Abstract
A honeybee hive serves as an information centre in which communication among bees allows the colony to exploit the most profitable resources in a continuously changing environment. The best-studied communication behaviour in this context is the waggle dance performed by returning foragers, which encodes information about the distance and direction to the food source. It has been suggested that another information cue, floral scents transferred within the hive, is also important for recruitment to food sources, as bee recruits are more strongly attracted to odours previously brought back by foragers in both honeybees and bumble-bees. These observations suggested that honeybees learn the odour from successful foragers before leaving the hive. However, this has never been shown directly and the mechanisms and properties of the learning process remain obscure. We tested the learning and memory of recruited bees in the laboratory using the proboscis extension response (PER) paradigm, and show that recruits indeed learn the nectar odours brought back by foragers by associative learning and retrieve this memory in the PER paradigm. The associative nature of this learning reveals that information was gained during mouth-to-mouth contacts among bees (trophallaxis). Results further suggest that the information is transferred to long-term memory. Associative learning of food odours in a social context may help recruits to find a particular food source faster.
Keywords: Apis mellifera, social learning, trophallaxis, information transfer, proboscis extension response
1. Introduction
Recruitment dynamics to scented food sources strongly suggest that information transfer about floral odours inside the hive is an important component of honeybee (Apis mellifera) recruitment (von Frisch 1967; Wenner et al. 1969). The role of this information transfer seemed so important that controversy has arisen about whether bees that follow dances decode the vector information, or instead rely exclusively on odour (von Frisch 1967; Wenner et al. 1969; Gould 1974). This controversy is now considered to be resolved (Gould 1974; Esch et al. 2001; Sherman & Visscher 2002) and the new findings strongly indicate that the dance as well as the olfactory information transferred inside the hive are used to find a particular food source (von Frisch 1967; Wenner et al. 1969; Seeley 1995; Kirchner & Grasser 1998; Esch et al. 2001; Sherman & Visscher 2002). Despite the importance of this olfactory information transfer for recruitment to food sources, questions remain unanswered with respect to whether or not recruits do indeed learn the association between odour and food, when they learn it and what kind of properties the established memory has.
Gerber et al. (1996) showed that olfactory memories of free flying bees established during flower visits in an operant context can be transferred to the proboscis extension response (PER) paradigm in the laboratory in which harnessed bees may extend their proboscis when presented with odorants, depending on their previous experiences with this odour. When the antennae of bees are touched with sucrose solution (unconditioned stimulus; US), they will reflexively extend their proboscis to drink the solution. If an odour (conditioned stimulus; CS) is presented shortly before it becomes associated with the US and subsequently elicits the response (Bitterman et al. 1983). This associative learning paradigm offers a convenient method to quantify retention for an odour in single bees, by testing whether associations between the nectar reward and odours have been acquired during flower visits or within the hive (Gerber et al. 1996; Menzel & Giurfa 2001).
Foraging bees that return from nectar sources transfer the gathered liquid to hive mates through several trophallactic contacts (von Frisch 1967; De Marco & Farina 2001; Farina & Wainselboim 2001a). It has been suggested that recruits may learn the odour of nectar brought back in the honey stomach during these contacts and most honeybees recruited to a source containing an artificial dye were observed to have received a sample from the forager inside the hive (von Frisch 1967). Even during short contacts (less than 4 s long) the regurgitated food may be transferred or just probed and thereby allow receivers to taste the incoming nectar (Farina & Wainselboim 2001b). Using the PER paradigm, we tested whether bees recruited to a scented food source extended their proboscis on the first presentation of the corresponding odour (spontaneous response) and, therefore, had learned the association between food and odour inside the hive during trophallactic contacts. We further analysed the development of retention for the learned odour during 3 consecutive days. Bees may have experienced the combination of odour and solution even if they did not respond spontaneously to the odour (Menzel 1999). In such a case, these bees should learn the odour faster as a consequence of a previous experience. Therefore, we tested their learning performance for the solution odour in a differential PER conditioning (Bitterman et al. 1983): one odour (the odorant diluted in the sugar solution) is paired with sucrose (CS+, CS) and the other odour (the odorant presented at the hive entrance) is presented unpaired (CS−) between CS+ trials. The bees learn to respond to the CS+ and not to the CS−.
2. Material and methods
(a) Study site and animals
The experiment was performed at the end of the nectar flow season (March–April 2004) at the experimental field of the University of Buenos Aires. We used two two-frame observation hives containing a colony of about 4000 European honeybees (hybrid descendants of A. mellifera ligustica) each. Hive bees were marked with coloured paint on the thorax. A group of bees was trained to collect 0.5 M (5 μl min−1 flow rate) unscented sucrose solution at an artificial feeder located 160 m from the hive. These bees were given individual marks and the group was renewed every 3–6 days to maintain a number of about 5 to 10 foragers.
(b) Hive and solution odour
The hive odour served two purposes. First, we wanted to reduce the effect of odour molecules clinging onto the forager's body, which may be perceived during trophallaxis as well. Our aim was that bees would associate the odour covering the forager with a non-appetitive hive context. Second, we were interested to see whether bees also would respond to the hive odour. The hive and its entrance were scented by putting absorbent paper (diameter 3 cm) soaked with 50 μl pure odorant inside a box connected to the entrance by a wire mesh. The paper was renewed once a week. Returning foragers passed the box when entering the hive. In two parts of the experiments we used two different sets of odorants. In part 1 (beginning on day 1), we used phenylacetaldehyde as the hive odour and linalool (LIO) as the odorant diluted in the reward (henceforth, solution odour). In part 2 (beginning on day 19) we used LIO as the hive odour and 2-nonanone as the solution odour (figure 1b,e). We avoided using Phenylacetaldehyde as the solution odour in part 2 because its use as hive odour in part 1 could affect olfactory conditioning of this odour, for instance by latent inhibition (Chandra et al. 2000). The three odours are natural flower odours (Knudsen et al. 1993). We used the same odour combinations for the differential conditioning. In this way, we repeated the experimental situation, where bees experienced one odour in an appetitive context (solution odour) and one in a non-appetitive context (hive odour) in the laboratory. Odours were obtained from Sigma-Aldrich, Steinheim, Germany.
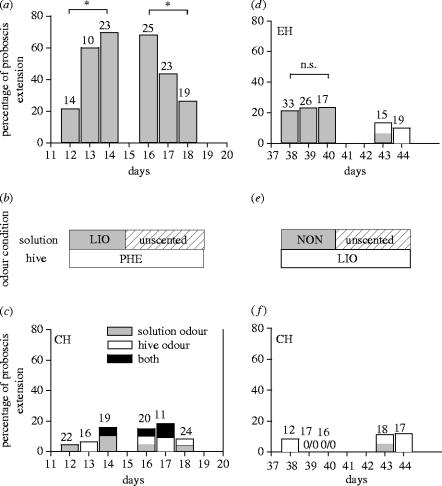
Figure 1.
Percentage of bees that extended the proboscis on the first presentation of the odour. (a) Responses from the experimental hive (EH) during part 1. (b) Its corresponding odour condition: linalool in solution and phenylacetaldehyde in the EH during days 12, 13 and 14; unscented solution and phenylacetaldehyde in the EH between 15 and 18 days. (c) Responses from the control hive (CH) during part 1. (d) Responses from the EH during part 2. (e) Its corresponding odour condition: 2-nonanone in solution and linalool in the EH during days 38, 39 and 40, unscented solution and linalool in the EH between 41 and 44 days. (f) Responses from the CH during part 2. Responses for solution odour (grey), hive odour (white) and for both odours (black) for the EH and the CH. The CH was untreated in both situations. Asterisks indicate statistical differences (Gadj-test, *p<0.05, n.s., not significant; see §3 for details). Number of tested bees above bars.
(c) Bee capture
Bees were captured during 5 periods. Part 1 of the experiment consisted of period 1 (days 3–5), period 2 (days 12–14) and period 3 (days 16–18). Part 2 consisted of period 4 (days 38–40) and period 5 (days 43, 44).
Experimental hive: during sampling periods of 3 h, a group of 5–8 marked bees from the experimental hive collected a 2.0 M sucrose solution (40 μl min−1 flow rate) at a feeder and recruited hive-mates. The solutions were scented with 50 μl pure odour per litre. Several days prior to periods 2 and 4, trained bees were already foraging small amounts (6 ml before period 2 and 4, respectively) of solution scented with the same odour as was used afterwards. After periods with scented solution, the feeders were replaced by clean feeders. Arriving recruits were captured with plastic tubes before they touched the solution; otherwise they were killed with alcohol. Captured bees were fed a drop of a 1.8 M unscented sucrose solution. The interval between capture and feeding was 30–60 min.
Control hive: changes in spontaneous response probabilities to odours and learning of odours could reflect changes in the availability of natural food source. To exclude this possibility we used a control hive placed about 5 m from the experimental hive. During the periods 2–5, bees leaving the control hive were captured and fed after the same interval mentioned above with a drop of 1.8 M sucrose solution.
(d) Harnessing
After 1–3 h in captivity, bees were harnessed in plastic tubes so that they could move their mouthparts and antennae freely (Bitterman et al. 1983). They were fed 1.8 M sucrose solution for about 3 s and kept in an incubator (25 °C, 55% relative humidity, darkness) for at least 3 h.
(e) Differential PER conditioning
We subjected the harnessed bees to standard differential PER conditioning (Bitterman et al. 1983), in which two pure odours are presented, one rewarded (CS+) with 1.8 M sucrose solution (US) and the other unrewarded (CS−), four times each, in a pseudo-randomized order. The inter-trial interval lasted 10–15 min. Only bees that showed the unconditioned response (the reflexive extension of the proboscis after applying a 1.8 M sucrose solution to the antennae) and that did not respond to the mechanical air flow stimulus were used. A device that delivered a continuous airflow was used for odorant application. Trials lasted for 46 s and consisted of 20 s of air flow, 6 s of odour (CS) and 20 s of air flow. During rewarded trials, the reward (US) was delivered upon the last 3 s of CS. Bees that responded to the first presentation of the CS (spontaneous response) were not used in the PER conditioning.
In part 1, the CS+ was LIO (solution odour) and the CS− was phenylacetaldehyde (hive odour). In part 2, the CS+ was 2-nonanone (NON; solution odour), and the CS− was LIO (hive odour). In this way, the experimental situation, where bees experienced one odour in an appetitive context (solution odour) and one in a non-appetitive context (hive odour) was repeated in the laboratory.
(f) Statistical analyses
G-tests were used to compare proboscis extension frequencies between groups. We corrected G-values for multiple comparisons within hive and indicated corresponding p-values with (*). Performance during conditioning was analysed using a discrimination index (Pelz et al. 1997), that was calculated as the cumulative sum of a bee's responses to the CS+ minus the cumulative sum of that bee's responses to the CS−. This index was then used in Kruskal–Wallis ANOVAs. A Dunn's test was used for multiple comparisons between groups.
3. Results
(a) Spontaneous response
The percentage of recruits that extended the proboscis on the first presentation of the odour differed among days in the experimental hive over the experiment, but not in the control hive (experimental hive: G-test, Gadj=88.7, d.f.=13, p<0.001; control hive: G-test, Gadj=6.25, d.f.=10, p>0.5). During the scented period with LIO (period 2) the spontaneous response increased from day 1 to 3 (day 12 versus day 14 of the experimental period: G-tests, LIO, Gadj=8.45, d.f.=1, *p<0.05; figure 1a). On day 3 of the LIO period (day 14), the spontaneous response shown by recruits was significantly higher than that of the foragers coming from a control hive captured the same day (G-test, Gadj=15.7, d.f.=1, p<0.001, figure 1a,c), which shows that bees learned the scents from their hive companions. Additionally, spontaneous responses on day 3 of the LIO period were higher than on day 3 (day 40) of the NON period (G-test, Gadj=8.31, d.f.=1, *p<0.05, figure 1d). After the LIO period, we captured bees recruited to unscented solution for 3 days (period 3) to analyse whether bees recruited several days later also remember the odour. We found a high spontaneous response on the first day (day 16), which decreased by the third day (day 18, G-test, Gadj=7.78, d.f.=1, *p<0.05; figure 1a).
During the period when solution was scented with NON (period 4), the spontaneous response probability did not differ between days 1 and 3, in either the experimental hive or the control hive (table 1). However, more recruits from the experimental hive than the control hive responded to NON during this period (G-test, Gadj=16.85, d.f.=1, p<0.001; figure 1d,f). This suggests that recruits also learned the odour of NON from their companions. After the NON period, we also captured bees recruited to unscented solution for 2 days (period 5, figure 1d). We found a decreasing spontaneous response, attaining a null spontaneous response after 4 days with no NON in the solution (day 44; figure 1d).
Table 1.
Comparison of PER frequencies between days 1 and 3 of a given period.
| analysis | N | Gadj | p |
|---|---|---|---|
| spontaneous response | |||
| NON treatment EH | 45 | 0.044 | 0.98 |
| NON treatment CH | 76 | 0.0 | 1.0 |
EH, experimental hive; CH, control hive; NON, 2-nonanone.
(b) Differential PER conditioning
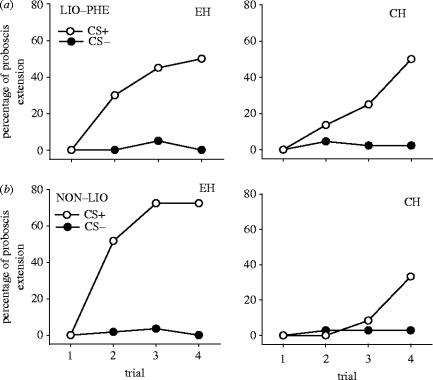
The bees that showed no spontaneous response (above) were used in a differential PER conditioning. There were strong differences in acquisition performance between the bees captured during the two periods when the solution was scented (periods 2 and 4), and the bees from the control hive captured during the corresponding periods (acquisition: Kruskal–Wallis ANOVA for the four groups of bees, N=158, d.f.=3, H=38.5, p<0.0001; comparison between days 1 and 3 for LIO period: experimental hive, N=20, d.f.=2, H=1.58, p=0.45; control hive, N=44, d.f.=2, H=0.93, p=0.63; pooled data for day 1–3 for NON period: experimental hive, N=58, d.f.=2, H=4.05, p=0.13; control hive, N=36, d.f.=2, H=0.22, p=0.89; figure 2a,b). Bees recruited by foragers collecting solution with NON showed better learning of the solution odour than bees from the two control groups (figure 2, table 2) but did not differ from recruits in the LIO treatment (table 2). On day 44, 4 days after the end of the NON period, learning performance in the experimental hive was still higher than in the control hive bees (Mann–Whitney U-test, U=44, z=3.1, p<0.005).
Figure 2.
Percentage of bees that extended the proboscis during a given trial. (a) Acquisition of bees from the experimental hive (EH) and control hive (CH) during the course of the differential PER conditioning. LIO was the CS+ and PHE was the CS−. (b) For conditioning with NON as CS+ and LIO as CS−.
Table 2.
Multiple comparisons of acquisition performance between days 1 and 3 of a given period.
| analysis | N | Q | p |
|---|---|---|---|
| acquisition (DI) | |||
| Dunn's test for multiple-comparisons | |||
| LIO EH versus LIO CH | 20/44 | 1.18 | n.s. |
| LIO EH versus NON EH | 20/58 | 2 | n.s. |
| LIO CH versus NON CH | 44/36 | 1.5 | n.s. |
| NON EH versus NON CH | 58/36 | 5.53 | <0.001 |
EH, experimental hive; CH, control hive; DI, discrimination index; NON, 2-nonanone; LIO, linalool.
4. Discussion
A honeybee colony must rapidly deploy its foragers among many different flower patches in the surrounding environment and therefore needs to acquire information about the different foraging opportunities. Previous studies suggested that the floral odour brought back to the hive by successful foragers is an important information cue for information flow in the control of a colony's foraging operation (von Frisch 1967; Wenner et al. 1969). However, despite the importance of olfactory information transfer inside the hive for recruitment to food sources, the mechanisms underlying this information transfer are poorly understood.
We used two procedures to document that recruited bees learned odours that had been brought back by other foragers. Applying the PER paradigm, we also show that recruits transfer the odour learned in a social context to the classical conditioning situation of the PER test. The associative nature of the PER paradigm (Bitterman et al. 1983) reveals that the learned association took place during trophallactic interactions where the transferred solution functioned as a US and the odour (in the nectar or clinging onto the bee's body) as the CS. We cannot exclude the possibility that recruits perceived odour molecules clinging onto the body of the donor, but our experimental design, as well as that used in earlier studies by von Frisch (1967), suggest that the odour present in the solution was perceived during food transfer.
The spontaneous response levels in the PER test (figure 1) differed between the two odours used. Although the same volumes of LIO and NON solution were collected by the recruiting bees from the experimental hive, recruits to NON solution showed a lower spontaneous response probability than recruits to LIO. On the other hand, recruits showed faster acquisition functions during differential conditioning as compared to bees from the control hive. The difference between the spontaneous response frequencies of recruits to LIO and NON may result from prior learning in the natural context (Bitterman et al. 1983; Menzel & Giurfa 2001) or from innate differences reflecting the biological relevance of the odours used (Knudsen et al. 1993). It may also be that the use of LIO in both parts of the experiments (as solution odour in part 1 and as hive odour in part 2) impaired the learning abilities of the bees in the experimental hive in the second part of the experiment. This, however, seems unlikely because during the 24 days between parts 1 and 2 the colony was almost completely renewed. That bees did not confuse the two contexts in which LIO was used is also supported by the observation that bees did not respond to LIO in part 2.
The potential role of mouth-to-mouth contacts as an information channel for food source characteristics and as a mechanism to efficiently direct the foraging activity of the colony has been suggested in earlier studies, which showed that aspects of trophallaxis correlate with food source profitability, such as nectar unloading rate (Farina & Núñez 1991), frequency and duration of contacts (Farina 1996; De Marco & Farina 2001) or thoracic temperature of food donors (Farina & Wainselboim 2001a). While information on distance and direction transferred during dancing is perceived only by bees following the dancers, information about food source characteristics, such as its odour, may be transferred to most members of the colony through a rapid (within a few hours) distribution of small quantities of food inside the hive (Nixon & Ribbands 1952). This could explain the high response frequency (68%) of foragers that were recruited to unscented solution 2 days after the end of the LIO period. Since the total amount of scented solution carried into the hive during each scented period is very small (approx. 28 ml of sugar solution), the high spontaneous response 2 and 3 days after the LIO period and the higher acquisition rate in the experimental hive than in the control hive 4 days after the end of the NON period may be interpreted in two ways. This olfactory information could be transferred to an early long-term memory, even after a single trophallaxis as it was recently reported (Gil & De Marco 2005). This memory trace is stable over 1–2 days but needs updating on a regular basis for transfer into late long-term memory, a form of memory that controls behaviour 3 days after learning (Menzel 1999). Or the receiving bee may be exposed to multiple experiences within a short period of time, e.g. attending several recruitment dances in a row. In that case, memory consolidation would undergo a sequential transfer from early to late long-term memory (Menzel 1999). Because our bees were killed after the differential PER conditioning, we probably underestimated the stability of the established memories if the bees exposed to the PER test represent a large proportion of the nest mates that learned the odour.
Learning floral odours in a social context such as a hive leads to long-lasting preferences for communicated odours and may affect a larger proportion of foragers and thereby influencing flower choice in the field for several days. Social learning of nectar scents in bees is thus remarkably similar to social transmission of information regarding food odours in some mammals such as the Norway rats, Rattus norvegicus. Rats learn food odours on the breath of co-specifics that have recently eaten and will show a preference for this food, even after weeks (Galef & Giraldeau 2001).
Acknowledgments
We are deeply indebted to M. Spivak, M. Giurfa, I.M. Hamilton and D. Heg for suggestions and valuable comments on the original manuscript. We also thank M. Giurfa for the donation of the PER set-up, and A. Arenas, A. Martinez and H. Verna for technical assistance.
This study was supported by funds from ANPCyT (01-12319), CONICET (02049), UBACyT (X 036) and Fundación Antorchas to W.M.F. C.G. was supported by the Dr. De Jacomi Stiftung and the Janggen-Pöhn Stiftung.
Footnotes
Present address: Division of Behavioural Ecology, University of Bern, Ethologische Station Hasli, Wohlenstrasse 50a, 3032 Hinterkappelen, Switzerland.
These authors contributed equally to this work.
References
- Bitterman M.E, Menzel R, Fietz A, Schafer S. Classical-conditioning of proboscis extension in honeybees (Apis mellifera) J. Comp. Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- Chandra S.B, Hosler J, Smith B. Heritable variation for latent inhibition and its correlation with reversal learning in honeybees (Apis mellifera) J. Comp. Psychol. 2000;114:86–97. doi: 10.1037/0735-7036.114.1.86. [DOI] [PubMed] [Google Scholar]
- De Marco R.J, Farina W.M. Changes in food source profitability affect the trophallactic and dance behavior of forager honeybees (Apis mellifera L.) Behav. Ecol. Sociobiol. 2001;50:441–449. [Google Scholar]
- Esch H.E, Zhang S.W, Srinivasan M.V, Tautz J. Honeybee dances communicate distances measured by optic flow. Nature. 2001;411:581–583. doi: 10.1038/35079072. [DOI] [PubMed] [Google Scholar]
- Farina W.M. Food-exchange by foragers in the hive—a means of communication among honey bees? Behav. Ecol. Sociobiol. 1996;38:59–64. [Google Scholar]
- Farina W.M, Núñez J.A. Trophallaxis in the honeybee, Apis mellifera (L.) as related to the profitability of food sources. Anim. Behav. 1991;42:389–394. [Google Scholar]
- Farina W.M, Wainselboim A.J. Changes in the thoracic temperature of honeybees while receiving nectar from foragers collecting at different reward rates. J. Exp. Biol. 2001a;204:1653–1658. doi: 10.1242/jeb.204.9.1653. [DOI] [PubMed] [Google Scholar]
- Farina W.M, Wainselboim A.J. Thermographic recordings show that honeybees may receive nectar from foragers even during short trophallactic contacts. Insectes Soc. 2001b;48:360–362. [Google Scholar]
- Galef B.G, Giraldeau L.A. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Gerber B, Geberzahn N, Hellstern F, Klein J, Kowalksy O, Wustenberg D, Menzel R. Honey bees transfer olfactory memories established during flower visits to a proboscis extension paradigm in the laboratory. Anim. Behav. 1996;52:1079–1085. [Google Scholar]
- Gil M, De Marco R. Olfactory learning by means of trophallaxis in Apis mellifera. J. Exp. Biol. 2005;208:671–680. doi: 10.1242/jeb.01474. [DOI] [PubMed] [Google Scholar]
- Gould J.L. Honey bee communication. Nature. 1974;252:300–301. [Google Scholar]
- Kirchner W.H, Grasser A. The significance of odor cues and dance language information for the food search behavior of honeybees (Hymenoptera: Apidae) J. Insect Behav. 1998;11:169–178. [Google Scholar]
- Knudsen J.T, Tollsten L, Bergstrom L.G. Floral scents—a checklist of volatile compounds isolated by headspace techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1999;185:323–340. [Google Scholar]
- Menzel R, Giurfa M. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn. Sci. 2001;5:62–71. doi: 10.1016/s1364-6613(00)01601-6. [DOI] [PubMed] [Google Scholar]
- Nixon H.L, Ribbands C.R. Food transmission within the honeybee community. Proc. R. Soc. B. 1952;140:43–50. doi: 10.1098/rspb.1952.0042. [DOI] [PubMed] [Google Scholar]
- Pelz C, Gerber B, Menzel R. Odorant intensity as a determinant for olfactory conditioning in honeybees: roles in discrimination, overshadowing and memory consolidation. J. Exp. Biol. 1997;200:837–847. doi: 10.1242/jeb.200.4.837. [DOI] [PubMed] [Google Scholar]
- Seeley T.D. Harward University Press; Cambridge, MA: 1995. The wisdom of the hive: the social physiology of honey bee colonies. [Google Scholar]
- Sherman G, Visscher P.K. Honeybee colonies achieve fitness through dancing. Nature. 2002;419:920–922. doi: 10.1038/nature01127. [DOI] [PubMed] [Google Scholar]
- von Frisch K. Harvard University Press; Cambridge, MA: 1967. The dance language and orientation of bees. [Google Scholar]
- Wenner A.M, Wells P.H, Johnson D.L. Honey bee recruitment to food sources—olfaction or language? Science. 1969;164:84–86. doi: 10.1126/science.164.3875.84. [DOI] [PubMed] [Google Scholar]