Abstract
Whilst there is an abundance of studies revealing how dominance interactions affect access to resources critical for survival and reproductive success, very little is known about how dominance status is influenced by early life experiences. However, there is increasing evidence that early developmental trajectories can shape the physiology and behaviour of the adult. In particular, compensatory growth following a period of poor nutrition can have long-term effects on the phenotype. Since catch-up growth increases daily energy requirements and hence the motivation to acquire sufficient resources, it might either increase or decrease competitive ability and aggression. Here we test whether growth compensation early in life subsequently affects the dominance status of adult male swordtail fishes Xiphophorus helleri, a species with strong sexual dimorphism and male–male competition. Males that experienced a period of restricted food early in life subsequently caught up and achieved the same adult body and ornament size as control males that had been raised on ad libitum food throughout development, but were subordinate to size-matched controls, suggesting a trade-off between sexual attractiveness and competitive ability. This indicates that early life history and/or growth trajectory can be an important determinant of competitive ability independent of current body size.
Keywords: growth, signalling, life history traits, social status, aggression, nutrition
1. Introduction
Dominance status, which can be defined as success in contests over critical resources (Qvarnström & Forsgren 1998), provides many potential benefits. Dominant individuals gain greater access to contested resources, such as sexual partners (Rantala & Kortet 2004), or food (Maclean & Metcalfe 2001). However, dominance may also be costly (Creel 2001), so if only males of high quality can afford the costs of dominance (Zahavi 1975) then a male's relative competitive ability should provide a reliable indicator of male quality (Qvarnström & Forsgren 1998). Winners of male–male competition are consequently expected to be of higher quality, and be preferred by females in mate choice (Berglund et al. 1996).
In addition to being affected by prior agonistic experience (e.g. winner and loser effects; Beaugrand 1997; Earley & Dugatkin 2002), dominance in adults has been linked to individual, size-independent, differences in behaviour during early life, such as boldness (Sundström et al. 2004). However, very little is known about the effects of early environmental conditions on the outcome of dominance interactions later in life. Poor conditions throughout development may lead to a stunted size at adulthood, which may in turn reduce competitive ability, since this is often related to body size (Huntingford & Turner 1987). Individuals may compensate for a poor start in life if conditions subsequently improve through accelerated growth and compensatory resource allocation (Metcalfe & Monaghan 2001). Such catch-up growth can be facilitated by social dominance (Maclean & Metcalfe 2001), but it is unclear what the consequences of compensatory growth might be for social status (i.e. if the arrow of causation is reversed). This is, however, likely to be an important determinant of life history trajectories and thus fitness, since compensatory responses may be more common than previously realized (Metcalfe & Monaghan 2001).
Johnsson et al. (1996) showed that stimulation of rapid growth through the administration of exogenous growth hormone (GH) increased dominance in trout, through a presumed mechanism whereby GH increases growth, and therefore energy demand, but also increases competitive ability. Alternatively, individuals that have experienced growth compensation might not be able to meet the energetic and physiological demands of aggression if compensatory resource allocation entails significant physiological costs (e.g. muscle damage—Christiansen et al. 1992).
We examined these alternative hypotheses using green swordtails, Xiphophorus helleri. This is a small, tropical, live-bearing fish which exhibits contrasting inter-sexual selection pressures on growth. In females, there is strong positive selection for large body size, since fecundity is correlated with body size. In contrast, in males selection is more focused on the secondary selected traits, particularly the ‘sword’, which is an extension of the caudal fin that develops at sexual maturity. Females prefer larger bodied males, but after controlling for body size they have a strong preference for males with longer swords (Basolo 1990, 1998). However, larger bodied males tend to be dominant over smaller males (Beaugrand et al. 1996), so there may be costs associated with investing in sword growth at the expense of body size. In natural situations swordtails are not seasonal breeders, so temporal effects mean mature males compete for territories against males of differing ages and consequently different early life histories. Here we test whether previous growth trajectory affects the dominance status of adult males.
2. Material and methods
Fish used in the behavioural contests were drawn from two of three treatment groups from a wider study of the behavioural consequences of compensatory resource allocation during growth. Details, in brief, of the rearing regime are as follows. The experiment was conducted in the laboratory under controlled conditions (stable temperature of 23±1 °C, 16L : 8D light regime). Twenty-one wild-caught females from Belize, South America were mated in the laboratory to wild-caught males from the same population. The resulting fry were removed from each female's breeding tank and reared in a separate rearing tank (one tank for each brood) until two months of age. Fry were then placed singly in one half of an acrylic plastic rearing tank (320×170×180 mm3; 10L) that was divided longitudinally by a transparent perspex partition. Fish were thus physically, but not visually isolated from their neighbours. Each tank had a gravel substrate, a plastic plant for cover and the water was aerated and filtered. Partial water changes were conducted on a weekly basis to maintain water quality.
Equal numbers of fish from each brood were allocated to two treatment groups:
Good/good (GG)—ad libitum food daily for the duration of the experimental period (two months of age onwards).
Poor/good (PG)—fed ad libitum three times a week from two to six months of age, then subsequently put onto the same daily ad libitum diet as GG fish from six months onwards.
Fish were fed twice a day (on days when fed) with commercial micro-pelleted food with a protein content of 44% and a lipid content of 4.8% (Hikari tropical micro-pellets; Kyorin, Japan). The different food regimes were designed, following initial trials, to stimulate a compensatory growth response in PG fish and maintain a steady growth trajectory in GG fish. Food was dispensed from a modified syringe with a wide gauge needle, such that GG fish received at least 4.5% of body mass daily (i.e. 0.036 g d−1 until they were at least 0.8 g in mass, then 0.072 g d−1 until they were 1.6 g in mass and 0.108 g d−1 if they were over 1.6 g). PG fish were initially fed 0.036 g d−1 3 days out of 7, so on average received the equivalent of 0.0154 g d−1 until six months, when they were put on the same feeding regime as similarly sized GG fish (see above).
Fish were weighed and measured initially at two months, and then subsequently every two weeks until 10 months of age. On each occasion they were anaesthetized in an aerated water bath using benzocaine in 95% alcohol at a concentration of 8 ml l−1 of water, weighed (±0.01 g) and the following linear measurements taken: standard length (i.e. excluding caudal fin), total length, and maximum body depth, all ±0.1 mm. Growth rate was quantified as the growth increment of standard length (SL, in mm) over successive growth periods (2–6 and 6–10 months). Once males were fully mature (i.e. with gonopodia and swords), 13 size-matched pairs were created by pairing single unrelated and unfamiliar males from the GG and PG groups. No fish had had social experience in competitive situations for a minimum of 15 months prior to this point (i.e. since they were two months old), nor had any prior visual or chemical communication with their sized-matched partner. This is important, since it has been previously shown that initial status (i.e. prior winner or loser) is a primary determinant of agonistic outcomes in size-matched male dyads in this species (Beaugrand & Cotnoir 1996; Earley & Dugatkin 2002). A total of 18 different families were represented in the experiment.
For the dominance experiments we used a protocol similar to that previously used by Beaugrand & Goulet (2000). Males were put into an unfamiliar tank (500×250×250 mm3) split into three equal sized sections using opaque Perspex partitions. Each male was placed into one of the two end sections and left to acclimatize for at least 3 h. Observations began once the partitions were removed so that both males had access to the whole tank, and the behaviour of each male was assessed using instantaneous scan sampling (Martin & Bateson 1992) each minute for 30 min. Males were readily individually identifiable within pairs from variation in flank and tail markings, so were not otherwise marked.
At each sampling point the behaviour of each fish was classified as aggressive, defensive or neutral. Aggressive behaviours were split into lower level (tail-beating and lateral-displaying) and escalated (attacking, biting, mouth-fighting) forms; the corresponding lower level and escalated defensive behaviours were adopting a folding position and fleeing. All other behaviours (e.g. approaching, fluttering, bottom immobility, rising from the bottom and breaking the surface) were considered neutral (Beaugrand & Goulet 2000). Dominance is defined in terms of defensive behaviour, thus a fish that expressed defensive behaviour towards its partner, but elicited no defensive behaviour itself was defined as being subordinate.
Dominance data were analysed with non-parametric Wilcoxon signed-ranks tests, whilst all morphometric data were analysed using paired t-tests. Data used in parametric tests were first tested for normality using one-sample Kolmogorov–Smirnov tests, and transformed where appropriate. We used restricted maximum likelihood estimation (REML) and the penalized log likelihood (Akaike information criterion; AIC) in mixed effects models to compare fit of different models of growth, following sequential dropping of non-significant terms from a full (maximal) model (Crawley 2002). Successive models were compared using the AIC—the smaller the AIC the better the model fit—and the log likelihood ratio test used to check that dropping terms did not significantly reduce the fit of the model. All statistical tests were conducted using S-Plus 6 for Windows or SPSS 10 for Windows.
3. Results
(a) Growth and development
Paired GG and PG males came from similar initial brood sizes (t12=0.79, p=0.44), so there is no reason to expect there to have been differences in the social environments that they experienced prior to the growth manipulations. GG and PG males were also similar initial sizes before the diet manipulation (figure 1). However, as a consequence of the different food regimes, males in the PG treatment grew more slowly during the first four months (figure 2) and so were significantly smaller at six months of age (figure 1). When switched onto the same diet as the fish on the GG treatment, the PG males accelerated their growth rate (figure 2), such that they had almost completely compensated in size by the age of 10 months, and had completely caught up with GG males by the time of testing (figure 1). There was thus no difference between pairs of males in any measured morphological trait at the time of dominance testing (table 1 and figure 1).
Figure 1.
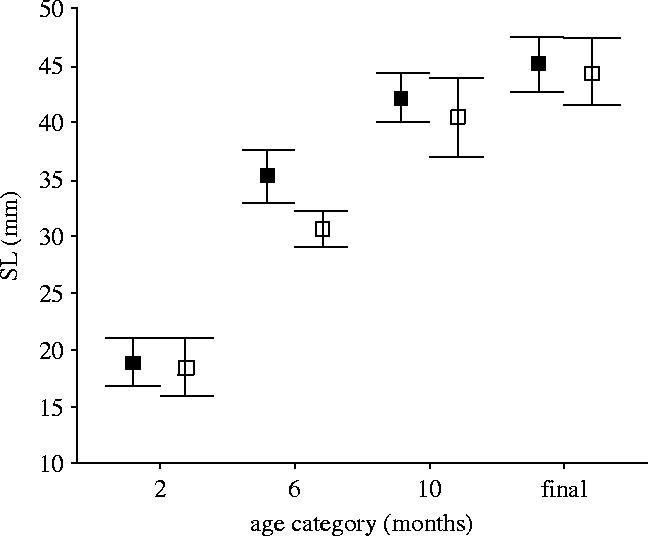
Body size (SL) of GG (filled squares) and PG (open squares) males in relation to age. Repeated measures ANOVA on SL at 2, 6 and 10 months of age; SL×treatment, F2,48=4.55, p=0.016. Contrasts indicate that PG males are smaller at six months than GG males, but not at two or 10 months; quadratic F1,24=20.47, p<0.0005. Means±95% confidence intervals.
Figure 2.
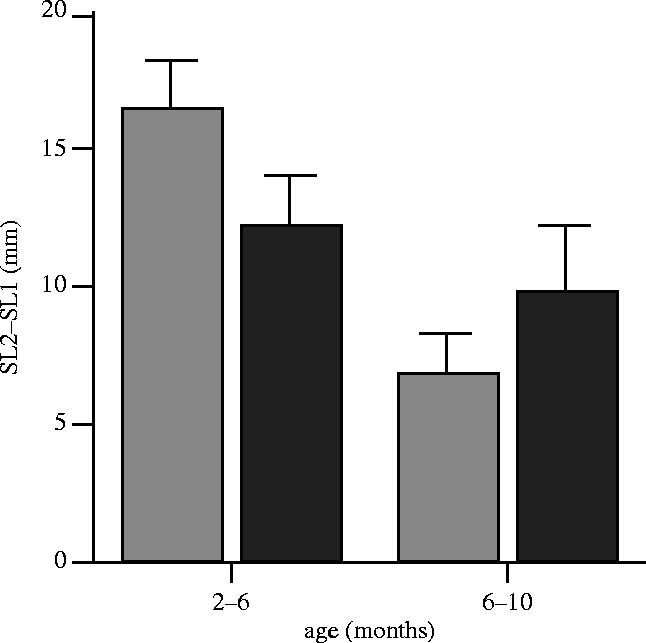
Absolute growth increments (where SL2 is final size, and SL1 is initial size, in mm) for GG (light shaded bars) and PG (dark shaded bars) males for successive periods of growth (2–6 and 6–10 months). Bars represent means with ±95% confidence intervals.
Table 1.
Measured trait values of males raised under ad libitum food treatment (GG), and males that compensated for a poor start in life (PG) at time of dominance testing.
| GG | PG | paired t-test | |
|---|---|---|---|
| total length (mm) | 79.34±8.78 | 79.17±8.30 | t12=0.16, p=0.88 |
| sword length (mm) | 21.72±4.90 | 21.99±4.41 | t12=0.20, p=0.85 |
| maximum body depth (mm) | 11.99±1.37 | 11.88±1.95 | t12=0.51, p=0.62 |
| mass (g) | 1.86±0.57 | 1.81±0.72 | t12=1.00, p=0.34 |
| age (months) | 20.38±1.98 | 20.54±2.26 | t12=0.16, p=0.87 |
See figure 1 for data on standard length. Means ±1 s.d.
A mixed effects analysis (REML) of the absolute growth increment during early development (2–6 months of age), with ‘pair’ as a random effect and treatment and initial SL (at two months) as fixed effects, showed negative effects of both treatment (t11=4.49, p=0.0009; i.e. PG<GG) and initial SL (t11=3.19, p=0.0086), but no significant interaction (p=0.2; which was dropped from the maximal model; maximal model AIC=129.77, minimal model AIC=128.69; log likelihood ratio test, p=0.34); thus GG males had a higher increment of growth than PG males during this period. A similar analysis for the growth increment between 6–10 months showed that PG males had a higher growth increment (treatment t10=2.38, p=0.039). There was also a significant negative overall effect of SL at six months (SL6; t10=2.43, p=0.036), and a significant treatment×SL6 interaction (t10=2.67, p=0.024), with a negative effect of SL at six months on GG fish (as might be expected, as larger fish were further up the growth curve), but a positive effect of SL6 on PG fish. This suggests that only the individuals that had managed to achieve the largest size at six months when on restricted rations during early growth (i.e. 2–6 months) could afford to put on a big growth spurt subsequently.
(b) Dominance
Males from the GG treatment regime were significantly more likely to show aggressive behaviour than their size-matched PG opponents (Wilcoxon, Z=2.67, n=13, p=0.008; figure 3a). Conversely, males that had experienced a poor nutritional start in life and subsequently showed compensatory growth (PG males) exhibited more defensive, subordinate behaviour than GG males (Wilcoxon, Z=2.30, n=13, p=0.021). This difference was so pronounced that in only one GG–PG dyad did the GG male exhibit any defensive behaviour at all (figure 3b). Consequently, GG fish were dominant to PG fish of equal size in 12 out of the 13 pairs (Binomial test, p=0.003). Trends were similar for summed behaviours (i.e. aggressive or defensive) as for their constituent parts (aggressive=offensive+menacing; defensive=fleeing+folding), so data are presented for summed behaviours only.
Figure 3.
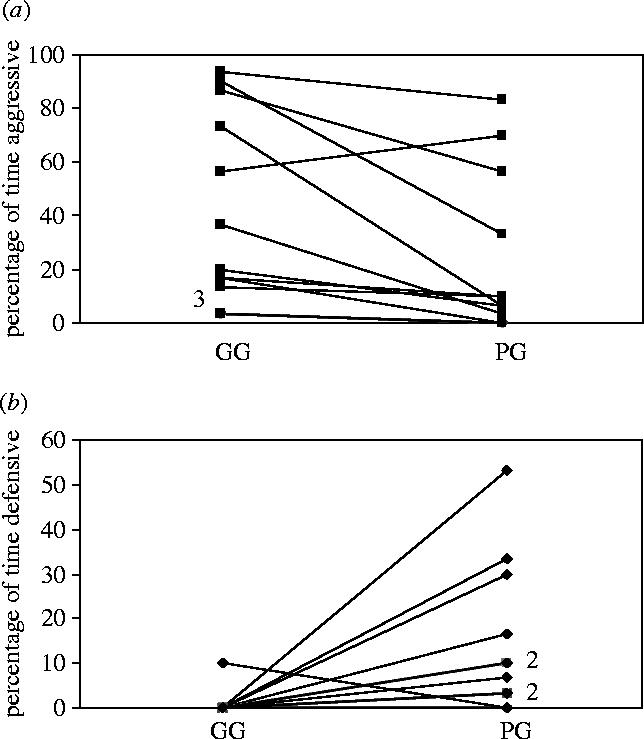
Percentage of total time spent on (a) aggressive and (b) defensive behaviours in relation to treatment. Lines connect individuals within dyads. Three pairs in (a) and two in (b) had identical relationships within dyads, so the total number of lines shown is less than 13; shared lines are marked.
4. Discussion
In paired contests, males that experienced good nutrition throughout development (GG fish) were socially dominant over their size-matched partners that had earlier compensated for a poor nutritional start in life (PG fish). There was no difference in initial size or final body or ornament size between pairs of males from the different treatments, and all males were raised in the absence of social competition until the social dominance assay, so there were no opportunities for males to assess their status prior to testing. Consequently, the only difference between males in the two treatments was the difference in growth trajectories brought about by the difference in resource availability during early development (2–6 months of age). GG males had more rapid initial growth than PG fish, but following the introduction of ad libitum food at six months, PG males had greater growth increment than GG fish and re-aligned on their previous growth trajectory. PG males caught up because the growth of GG males was slowing to an asymptote by this time, and not because PG males accelerated growth rate above a level expected for their size. However, the growth of PG males was more rapid than that expected for GG fish of similar age, so growth was accelerated with respect to developmental age. Hence the differences in competitive ability and dominance status were due to nutritional experience during early life and the effect of this perturbation of growth trajectory, rather than size achieved per se.
(a) Costs of growth compensation
Although PG males had lower social status than GG males they were phenotypically indistinguishable from one another (at least to our eyes). This suggests that growth compensation extracts a cost at the physiological level that may adversely affect physiological performance. Faster growing, but size-matched transgenic adult coho salmon, Oncorhynchus kitsutch had higher routine oxygen consumption, lower critical swimming speeds (Ucrit) and were less efficient swimmers (oxygen consumption as a function of swimming speed) than slower growing control fish, and had poorer rates of recovery following exercise (Lee et al. 2003). In related work Devlin et al. (2004) showed that the faster growth of transgenic fish was associated with an increased competitive ability relative to non-transgenic fish in mixed populations, but under sub-optimal food availability transgenic fish had much lower survival than non-transgenic fish. Costs of rapid growth are not just restricted to genetically modified individuals, however. Munch & Conover (2003) used two populations of Atlantic silverside fishes, Menidia menidia, that differed in intrinsic growth rate, to test whether rapid growth increased susceptibility to predation. This showed that neither fast or slow growth populations modified their growth in response to levels of predation, but the fast growth population had higher mortality, related to increased risk taking, despite individuals being 40% larger by the end of the trial (Munch & Conover 2003). Previous work had shown that fast growing silversides were more susceptible to predation and had poorer swimming performance compared to slow-growing individuals of the same size suggesting that increased mortality was a consequence of variation in growth rate and not just due to differences between populations (Billerbeck et al. 2001; Lankford et al. 2001). These studies illustrate the long-term nature of costs of growth, and show that these costs are not necessarily ameliorated by achieving a large size (Munch & Conover 2003).
Given that growth rate can have substantial effects on swimming performance, which is dependent upon muscle power and development, it is possible that male swordtails can detect weaknesses relating to competitive ability from swimming performance during displays. Lateral displays are known to be energetically costly, with costs higher for males with longer swords (Basolo & Alcaraz 2003). Since PG males had similar length swords to GG males, but compensated for poorer nutrition during early development, which can result in muscle damage (Christiansen et al. 1992), it seems possible that lateral displays would be more energetically costly for PG males than for GG males, and that this could have been assessed by rival males. Certainly, another form of assessment known to be used in the establishment of social rank, body depth (Swain & Holtby 1989; Holtby et al. 1993), is not applicable here, since there were no differences in body depth of males in the different treatments. Although the means by which males assess competitive ability is not clear, the results indicate that secondary sexual characteristics are not necessarily a good indicator of competitive ability per se, and that the sword is not a badge of status (sensu Rohwer 1975).
(b) Costs and benefits of dominance status
Females do not always prefer dominant males (Qvarnström & Forsgren 1998; Moore et al. 2001) and social dominance is not always a good indicator of fitness prospects (Verhulst & Salomons 2004), but the suggestion that energy costs of dominance make dominant individuals more likely to starve (Qvarnström & Forsgren 1998; Devlin et al. 2004) is unlikely to apply here since there were no differences in the size of GG and PG males. It is possible that PG fish compensated for their earlier poor nutrition by trading off their adult size and attractiveness with competitive ability. Consequently, if anything it seems more likely that PG fish would be more susceptible to starvation than GG fish. Conversely, dominance status can have a positive effect not just on resource acquisition ability, but also on behaviour (e.g. spatial awareness and learning; Barnard & Luo 2002), so there are numerous benefits to offset the potential costs. The available data on the social structure of green swordtails suggests that reduced dominance status may be an important cost to fitness of individuals that have compensated for a poor start in life. In the wild, males maintain a semi-territorial system of overlapping home ranges, which are defended vigorously from other males (Franck & Ribowski 1993). Their social behaviour is therefore characterized by a high frequency of male–male competitive interactions and the formation of stable dominance hierarchies (Franck & Ribowski 1993). This can also be seen in the laboratory, where subordinate adult males are frequently harassed by more dominant individuals (personal observation). Consequently, growth compensation, although increasing a male's potential attractiveness to females, results in clear costs of reduced competitiveness in male–male interactions in swordtails independent of their body size.
Acknowledgments
We thank John Laurie, Helcia Lepatik, June Freel, Craig Walling and Graham Adam for help with fish husbandry, and two referees for helpful comments on the manuscript. This work was funded by a grant from the BBSRC.
References
- Barnard C.J, Luo N. Acquisition of dominance status affects maze learning in mice. Behav. Process. 2002;60:53–59. doi: 10.1016/s0376-6357(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Basolo A.L. Female preference for male sword length in the green swordtail, Xiphophorus helleri (Pisces, Poeciliidae) Anim. Behav. 1990;40:332–338. [Google Scholar]
- Basolo A.L. Shift in investment between sexually-selected traits: tarnishing of the silver spoon. Anim. Behav. 1998;55:665–671. doi: 10.1006/anbe.1997.0634. [DOI] [PubMed] [Google Scholar]
- Basolo A.L, Alcaraz G. The turn of the sword: length increases male swimming costs in swordtails. Proc. R. Soc. B. 2003;270:1631–1636. doi: 10.1098/rspb.2003.2388. 10.1098/rspb.2003.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugrand J.P. Resolution of agonistic conflicts in dyads of acquainted green swordtails (Xiphophorus helleri): a game with perfect information. Behav. Process. 1997;41:293–310. doi: 10.1016/s0376-6357(97)00038-7. [DOI] [PubMed] [Google Scholar]
- Beaugrand J.P, Cotnoir P.-A. The role of individual differences in the formation of triadic dominance orders of male green swordtail fish (Xiphophorus helleri) Behav. Process. 1996;38:287–296. doi: 10.1016/s0376-6357(96)00039-3. [DOI] [PubMed] [Google Scholar]
- Beaugrand J.P, Goulet C. Distinguishing kinds of prior dominance and subordination experiences in males of green swordtail fish (Xiphophorus helleri) Behav. Proc. 2000;50:131–142. doi: 10.1016/s0376-6357(00)00096-6. [DOI] [PubMed] [Google Scholar]
- Beaugrand J.P, Payette D, Goulet C. Conflict outcome in male green swordtail fish dyads (Xiphophorus helleri): Interaction of body size, prior dominance/subordination experience, and prior residency. Behaviour. 1996;133:303–319. [Google Scholar]
- Berglund A, Bisazza A, Pilastro A. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 1996;58:385–399. [Google Scholar]
- Billerbeck J.M, Lankford T.E, Conover D.O. Evolution of intrinsic growth and energy acquisition rates. 1. Trade-offs with swimming performance in Menidia menidia. Evolution. 2001;55:1863–1872. doi: 10.1111/j.0014-3820.2001.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Christiansen J.S, Martinez I, Jobling M, Amin A.B. Rapid somatic growth and muscle damage in a salmonid fish. Basic Appl. Myol. 1992;2:235–239. [Google Scholar]
- Crawley M.J.Statistical computing. An introduction to data analysis using S-Plus2002John Wiley & Sons; Sussex, UK [Google Scholar]
- Creel S. Social dominance and stress hormones. Trends Ecol. Evol. 2001;16:491–497. [Google Scholar]
- Devlin R.H, D'Andrade M, Uh M, Biagi C.A. Population effects of growth hormone transgenic coho salmon depend on food availability and genotype by environment interactions. Proc. Natl Acad. Sci. USA. 2004;101:9303–9308. doi: 10.1073/pnas.0400023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley R.L, Dugatkin L.A. Eavesdropping on visual cues in green swordtail (Xiphophorus helleri) fights: a case for networking. Proc. R. Soc. B. 2002;269:943–952. doi: 10.1098/rspb.2002.1973. 10.1098/rspb.2002.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck D, Ribowski A. Dominance hierarchies of male green swordtails (Xiphophorus helleri) in nature. J. Fish Biol. 1993;43:497–499. [Google Scholar]
- Holtby L.B, Swain D.P, Allan G.M. Mirror-elicited agonistic behaviour and body morphology as predictors of dominance status in juvenile coho salmon (Oncorhynchus kisutch) Can. J. Fish. Aquat. Sci. 1993;50:676–684. [Google Scholar]
- Huntingford F.C, Turner A. Chapman & Hall; London: 1987. Animal conflict. [Google Scholar]
- Johnsson J.I, Jönsson E, Björnsson B.T. Dominance, nutritional state, and growth hormone levels in rainbow trout (Oncorhynchus mykiss) Horm. Behav. 1996;30:13–21. doi: 10.1006/hbeh.1996.0003. [DOI] [PubMed] [Google Scholar]
- Lankford T.E, Billerbeck J.M, Conover D.O. Evolution of intrinsic growth and energy acquisition rates. 2. Trade-offs with vulnerability to predation in Menidia menidia. Evolution. 2001;55:1873–1881. doi: 10.1111/j.0014-3820.2001.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Lee C.G, Devlin R.H, Farrell A.P. Swimming performance, oxygen consumption and excess post-exercise oxygen consumption in adult transgenic and ocean-ranched coho salmon. J. Fish Biol. 2003;62:753–766. [Google Scholar]
- Maclean A, Metcalfe N.B. Social status, access to food, and compensatory growth in juvenile Atlantic salmon. J. Fish Biol. 2001;58:1331–1346. [Google Scholar]
- Martin P, Bateson P. Cambridge University Press; 1992. Measuring behaviour: an introductory guide. [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Moore A.J, Gowaty P.A, Wallin W.G, Moore P.J. Sexual conflict and the evolution of female mate choice and male social dominance. Proc. R. Soc. B. 2001;268:517–523. doi: 10.1098/rspb.2000.1399. 10.1098/rspb.2000.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch S.B, Conover D.O. Rapid growth results in increased susceptibility to predation in Menidia menidia. Evolution. 2003;57:2119–2127. doi: 10.1111/j.0014-3820.2003.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Qvarnström A, Forsgren E. Should females prefer dominant males? Trends Ecol. Evol. 1998;13:498–501. doi: 10.1016/s0169-5347(98)01513-4. [DOI] [PubMed] [Google Scholar]
- Rantala M.J, Kortet R. Male dominance and immunocompetence in a field cricket. Behav. Ecol. 2004;15:187–191. [Google Scholar]
- Rohwer S. The social significance of avian winter plumage variability. Evolution. 1975;29:593–610. doi: 10.1111/j.1558-5646.1975.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Sundström L.F, Petersson E, Höjesjö J, Johnsson J.I, Järvi T. Hatchery selection promotes boldness in newly hatched brown trout (Salmo trutta): implications for dominance. Behav. Ecol. 2004;15:192–198. [Google Scholar]
- Swain D.P, Holtby L.B. Differences in morphology and behavior between juvenile coho salmon (Oncorhynchus kisutch) rearing in a lake or in its tributary stream. Can. J. Fish. Aquat. Sci. 1989;46:1406–1414. [Google Scholar]
- Verhulst S, Salomons H.M. Why fight? Socially dominant jackdaws, Corvus monedula, have low fitness. Anim. Behav. 2004;68:777–783. [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]


