive effect on the development and organization of immune function. There is some evidence from studies on adult birds that experimentally elevated testosterone levels suppressed the humoral immunity measured as the response to a novel antigen (Duffy et al. 2000; Peters 2000; Casto et al. 2001; but see Hasselquist et al. 1999) and CMI (Duffy et al. 2000). In the chick, yolk androgens may affect the size of immune organs such as the bursa of Fabricius, which has high-affinity androgen receptors (Sullivan & Wira 1979). Indeed, experimental prenatal treatment with relatively high (pharmacological) doses of testosterone in birds leads shown to cause regression of the bursa that leads to impaired antibody production in the domestic chicken (Gallus gallus domesticus) (Hirota et al. 1976; Glick 1983). However, the effect of prenatal exposure to physiologically relevant concentrations of androgens on humoral immune function in birds is not known. Embryonic androgen exposure may also directly affect other branches of the avian immune system, such as CMI. Clearly, prenatal androgen exposure may impose a severe immunological cost for the developing chick.
We examined the effect of yolk androgens on the chick's immune function in black-headed gulls (Larus ridibundus). This species is highly appropriate for the study of hormone-mediated maternal effects. The eggs of this species contain high levels of maternal androgens that vary systematically between and within clutches (Groothuis & Schwabl 2002; Müller et al. 2004a). Furthermore, the functional consequences of embryonic androgen exposure in terms of enhanced growth and begging behaviour have been convincingly demonstrated (e.g. Eising et al. 2001; Eising & Groothuis 2003). In this study, yolk androgen levels of freshly laid eggs were experimentally manipulated by in ovo injection of either androgens (testosterone and androstenedione) dissolved in sesame oil within the physiological range or vehicle only (control). Subsequently, we challenged two branches of the avian immune system, CMI and humoral immunity, during the early developmental period, 1 and 7 days after hatching, respectively. Taking into account different branches of the immune system is particularly important in this case, since testosterone may induce a shift from one component to the other (Braude et al. 1999; Norris & Evans 2000; Buchanan et al. 2003). We expected that chicks hatching from androgen treated eggs would have lowered cell-mediated and humoral immunity compared to chicks from control treated eggs.
2. Material and methods
(a) Fieldwork and egg treatment
In 2003, three sub-colonies of 200–300 breeding pairs each within a large black-headed gull colony (6000 breeding pairs) in the ‘Workumer Waard’ adjacent to the IJsselmeer (The Netherlands) were checked daily for freshly laid eggs. Eggs were individually marked with a non-toxic marker referring to the position within the laying order and date of laying. At the day of clutch completion (modal clutch size three eggs), we manipulated hormone levels of the first laid egg of a clutch, which contains the lowest maternal androgen concentration (Groothuis & Schwabl 2002). Eggs were injected with either 50 μl of sesame oil (control, sterile cold-pressed sesame oil, subsequently called control-eggs) or 50 μl of sesame oil containing a mixture of 0.12 μg testosterone and 10.0 μg androstenedione (equals androgen-eggs). Hormone levels in androgen-eggs were elevated to resemble those naturally occurring in last laid eggs (see Eising & Groothuis 2003 for details on the injection procedure and androgen levels). After injection, all eggs were returned to their original nest.
Within 3 days 224 eggs were injected (107 control-eggs, 117 androgen-eggs). Of these eggs 14 (9 control-eggs, 5 androgen-eggs) were predated, while 58 eggs (26 control-eggs, 32 androgen-eggs) failed to hatch. Thus 72% of all injected eggs successfully hatched (68 chicks hatched from control-eggs (equals control-chicks), 82 chicks from androgen-eggs (equals androgen-chicks)) which was not different among treatments (Yates' corrected χ2=0.80, p=0.37) and is similar to previous studies (e.g. Eising et al. 2001).
(b) Experimental nests
About 5 days prior to hatching, all experimental eggs were cross-fostered to a few restricted areas (first cross-fostering). This allowed efficient recording of the hatching process and reduced the disturbance to the colony to the minimum. Three eggs of the same treatment and the same laying date were placed in one nest. These nests had been surrounded by mini-enclosures 1 day before cross-fostering the eggs to facilitate the adoption procedure. All mini-enclosures were situated within larger enclosures (wire mesh, 40–50 cm high) varying in size from 30 to 60 m2. Enclosures contained from 10 to 15 nests and enabled us to follow chick development when the mini-enclosures were removed after the parent chick bond was established (5–7 days after cross-fostering). At hatching, we registered which chick came from which particular egg and chicks received a numbered colour band for individual identification. A blood sample (60 μl) was taken from the ulnar vein and stored in 100% ethanol for sex determination. Within 24 h all sexes were determined using molecular sex determination according to Griffiths et al. (1998), which has been validated for our study species (Müller et al. 2003).
On the day of hatching, all experimental chicks were again cross-fostered (second cross-fostering), allocating one chick of each treatment to an experimental nest, matched for body mass. The next day, when the sex of the chicks was known, we created additional same sex nests in those cases where body mass could be matched. None of the chicks remained in the nest where it hatched. We created 48 weight-matched broods each containing one control- and one androgen-chick (control-chicks: 27.38±0.40 g (s.e.); androgen-chicks: 27.36±0.36 g (s.e.); paired sample t-test, t=0.03, p=0.97), of which 33 nests were also sex-matched.
(c) Immunity-tests
(i) Cell-mediated immunity: PHA-challenge
One day after hatching CMI was measured via in vivo injection of phytohemagglutinin-P (PHA, Sigma). This method is considered to reliably measure CMI (reviewed by Norris & Evans 2000) as it produces a local swelling due to a prominent perivascular accumulation of T-lymphocytes followed by macrophage infiltration (Smits et al. 1999). At the age of 1 day we injected intradermally 0.04 ml of 1 mg ml−1 PHA dissolved in phosphate-buffered saline (PBS) into the ball of the foot (for a detailed description see Müller et al. 2003). Three repeated measurements of the height of the ball of the foot (the distance between the base of the hind toe and the top of the ball when holding the foot at an angle of 90° to the tarsus) were taken with a sliding caliper (to the nearest 0.05 mm) just prior to injection (initial), and a further three, 24 h (±1 h) after injection (final). Because the repeatability of the successive measurements was high (field: initial F85,172=30.04, r=0.91, final F85,172=29.31, r=0.90; Lessells & Boag 1987) we used the mean value of the three measurements for analysis. The difference between pre-injection and post-injection measurements was used as the response estimate for CMI (Smits et al. 1999).
In 43 out of the 48 nests we were able to measure the response of both chicks 24 h after the PHA-challenge (age 2 days). We measured growth in terms of increase in body mass over 24 h (age 2 days) and 48 h (age 3 days) after the challenge. Growth was assessed as a potential trade-off between a response to an immune challenge and growth (Brommer 2003; Soler et al. 2003) may differ between androgen- and control-chicks. We also evaluated body mass gain within 48 h post-injection as it has been shown that 48 h post-injection of PHA resting metabolic rate (RMR) was enhanced, indicating that this trade-off may last longer than 24 h (Martin et al. 2002).
(ii) Humoral immunity: LPS challenge
To evaluate the effects on the humoral immunity at a very early developmental stage of the immune system, chicks were challenged at day 7±2 days after hatching with lipopolysaccharides (LPS) from the cell wall of Escherichia coli (LPS, serotype O55:B5, Sigma). LPS induce an immune response that mimics the immune response to a bacterial infection, but as it is an inert antigen the negative effects of the pathogen are temporary. LPS increase the release of cytokines and induce an inflammatory response, which is followed by the production of antibodies (Poxton 1995; Leshchinsky & Klasing 2001). As a bacterial antigen, LPS give rise to an antibody response of the IgM type (Janeway & Travers 1999). Furthermore, because T-cell independent antibody responses shortly after birth are higher than T-cell dependent responses, LPS are an appropriate antigen to use at this early developmental stage (Apanius 1998). Since the acute phase response such as caused by a challenge with LPS differs significantly between species (see e.g. domestic chicken, Leshchinsky & Klasing 2001; Japanese quail (Coturnix coturnix japonica), Koutsos & Klasing 2001), we first investigated this for our study species. To this end we injected black-headed gull chicks of similar age and size as in the final experiment with different concentrations of LPS dissolved in PBS (range: 0.1–2.0 mg ml−1). Sickness behaviour, which resulted in a reduced alertness within 6–12 h after the challenge and (slight) weight loss within 24 h, was induced by injection of 0.1 ml PBS with concentrations more than or equal to 2 mg LPS per 1 ml PBS. In the final experiment, we consequently used a lower concentration of LPS, which did not induce sickness behaviour, to reduce the effect of an acute phase response on the humoral immune response.
In twenty-three nests we were able to measure the humoral immune response of both chicks. Within each nest both chicks were intraperitoneally injected either with 0.1 ml PBS (control treatment, n=11 nests) or with 0.1 ml PBS containing LPS antigen (concentration 1 mg ml−1, LPS treatment, n=12 nests).
Chicks from these nests were blood sampled shortly before injection (initial) and 48 h after injection (final) by venipuncture of the brachial vein in the left wing with a 25G needle. Blood (60 μl) was collected in a capillary. The capillary was sealed and stored cool (about 8 °C) for the rest of the day (maximum 6 h). In the laboratory, capillaries were centrifuged for 10 min at 10 000 r.p.m. The separated plasma was then stored in eppendorff tubes at −20 °C until analysis. Antibody titres were determined using enzyme-linked immunosorbent assay (ELISA; see below) and the change in concentration between initial and final sample was used as an estimate for the antibody response.
The second blood sample was taken after a comparatively short time period to reduce the probability of a confounding variation in plasma immunoglobulins due to the maturation of the antibody mediated immunity and the consequent increase in plasma immunoglobulin concentrations during the nestling period (Müller 2004). As we measured non-specific immunoglobulin concentrations, the change in plasma concentrations might also relate to the mitogenic activity of LPS. LPS has been shown to directly activate B-lymphocytes in vitro (Andersson et al. 1972). LPS-activated B-lymphocytes produce antibodies of diverse specificities, mainly of the IgM type within a short time period (Andersson et al. 1978), which are measured in the ELISA.
(d) Enzyme-linked immunosorbent assay for antibody determination
Antibody concentrations were determined using an indirect ELISA with commercial anti-chicken antibodies (Sigma C-6409). This method provides a sensitive measurement of antibody concentrations that bind to a specific antigen (Janeway & Travers 1999). Using indirect ELISA has been validated for our study species (see also Martinez et al. 2003 for a variety of other bird species, Müller et al. 2004b). However, the anti-chicken IgG antibody binds to the light chain of the IgM and IgG (I. Jokinen, unpublished data). In the analysis we are therefore unable to distinguish between an IgM and an IgG response. As the primary immune response, which is assessed here, consists primarily of IgM, we probably measured to a great extent IgM.
To assess antibody concentrations, a standard of pooled plasma of all individuals was used, which was given an arbitrary concentration of 106. All values were subsequently expressed in units relative to this standard (U ml−1). Plasma samples were diluted 1 : 5000, 1 : 10 000 or 1 :15 000 in 1% BSA–PBS for analysis to ensure that the samples were on the linear part of the standard curve. 50 μl of this dilution as well as the standard was added in replicate to the wells including a negative control (for a detailed description see Müller et al. 2004b). All samples were analysed within the same assay; the intra-assay coefficient of variation was 7.3%.
(e) Statistical procedures
All data were checked for normality and in case of plasma antibody concentrations at the age of 7 days log transformations were applied to obtain a normal distribution. During the nestling period the data represented a nested structure and we therefore used hierarchical linear models (MLwiN 1.10, Rasbash et al. 2000). Statistical significance was tested using the increase in deviance when a factor was removed from a model (Wald statistic), which follows a χ2-distribution. For CMI we tested the effect of treatment, sex and the interaction term of these two variables. In the case of the humoral response, we tested the effect of treatment, sex, LPS-challenge, age (given the slight variation in age of injection), body mass and all possible interactions. All factors with a significance level of p<0.05 were retained in the final model. For post hoc analyses we used parametric statistics (Pearson correlations, independent t-test, and paired sample t-test) in SPSS. All tests were two tailed and significance was accepted at p<0.05.
3. Results
(a) T-cell-mediated immunity
CMI (mean±s.e. throughout the paragraph) measured shortly after hatching was significantly higher in chicks hatching from control-eggs (0.59±0.07 mm) than in their foster nest mates from androgen injected eggs (0.37±0.08 mm) (treatment: estimate 0.22, error 0.09, Δdev 5.39, d.f. 1, p=0.02; figure 1). There was no effect of offspring sex on CMI (sex: estimate 0.09, error 0.05, Δdev 2.57, d.f. 1, p=0.11) or sex in interaction with androgen treatment (treatment×sex: estimate 0.007, error 0.096, Δdev 0.006, d.f. 1, p=0.94). There was no significant difference in body mass gain between control-chicks (8.45±0.46 g) and androgen-chicks (8.36±0.46 g) within 24 h after the PHA injection (paired sample t-test, n=43, t=0.17, p=0.86). In 38 out of the 43 nests included in the PHA-challenge we also measured the body mass 48 h after injection. Again, there was no difference in body mass gain between control-chicks (21.74±0.77 g) and androgen-chicks (21.80±1.23 g) (paired sample t-test, n=38, t=−0.05, p=0.96). CMI did not correlate with body mass at the day of injection or body mass gain during the time of the challenge either in control-chicks or in androgen-chicks (Pearson p>0.16 in all cases).
Figure 1.
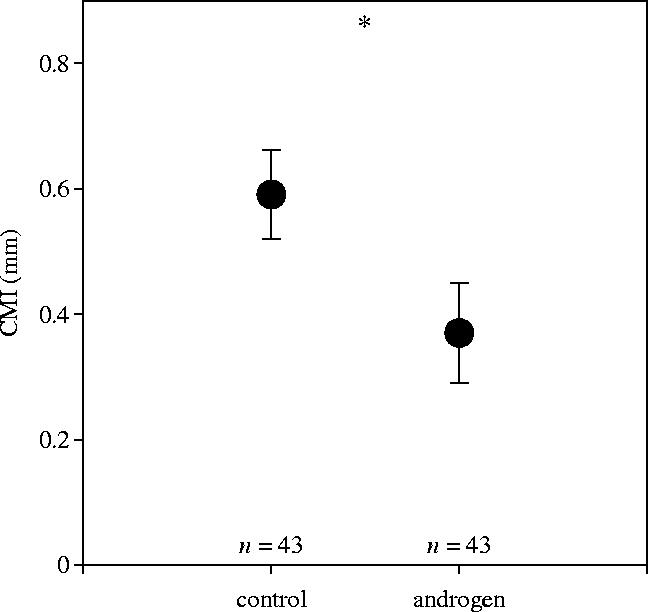
T-cell-mediated immunity (CMI, mean±s.e.) in relation to in ovo androgen or control treatment at day 1 (n=43 nests).
(b) Humoral immune response
Plasma antibody concentrations (mean±s.e. throughout the paragraph) at the age of 7±2 days did not differ between control-chicks (35 415±3858 U ml−1) and androgen-chicks (41 066±4867 U ml−1) (p>0.36).
The change in antibody concentrations from pre- to post-injection differed significantly between LPS- and PBS-challenged chicks in interaction with embryonic androgen treatment (LPS-challenge×androgen treatment: estimate 679 692 U ml−1, error 21 535, Δdev 8.54, d.f. 1, p=0.003, figure 2). In subsequent post hoc analyses, androgen treatment turned out not to affect the change in plasma antibodies within the PBS-group (PBS-treated control-chicks: 13 324±6000 U ml−1; PBS-treated androgen-chicks: 12 149±13 981 U ml−1; paired sample t-test, t=0.07, d.f. 10, p=0.94; figure 2a). Two control-chicks and five androgen-chicks did not increase their plasma immunoglobulin concentrations, and the proportion of non-responders did not differ between treatments (Yates' corrected χ2=0.35, p=0.55). The change in plasma antibody concentrations tended to deviate significantly from zero in control- but not androgen-chicks (PBS-treated control-chicks: t=2.22, p=0.051; PBS-treated androgen-chicks: t=0.87, p=0.40).
Figure 2.
Change in plasma immunoglobulins (U ml−1) in relation to in ovo androgen treatment within 48 h after experimental challenge (n=23 nests), (a) PBS (control-challenge; n=11 nests) (b) lipopolysaccharides (LPS from E. coli) challenge (n=12 nests). Chicks were 7(±2) days old at the day of injection. Initial plasma immunoglobulin concentrations were 35 415 U ml−1 (control-chicks) and 41 066 U ml−1 (androgen-chicks), respectively.
LPS-treated control-chicks showed a much stronger immune response than LPS-treated androgen-chicks (LPS-treated control-chicks: 95 232±14 185 U ml−1; LPS-treated androgen-chicks: 26 369±9947 U ml−1; paired sample t-test, t=4.30, d.f. 11, p=0.001; figure 2b). LPS-treated androgen-chicks did not differ from PBS-treated androgen-chicks (independent sample t-test, t=−0.84, d.f. 21, p=0.41; figure 2a,b). In contrast, LPS-treated control-chicks significantly differed in their change from PBS-treated control-chicks (independent t-test, t=−5.15, d.f. 21, p<0.001; figure 2a,b). One androgen-chick did not show a positive response, while the change in antibody concentrations was significantly different from zero for both control- and androgen-chicks (LPS-treated control-chicks: t=6.71, p<0.001; LPS-treated androgen-chicks: t=2.65, p=0.02). There was no effect of age (age: estimate 688 U ml−1, error 5078, Δdev 0.01, d.f. 1, p=0.93), sex (sex: estimate 3372 U ml−1, error 5734, Δdev 0.14, d.f. 1, p=0.71) or body mass at the time of injection (body mass: estimate 839 U ml−1, error 505, Δdev 2.67, d.f. 1, p=0.10) on the humoral response in reaction to the LPS challenge. The change in antibody concentrations did not correlate with body mass at the day of injection or body mass gain during the time of the challenge, either in control-chicks or in androgen-chicks (Pearson p>0.40 in all cases).
The change in body mass within 48 h after the challenge tended to differ between LPS- and PBS-challenged chicks in interaction with embryonic androgen treatment (LPS-challenge×androgen treatment: estimate 10.70 g, error 5.91, Δdev 3.06, d.f. 1, p=0.08). However, there were no differences in body mass gain between control-chicks and androgen-chicks (PBS-treated group: control-chicks 18.91±2.96 g, androgen-chicks: 24.27±4.41 g; LPS-treated group: control-chicks 28.25±5.37 g, androgen-chicks 22.92±3.30 g; paired sample t-test, p>0.19 in all cases). LPS did not affect body mass gain either in androgen-chicks or control-chicks compared to the PBS challenged group (independent sample t-test, p>0.15, in all cases).
4. Discussion
This experimental field study demonstrates that embryonic exposure to elevated yolk androgen concentrations within the physiological range affects cell-mediated as well as humoral immunity, measured at an early developmental stage of the nestling period in a semi-precocial bird species, the black-headed gull (figures 1 and 2).
We measured CMI shortly after hatching and found that androgen-chicks had a significantly lower CMI when compared to their control-chick nest mates (figure 1). The increase in yolk androgen concentrations within the laying sequence as reported for our study species (Eising et al. 2001; Groothuis & Schwabl 2002) may hence be causally involved in the reduction of the chick's CMI over the hatching order (Müller et al. 2003; Groothuis et al. 2005b). The reduction in CMI as a consequence of experimental elevation of the yolk androgen concentrations in first-laid eggs to the levels of naturally last-laid eggs was somewhat smaller than these data in the natural situation had suggested. This indicates that the decrease in CMI within the hatching order is not exclusively due to changes in yolk androgen concentrations, but may also depend on other egg components such as yolk carotenoids, which have been shown to decrease with position in the laying order in gulls (Royle et al. 2001; Blount et al. 2002) and have recently been shown to enhance CMI of barn swallow chicks (Saino et al. 2003).
Our results support the proposal that elevated yolk androgen levels are associated with suppressing CMI and this occurs at a stage of development when large parts of the immune system are still immature and when the newly hatched chicks depend highly on the CMI (Apanius 1998). We have previously shown that CMI positively correlates with early survival probability in this species (Müller et al. 2003), and suppression of the CMI through embryonic androgen exposure is therefore likely to be biologically relevant (see also Christe et al. 1998).
When the chicks were about one week old, we challenged the humoral immune system with LPS. As a bacterial antigen LPS induces a T-cell independent immune response that was evaluated 48 h after injection. The results are in line with our hypothesis. In contrast to their control-chick nest mates, which raised a steep humoral immune response, androgen-chicks only slightly responded to the challenge (figure 2a,b). An LPS-challenge mimics the immune response to a bacterial infection (Poxton 1995). The lack of a humoral response in androgen-chicks may hence indicate their difficulties to deal with natural bacterial infections. We measured the change in immunoglobulin concentrations 48 h post-injection and therefore did not evaluate peak antibody levels in response to the LPS-challenge (Sunwoo et al. 1996), but rather immunoglobulin concentrations at an early stage of the humoral immune response. Thus, theoretically androgen-chicks may be able to reach a similar peak concentration. However, they still show a delayed increase in immunoglobulin concentration at an early phase of the immune response. In any case, embryonic androgen exposure within the natural range negatively affects the humoral immunity.
During the nestling period chicks may face a trade-off between body mass gain, important for the maintenance of a size advantage in sibling competition, and raising an immune response (Brommer 2003; Soler et al. 2003). Both are energetically costly (reviewed in Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000). Thus, differences in immune function due to in ovo androgen treatment may relate to the fact that androgens alter the allocation of resources. Indeed, two recent studies provide evidence that a reduction in CMI such as that induced by yolk androgens may result from a reallocation of resources mediated by yolk androgens (Andersson et al. 2004; Groothuis et al. 2005b). However, in this study we did not find evidence in the case of either CMI or humoral immunity that would indicate a trade-off between growth and immunity. The embryonic exposure to maternal androgens may affect growth and immunity independently and it may depend on the nutritional circumstances and the time point of the challenge, whether growth is affected.
Our data clearly show that the ability to respond to an immune challenge is reduced in androgen-chicks. From the mechanistic point of view, this may be due to differences in the development of immune organs such as the bursa of Fabricius in case of the humoral immunity (Hirota et al. 1976; Glick 1983). LPS has a mitogenic function and has been shown to directly induce immunoglobulin secretion in vitro (Andersson et al. 1972, 1978). This has been shown to particularly occur at low LPS concentrations (Andersson et al. 1972), such as used in this study. The lack of an increase in plasma immunoglobulins in androgen-chicks may thus relate to a lower maturity of their B-lymphocytes due to negative effects on the bursa of Fabricius (Hirota et al. 1976; Glick 1983), which may be less inducible by LPS as suggested by Kühlmann-Rabens et al. (1987).
The mechanism is less clear in case of the CMI. Although it is likely that the negative effect of androgens is on thymic development and maturation of the T-lymphocytes (Gause & Marsh 1986), it may also relate to a reduced phagocytic activity of the macrophages (Al-Afaleq & Homeida 1998). However, both studies applied post-hatching androgen manipulations.
Alternatively or in addition, as black-headed gull chicks endogenously produce testosterone from an early age onwards (Ros et al. 2002) chicks hatching from eggs with elevated androgen levels may produce higher androgen levels after hatching, which may suppress their immunity, which has not been investigated yet. Finally, enhanced androgen sensitivity as a consequence of previous androgen exposure (Ros et al. 2002) may also apply to their immune cells.
In conclusion, in addition to the repeatedly reported beneficial effects of maternal androgens for the chick, these hormones also entail costs in terms of immunosuppression. The outcome of this trade-off is likely to depend on environmental circumstances such as food availability, degree of sibling competition and pathogen exposure. If the nutritional circumstances are harsh, competitiveness will be essential to guarantee survival as the risk of starvation might be higher than the likelihood of dying from an infectious disease. On the other hand, reducing the immune function when parasites and pathogens are abundant might strongly reduce survival probability. In addition, consequences way into adulthood may contribute to the selective costs and benefits of maternal yolk androgens. Yolk androgens positively affected dominance status in juvenile canaries as well as in five month old house sparrows (Schwabl 1993; Strasser & Schwabl 2004). Potentially also the negative effects on immune function may go beyond the nestling period, which clearly requires further investigation to evaluate the ultimate costs and benefits of maternal androgen deposition for avian offspring.
Acknowledgments
We thank ‘It Fryske Gea’, who granted us permission to work on their properties. Fanny Stavasius and Sebastiaan Dusseljee contributed to the successful progress during the fieldwork and Elina Virtanen and Ilmari Jokinen were invaluable during the laboratory work. Serge Daan, Simon Marquis, Suzie Mills, Victor Apanius provided helpful comments on an earlier draft of this manuscript. This project was approved by the animal experimentation committee of the University of Groningen under license DEC 3012. W.M. was supported by a Ph.D. studentship from the University of Groningen and a Marie Curie Host fellowship to the University of Jyväskylä.
References
- Al-Afaleq A.I, Homeida A.M. Effects of low doses of oestradiol, testosterone and dihydrotestosterone on the immune response of broiler chicks. Immunopharmacol. Immunotoxicol. 1998;20:315–327. doi: 10.3109/08923979809038547. [DOI] [PubMed] [Google Scholar]
- Andersson J, Sjöberg O, Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur. J. Immunol. 1972;2:349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Andersson J, Coutinho A, Melchers F. Stimulation of murine B lymphocytes to IgG synthesis and secretion by the mitogens lipopolysaccharide and lipoprotein and its inhibition by anti-immunoglobulin antibodies. Eur. J. Immunol. 1978;8:336–343. doi: 10.1002/eji.1830080509. [DOI] [PubMed] [Google Scholar]
- Andersson S, Uller T, Lõhmus M, Sundström F. Effects of yolk testosterone on growth and immunity in a precocial bird. J. Evol. Biol. 2004;17:501–505. doi: 10.1111/j.1420-9101.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- Apanius V. Ontogeny of immune function. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development—evolution within the altricial–precocial. ch. 8. Oxford University Press; 1998. pp. 203–222. [Google Scholar]
- Blanckenhorn W.U. The evolution of body size: what keeps organisms small. Q. Rev. Biol. 2000;75:385–407. doi: 10.1086/393620. [DOI] [PubMed] [Google Scholar]
- Blount J.D, Surai P.F, Nager R.G, Houston D.C, Møller A.P, Trewby M.L, Kennedy M.W. Carotenoids and egg quality in the lesser black-backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proc. R. Soc. B. 2002;269:29–36. doi: 10.1098/rspb.2001.1840. 10.1098/rspb.2001.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude S, Tang-Martinez Z, Taylor G.T. Stress, testosterone, and the immunoredistribution hypothesis. Behav. Ecol. 1999;10:345–350. [Google Scholar]
- Brommer J.E. Immunocompetence and its costs during development: an experimental study in blue tit nestlings. Proc. R. Soc. B. 2003;271(Suppl. 3):S110–S113. doi: 10.1098/rsbl.2003.0103. 10.1098/rsbl.2003.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K.L, Evans M.R, Goldsmith A.R. Testosterone, dominance signaling and immunosuppression in the house sparrow, Passer domesticus. Behav. Ecol. Sociobiol. 2003;55:50–59. [Google Scholar]
- Casto J.M, Nolan V, Ketterson E.D. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) Am. Nat. 2001;157:408–420. doi: 10.1086/319318. [DOI] [PubMed] [Google Scholar]
- Christe P, Møller A.P, de Lope F. Immunocompetence and nestling survival in the house martin: the tasty chick hypothesis. Oikos. 1998;83:175–179. [Google Scholar]
- Demas G.E. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duffy D.L, Bentley G.E, Drazen D.L, Ball G.F. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behav. Ecol. 2000;11:654–662. [Google Scholar]
- Eising C.M, Groothuis T.G.G. Yolk androgens and begging behavior in black-headed gull chicks: an experimental field study. Anim. Behav. 2003;66:1027–1034. [Google Scholar]
- Eising C.M, Eikenaar C, Schwabl H, Groothuis T.G.G. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. 10.1098/rspb.2001.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause W.C, Marsh J.A. Effect of testosterone treatment for varying periods on autoimmune development and on specific infiltrating leukocyte populations in the thyroid gland of obese strain chickens. Clin. Immunol. Immunopathol. 1986;39:464–478. doi: 10.1016/0090-1229(86)90174-1. [DOI] [PubMed] [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. 10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Glick B. Bursa of Fabricius. In: Farner D.S, King J.R, Parkes K.C, editors. Avian biology. vol. 7. Academic Press; New York: 1983. pp. 443–500. [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Schwabl H. The influence of laying sequence and habitat characteristics on maternal yolk hormone levels. Funct. Ecol. 2002;16:281–289. [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C.M. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Eising C.M, Dijkstra C, Müller W. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 2005b;1:78–81. doi: 10.1098/rsbl.2004.0233. 10.1098/rsbl.2004.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselquist D, Marsh J.A, Sherman P.W, Wingfield J.C. Is avian humoral immunocompetence suppressed by testosterone? Behav. Ecol. Sociobiol. 1999;45:167–175. [Google Scholar]
- Hirota Y, Suzuki T, Chazono Y, Bito Y. Humoral immune response characteristics of testosterone-propionate-treated chickens. Immunology. 1976;30:341–348. [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A, Travers P. 4th edn. Current biology; London: 1999. Immunobiology: the immune system in health and disease. [Google Scholar]
- Koutsos E.A, Klasing K.C. The acute phase response in Japanese quail (Coturnix coturnix japonica) Comp. Biochem. Physiol. C. 2001;128:255–263. doi: 10.1016/s1532-0456(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Kühlmann-Rabens I, Wanke R, Storandt F, Altmann B, Lösch U, Merkenschlager M. Attempts to localize the defect in dysgammaglobulinemia of UM-B19 chickens by studying the effect of immunomodulating substances on immunoglobulin and antibody production. Vet. Immunol. Immunopathol. 1987;14:123–143. doi: 10.1016/0165-2427(87)90048-1. [DOI] [PubMed] [Google Scholar]
- Leshchincky T.V, Klasing K.C. Divergence of the inflammatory response in two types of chickens. Dev. Comp. Immunol. 2001;25:629–638. doi: 10.1016/s0145-305x(01)00023-4. [DOI] [PubMed] [Google Scholar]
- Lessells C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Lipar J.L, Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Aegelius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. 10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Martin L.B, II, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2002;270:153–158. doi: 10.1098/rspb.2002.2185. 10.1098/rspb.2002.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Tomas G, Merino S, Arriero E, Moreno J. Detection of serum immunoglobulins in wild birds by direct ELISA: a methodological study to validate the technique in different species using antichicken antibodies. Funct. Ecol. 2003;17:700–706. [Google Scholar]
- Metcalfe N.B, Monaghan P. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 2003;38:935–940. doi: 10.1016/s0531-5565(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Müller, W. 2004 Maternal phenotypic engineering—adaptation and constraint in prenatal maternal effects. Ph.D. thesis, University of Groningen.
- Müller W, Dijkstra C, Groothuis T.G.G. Inter-sexual differences in T-cell-mediated immunity of black-headed gull chicks (Larus ridibundus) depend on the hatching order. Behav. Ecol. Sociobiol. 2003;55:80–86. [Google Scholar]
- Müller W, Eising C.M, Dijkstra C, Groothuis T.G.G. Within-clutch patterns of yolk testosterone vary with the onset of incubation in black-headed gulls. Behav. Ecol. 2004a;15:893–897. [Google Scholar]
- Müller W, Groothuis T.G.G, Dijkstra C, Siitari H, Alatalo R.V. Maternal antibody transmission and breeding densities in black-headed gulls (Larus ridibundus) Funct. Ecol. 2004b;18:719–724. [Google Scholar]
- Norris K, Evans M.R. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. [Google Scholar]
- Peters A. Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free-living males with high testosterone are more immunocompetent. Proc. R. Soc. B. 2000;267:883–889. doi: 10.1098/rspb.2000.1085. 10.1098/rspb.2000.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz K.M, Smith H.G. Egg yolk androgen levels increase with breeding density in the European starling Sturnus vulgaris. Funct. Ecol. 2004;18:58–67. [Google Scholar]
- Pilz K.M, Quiroga M, Schwabl H, Adkins-Regan E. European starling chicks benefit from high yolk testosterone levels during a drought year. Horm. Behav. 2004;46:179–192. doi: 10.1016/j.yhbeh.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Poxton I.R. Antibodies to lipopolysaccharide. J. Immunol. Methods. 1995;186:1–15. doi: 10.1016/0022-1759(95)00123-r. [DOI] [PubMed] [Google Scholar]
- Prati M, Calvo R, Morreale de Escobar G. l-Thyroxine and 3,5,3′-triodothyronine concentrations in the chicken egg and in the embryo before and after the onset of thyroid function. Endocrinology. 1992;130:2651–2659. doi: 10.1210/endo.130.5.1572286. [DOI] [PubMed] [Google Scholar]
- Rasbash J, Browne W, Healy M, Cameron B, Charlton C. University of London; London: 2000. Multilevel models project. [Google Scholar]
- Reed W.L, Vleck C.M. Functional significance of variation in egg-yolk androgens in the American coot. Oecologia. 2001;128:164–171. doi: 10.1007/s004420100642. [DOI] [PubMed] [Google Scholar]
- Rollo C.D. Growth negatively impacts the life span of mammals. Evol. Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Ros A.F.H, Dieleman S.J, Groothuis T.G.G. Social stimuli, testosterone, and aggression in gull chicks: support for the challenge hypothesis. Horm. Behav. 2002;41:334–342. doi: 10.1006/hbeh.2002.1768. [DOI] [PubMed] [Google Scholar]
- Royle N.J, Surai P.F, Hartley I.R. Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav. Ecol. 2001;12:381–385. [Google Scholar]
- Saino N, Ferrari R, Romano M, Martinelli R, Møller A.P. Experimental manipulation of egg carotenoids affects immunity of barn swallow nestlings. Proc. R. Soc. B. 2003;270:2485–2489. doi: 10.1098/rspb.2003.2534. 10.1098/rspb.2003.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Nat. Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the egg enhances postnatal growth. Comp. Biochem. Physiol. 1996;114:271–276. doi: 10.1016/0300-9629(96)00009-6. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defenses and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Smits J.E, Bortolotti G.R, Tella J.L. Simplifying the phytohemagglutinin skin-testing technique in studies of avian immunocompetence. Funct. Ecol. 1999;13:567–572. [Google Scholar]
- Sockman K.W, Schwabl H. Yolk androgens reduce offspring survival. Proc. R. Soc. B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. 10.1098/rspb.2000.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler J.J, de Neve L, Pérez-Contreras T, Soler M, Sorci G. Trade-off between immunocompetence and growth in magpies: an experimental study. Proc. R. Soc. B. 2003;270:241–248. doi: 10.1098/rspb.2002.2217. 10.1098/rspb.2002.2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Schwabl H. Yolk testosterone organizes behavior and male plumage coloration in house sparrows (Passer domesticus) Behav. Ecol. Sociobiol. 2004;56:491–497. [Google Scholar]
- Sullivan D.A, Wira C.R. Sex hormone and glucocorticoid receptors in the bursa of Fabricius of immature chickens. J. Immunol. 1979;122:2617–2623. [PubMed] [Google Scholar]
- Sunwoo H.H, Nakano T, Dixon W.T, Sim J.S. Immune response in chickens against lipopolysaccharide of E. coli and Salmonella typhimurium. Poult. Sci. 1996;75:342–345. doi: 10.3382/ps.0750342. [DOI] [PubMed] [Google Scholar]
- Verboven N, Monaghan P, Evans D.M, Schwabl H, Evans N, Whitelaw C, Nager R.G. Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the lesser black-backed gull (Larus fuscus) Proc. R. Soc. B. 2003;270:2223–2232. doi: 10.1098/rspb.2003.2496. 10.1098/rspb.2003.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]



