Abstract
Communication between G protein-coupled receptor (GPCR) and epidermal growth factor receptor (EGFR) signalling systems involves cell surface proteolysis of EGF-like precursors. The underlying mechanisms of EGFR signal transactivation pathways, however, are largely unknown. We demonstrate that in squamous cell carcinoma cells, stimulation with the GPCR agonists LPA or carbachol specifically results in metalloprotease cleavage and release of amphiregulin (AR). Moreover, AR gene silencing by siRNA or inhibition of AR biological activity by neutralizing antibodies and heparin prevents GPCR-induced EGFR tyrosine phosphorylation, downstream mitogenic signalling events, cell proliferation, migration and activation of the survival mediator Akt/PKB. Therefore, despite some functional redundancy among EGF family ligands, the present study reveals a distinct and essential role for AR in GPCR-triggered cellular responses. Furthermore, we present evidence that blockade of the metalloprotease-disintegrin tumour necrosis factor-α-converting enzyme (TACE) by the tissue inhibitor of metalloprotease-3, a dominant-negative TACE mutant or RNA interference suppresses GPCR-stimulated AR release, EGFR activation and downstream events. Thus, TACE can function as an effector of GPCR-mediated signalling and represents a key element of the cellular receptor cross-talk network.
Keywords: amphiregulin/EGFR/HNSCC/TACE/transactivation
Introduction
Interreceptor communication between G protein-coupled receptors (GPCRs) and the epidermal growth factor receptor (EGFR) occurs in diverse cell types including fibroblasts, keratinocytes, astrocytes, PC-12 cells and smooth muscle cells (Daub et al., 1997; Zwick et al., 1997; Eguchi et al., 1998). Treatment of cells with GPCR agonists results in activation and tyrosine phosphorylation of the EGFR and subsequently leads to the generation of an EGFR-characteristic, intracellular signal (Daub et al., 1996). Due to the rapid kinetics of the transactivation signal and the fact that release of EGFR ligands was not detectable after GPCR stimulation, the mechanism of EGFR transactivation was proposed not to involve the interaction of the EGFR with a ligand. Hence, EGFR activation by GPCR agonists was assumed to rely exclusively on intracellular elements such as Ca2+, protein kinase C (PKC) and Src (Daub et al., 1996; reviewed in Carpenter, 1999). In contrast, a novel mechanistic concept of EGFR transactivation involves the proteolytic release of heparin-binding EGF-like growth factor (HB-EGF) at the cell surface of GPCR-stimulated cells (Prenzel et al., 1999; reviewed in Carpenter, 2000). HB-EGF, as well as transforming growth factor-α (TGF-α) and amphiregulin (AR), belongs to a family of EGF-like ligands that directly activate the EGFR. These molecules are synthesized as transmembrane precursors, which are subject to proteolytic cleavage at the cell surface to produce the soluble and diffusible growth factors (reviewed in Massagué and Pandiella, 1993). Subsequently, the mature ligands activate receptor tyrosine kinases of the EGFR family by autocrine or paracrine stimulation.
Growing evidence points to transmembrane metalloproteases as the key enzymes of growth factor precursor shedding. The severe phenotype of mice lacking the metalloprotease-disintegrin tumour necrosis factor-α-converting enzyme (TACE)/ADAM17 suggests an essential role for TACE and soluble TGF-α in normal development and emphasizes the importance of protein ectodomain shedding in vivo (Peschon et al., 1998). In addition, the absence of functional TACE results in impaired basal solubilization of a variety of other EGF-like ligands and cell surface molecules such as AR and HB-EGF (Merlos-Suarez et al., 2001; Sunnarborg et al., 2002). ADAM10-deficient mice have been reported to die very early in embryogenesis with multiple defects of the developing central nervous system, somites and cardiovascular system (Hartmann et al., 2002). It is not known, however, whether these developmental defects are due to impaired growth factor precursor shedding. On the other hand, mice lacking MDC9/ADAM9 have no evident major abnormalities during development or adult life (Weskamp et al., 2002). Moreover, proHB-EGF processing is comparable in embryonic fibroblasts isolated from ADAM9(–/–) and wild-type mice, arguing against an essential role for ADAM9 in proHB-EGF shedding in these cells.
The HB-EGF-dependent mechanism of EGFR signal transactivation has gained further experimental support by studies on GPCR mitogenic signalling in vascular smooth muscle cells (Eguchi et al., 2001), cardiac endothelial cells (Fujiyama et al., 2001) and cardiomyocytes (Kodama et al., 2002). Importantly, recent data have implicated EGFR signal transactivation pathways in the aetiology of pathobiological processes such as cystic fibrosis (Lemjabbar and Basbaum, 2002), and cardiac (Asakura et al., 2002) and gastrointestinal hypertrophy (Keates et al., 2001). Furthermore, increasing evidence argues for a direct correlation between aberrant GPCR signalling and the development and progression of human cancers (reviewed in Marinissen and Gutkind, 2001). We recently have demonstrated that GPCR–EGFR cross-talk pathways are widely established in head and neck squamous cell carcinoma (HNSCC) cells and that GPCR agonists promote cell proliferation and motility of HNSCC cells via transactivation of the EGFR (Gschwind et al., 2002). Elucidation of the molecular mechanisms underlying EGFR signal transactivation may thus lead to novel strategies for the treatment of human cancers.
The results of this study indicate that treatment of squamous cell carcinoma cells with GPCR ligands such as LPA and carbachol leads to the rapid and specific cleavage of proAR at the cell surface by TACE. Moreover, release of mature AR is a prerequisite to EGFR stimulation, subsequent SHC and Grb2 adaptor protein recruitment, downstream activation of ERK1/2 and phosphatidylinositol 3-kinase (PI3K)-dependent phosphorylation of Akt/PKB. Finally, this triple membrane passing signal (TMPS) mechanism of EGFR transactivation provides a molecular explanation for the question of how GPCR ligands regulate the proliferative and migratory behaviour of cancer cells.
Results
EGFR signal transactivation in HNSCC cells involves a ligand-dependent mechanism
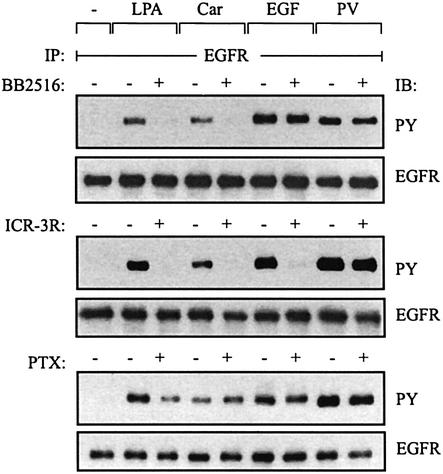
The transactivation signal induced by the GPCR ligands lysophosphatidic acid (LPA) and carbachol was sensitive to broad-spectrum metalloprotease inhibitors such as batimastat (BB94; Gschwind et al., 2002) and marimastat (BB2516; Figure 1, upper panel) in SCC-9 cells. In contrast, these compounds did not interfere with responses triggered by EGF or by pervanadate, a potent tyrosine phosphatase inhibitor (Huyer et al., 1997) which increases the tyrosine phosphorylation content of many intracellular proteins. Consistent with a ligand-dependent mechanism of EGFR signal transactivation, we found that the monoclonal anti-EGFR antibody ICR-3R, which prevents binding of EGF-like growth factors to the extracellular domain of the receptor (Mateo et al., 1997), abrogated GPCR- and EGF-induced EGFR tyrosine phosphorylation in HNSCC cells (Figure 1, middle panel).
Fig. 1. EGFR signal transactivation requires metalloprotease activity and the extracellular domain of the EGFR. SCC-9 cells were pre-incubated with marimastat (BB2516, 10 µM; 20 min), anti-EGFR antibody ICR-3R (20 µg/ml; 60 min) or PTX (100 ng/ml; 18 h) and treated with LPA (10 µM), carbachol (Car, 1 mM), EGF (7.5 ng/ml) or pervanadate (PV, 1 mM) for 3 min. Following immunoprecipitation (IP) of cell extracts with anti-EGFR antibody, proteins were immunoblotted (IB) with anti-phosphotyrosine antibody and re-probed with anti-EGFR antibody.
GPCR agonists stimulate proteolytic cleavage and release of AR
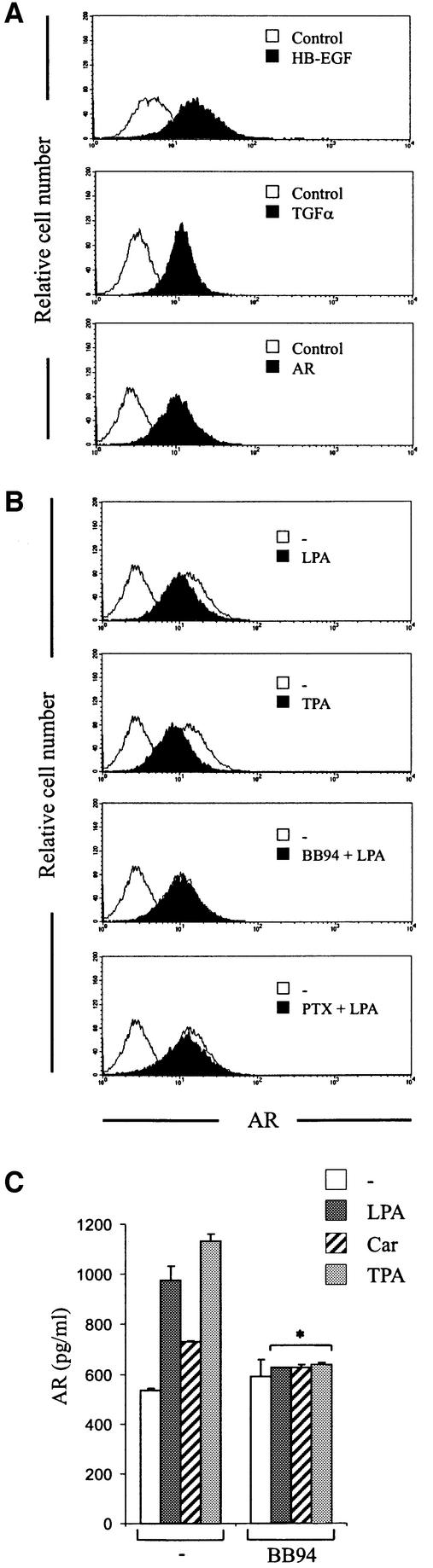
Previous reports demonstrating that GPCR-induced EGFR tyrosine phosphorylation requires proteolytic cleavage of proHB-EGF (Prenzel et al., 1999; Asakura et al., 2002; Lemjabbar and Basbaum, 2002) prompted us to ask whether HB-EGF or other EGF-like growth factors are involved in the EGFR transactivation pathway in head and neck cancer cells. By cDNA microarray analysis, we found the expression of proHB-EGF, proTGF-α and proAR mRNAs in SCC-4, SCC-9, SCC-15 and SCC-25 cells (data not shown). Moreover, the expression and cell surface localization of these ligands were confirmed by flow cytometry using ectodomain-specific antibodies (Figure 2A, representative data shown for SCC-9). Treatment of head and neck cancer cells with LPA or the phorbol ester 12-o-tetradecanoylphorbol-13-acetate (TPA), which acts as a general inductor of shedding events, resulted in rapid reduction in the cell surface content of endogenous proAR (Figure 2B). However, under these experimental conditions, we were not able to detect the proteolytic cleavage of proTGF-α or proHB-EGF in response to short-term LPA stimulation (up to 30 min), while stimulation with TPA resulted in ectodomain cleavage of both EGF-like growth factor precursors (data not shown). These findings suggested that LPA stimulation selectively induces shedding of proAR in HNSCC. In addition, batimastat completely abolished LPA-induced ectodomain cleavage of proAR (Figure 2B), confirming the requirement for metalloprotease activity for proAR shedding. In agreement with the observation that predominantly pertussis toxin (PTX)-sensitive G proteins of the Gi/o family are mediators of LPA-induced EGFR tyrosine phosphorylation (Figure 1, lower panel), PTX partially inhibited proAR shedding at the cell surface of SCC-9 cells (Figure 2B).
Fig. 2. GPCR stimulation results in metalloprotease-dependent cleavage and release of AR at the cell surface. (A) Flow cytometric analysis of EGF-like precursor expression. SCC-9 cells were collected and stained for surface HB-EGF, TGF-α or AR and analysed by flow cytometry. Control cells were labelled with FITC-conjugated secondary antibody alone. (B) LPA-induced proteolytic processing of proAR. SCC-9 cells were pre-incubated with batimastat (BB94; 10 µM) or PTX and stimulated with LPA or TPA (1 µM) for 5 min. Cells were collected and analysed for cell surface AR density by flow cytometry. (C) GPCR-induced proteolytic release of AR. SCC-9 cells were pre-incubated with batimastat or vehicle followed by stimulation with agonists as indicated for 120 min. Conditioned medium was collected and analysed for total AR amount by ELISA. Each bar is the average of triplicate values (mean ± SD). *P < 0.03 for the difference between agonists versus BB94 + agonists.
In addition to the decrease of cell surface proAR, GPCR stimulation resulted in the accumulation of mature AR in cell culture medium as determined by sandwich enzyme-linked immunosorbent assay (ELISA) (Figure 2C). The finding that AR release in response to carbachol was substantially lower compared with LPA stimulation suggested a direct correlation between the amount of released AR and EGFR tyrosine phosphorylation content in response to GPCR ligands (Figure 1). Moreover, pre-incubation with batimastat completely prevented GPCR- and TPA-induced accumulation of AR in cell culture medium (Figure 2C), confirming the metalloprotease dependency of AR release.
Ectodomain shedding of proAR is a prerequisite to GPCR-induced EGFR activation and EGFR-characteristic cellular responses
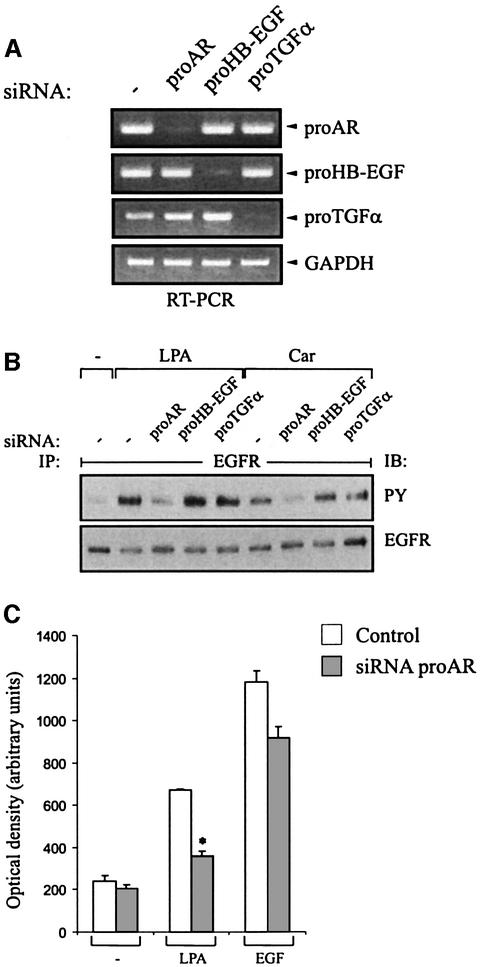
We used three approaches to determine if AR function is required for GPCR-induced EGFR tyrosine phosphorylation and downstream cellular responses. First, the endogenous expression of proAR, proHB-EGF and proTGF-α was silenced by small interfering RNA (siRNA) in SCC-9 cells. Efficient and specific knockdown of target gene expression was monitored by RT–PCR (Figure 3A), confirming that gene silencing occurred by mRNA degradation. Concomitantly, the effect of siRNAs on the EGFR transactivation signal was examined. As shown in Figure 3B, siRNA to proAR completely blocked GPCR-induced EGFR tyrosine phosphorylation. SiRNAs to proHB-EGF and proTGF-α, however, did not significantly alter the transactivation signal, demonstrating a specific requirement for proAR. In addition, we examined whether inhibition of proAR expression affects the GPCR-induced chemotactic migration of head and neck cancer cells towards fibronectin in vitro. In fact, proAR siRNA significantly suppressed LPA-induced migration of SCC-9 cells (Figure 3C). The finding that EGF-triggered transwell migration was partly inhibited by AR siRNA points toward a role for AR in autocrine-regulated cellular responses in HNSCC, as suggested before (O-Charoenrat et al., 2000).
Fig. 3. Effect of proAR siRNA on GPCR-induced EGFR activation and cell migration. (A) Blockade of EGF-like growth factor precursor expression by RNA interference (RNAi). SCC-9 cells were transfected with siRNA for proAR, proHB-EGF or proTGF-α, cultured for 2 days and analysed for gene expression by RT–PCR as indicated or (B) stimulated with LPA or carbachol and assayed for EGFR tyrosine phosphorylation content. (C) Requirement of AR for LPA-induced cell migration. SiRNA-transfected SCC-9 cells were analysed for transwell migration toward fibronectin as chemoattractant. Each bar is the average of quadruplicate values (mean ± SD). *P < 0.001 for control siRNA + LPA versus proAR siRNA + LPA.
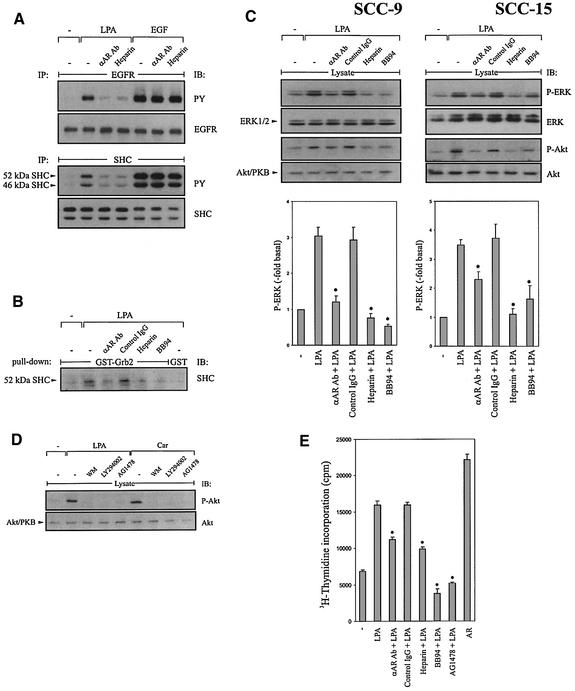
Secondly, we examined the effect of AR neutralizing antibodies on EGFR tyrosine phosphorylation by LPA in the squamous cell carcinoma cell lines SCC-4, SCC-9, SCC-15 and SCC-25. The results show that pre-treatment with either a polyclonal goat or a monoclonal mouse antibody raised against the ectodomain of human AR inhibited the EGFR transactivation signal (Figure 4A, upper panel; representative data shown for the polyclonal anti-AR antibody in SCC-9 cells). Similar results were obtained upon stimulation of head and neck cancer cells with carbachol (data not shown). In contrast, specific inhibition of HB-EGF by using the diphtheria toxin mutant CRM197 or anti-HB-EGF neutralizing antibodies showed no effect on LPA- or carbachol-induced EGFR transactivation (data not shown).
Fig. 4. Inhibition of AR bioactivity by anti-AR neutralizing antibodies and heparin abrogates EGFR tyrosine phorphorylation, mitogenic signalling events, activation of Akt/PKB and cell proliferation by GPCR ligands. (A) SCC-9 cells were pre-treated with anti-AR antibody (αAR Ab; 50 µg/ml, 60 min) or heparin (100 ng/ml, 15 min), and stimulated for 3 min (EGFR, upper panel) or 5 min (SHC, lower panel) as indicated. Precipitated EGFR and SHC were immunoblotted with anti-phosphotyrosine antibody followed by reprobing of the same filters with anti-EGFR and anti-SHC antibody, respectively. (B) Association of Grb2 with SHC in vitro. SCC-9 cells were pre-incubated with inhibitors and stimulated for 5 min as indicated. Lysates were incubated with GST–Grb-2 fusion protein or GST alone. Proteins were immunoblotted with monoclonal anti-SHC antibody. (C) AR is required for GPCR-induced ERK/MAPK activation and Akt/PKB phosphorylation. SCC-9 or SCC-15 cells were pre-incubated with inhibitors and stimulated for 7 min. Phosphorylated ERK1/2 was detected by immunoblotting total lysates with anti-phospho-ERK antibody. The same filters were re-probed with anti-ERK antibody. Quantitative analysis of ERK phosphorylation from three independent experiments (mean ± SD). *P < 0.05 for the difference between LPA versus inhibitors + LPA. Stimulation of Akt/PKB. Cell lysates were immunoblotted with anti-phospho-Akt/PKB antibody followed by reprobing of the same filters with anti-Akt/PKB antibody. (D) Effect of PI3K and EGFR inhibition on GPCR-induced Akt/PKB phosphorylation. Quiescent SCC-9 cells were pre-treated with wortmannin (WM, 100 nM), LY294002 (100 µM), AG1478 (250 nM) or vehicle for 30 min and stimulated with LPA or carbachol for 15 min. After lysis, activated Akt/PKB was detected by immunoblotting of total lysates with polyclonal anti-phospho-Akt/PKB (P-Akt) antibody, followed by reprobing of the same filter with polyclonal anti-Akt/PKB (Akt) antibody. (E) Effect of AR inhibition on LPA-induced DNA synthesis. SCC-15 cells were treated with inhibitors as indicated and incubated in the presence or absence of ligands (LPA; AR, 10 ng/ml) for 18 h. Cells were then pulse labelled with [3H]thymidine, and thymidine incorporation was measured by liquid scintillation counting. Quantitative analysis from three independent experiments (mean ± SD). *P < 0.001 for LPA versus inhibitors + LPA.
Thirdly, since AR contains a heparin-binding domain and the glycosaminoglycan heparin prevents AR-triggered mitogenic responses in keratinocytes (Cook et al., 1991), we evaluated the effect of heparin on the EGFR transactivation signal. As expected, heparin completely blocked EGFR tyrosine phosphorylation caused by LPA (Figure 4A, upper panel). Based on these findings, we next examined whether AR function is required for SHC activation downstream of the transactivated EGFR, since tyrosine phosphorylation of the adaptor protein SHC and formation of an SHC–Grb2–Sos complex is known to be a critical step in linking the activated EGFR to the Ras/MAPK cascade (reviewed in Bar-Sagi and Hall, 2000). In fact, AR blockade with anti-AR neutralizing antibodies and heparin completely prevented LPA-induced SHC tyrosine phosphorylation (Figure 4A, lower panel) and association of SHC with a GST–Grb2 fusion protein in vitro (Figure 4B).
Several studies have demonstrated previously that EGFR transactivation is one important mechanism whereby GPCR agonists activate the ERK/MAPK pathway (Faure et al., 1994; reviewed in Gschwind et al., 2001; Marinissen and Gutkind, 2001). To determine whether AR was required for LPA-stimulated ERK/MAPK activation in HNSCC cells, the effect of AR inhibition on ERK1/2 activation was studied. As shown on Figure 4C, AR neutralizing antibodies, heparin and batimastat prevented LPA-induced ERK1/2 activation in SCC-9 and SCC-15 cells. In addition to its mitogenic effect, LPA can act as a survival factor by activating both the ERK/MAPK pathway and the PI3K-dependent phosphorylation of Akt/PKB (Sautin et al., 2001). We therefore raised the question of whether LPA stimulation induces phosphorylation of Akt/PKB in head and neck cancer cells. The results indicate that LPA markedly increased phosphorylation of Akt/PKB at Ser473 (Figure 4D) and that activation of Akt/PKB was inhibited by the EGFR-specific tyrphostin AG1478. Moreover, Akt/PKB phosphorylation by LPA was sensitive to PI3K inhibition by wortmannin or LY294002 (Figure 4D) and was also abrogated by AR blockade or batimastat treatment in SCC-9 and SCC-15 cells (Figure 4C).
To extend our studies on AR function for growth-promoting GPCR signalling further, we assessed the effect of AR inhibition on LPA-induced DNA synthesis. As shown in Figure 4E, HNSCC cells displayed a significant reduction in the rate of DNA synthesis triggered by LPA upon AR inhibition, suggesting that a full proliferative response by LPA requires AR. Moreover, batimastat and AG1478 decreased DNA synthesis by LPA to below the basal level. Collectively, these data substantiate the requirement for AR for the generation of an EGFR-characteristic, mitogenic and motility-promoting transactivation signal in HNSCC.
TACE/ADAM17 is involved in GPCR-induced proAR cleavage and EGFR signal transactivation
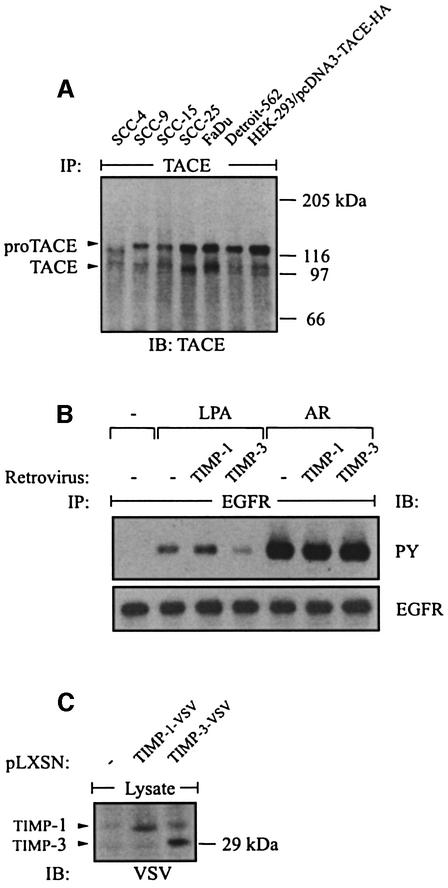
Recent observations have suggested a role for the metalloprotease-disintegrin TACE/ADAM17 in constitutive shedding of proAR and other EGF-like growth factor precursors in murine fibroblasts (Peschon et al., 1998; Sunnarborg et al., 2002). By immunoblot analysis, we found TACE to be widely expressed in HNSCC cells (Figure 5A). Similarly to other cell lines (Schlondorff et al., 2000), two forms of immunoprecipitated TACE were detected, the full-length precursor (proTACE) of ∼120 kDa (under reducing conditions) and the 100 kDa mature form (TACE) lacking the prodomain (Figure 5A). The proteolytic activity of TACE previously has been shown to be inhibited by the tissue inhibitor of metalloprotease-3 (Timp-3) but not Timp-1 in vitro (Amour et al., 1998). Timps are natural inhibitors of matrix metalloproteases (MMPs) and ADAMs. In contrast to TACE, ADAM10 can be inhibited by Timp-1 and only very poorly by Timp-3 (Amour et al., 2000). These observations suggest that the involvement of particular ADAMs can be distinguished from TACE through their differing sensitivity to Timps. Therefore, we investigated the effect of Timp-1 and Timp-3 on the EGFR transactivation signal. Indeed, ectopic expression of Timp-3 but not Timp-1 by retroviral transduction inhibited GPCR-induced EGFR tyrosine phosphorylation in SCC-9 cells (Figure 5B). However, Timp-3 had no effect on EGFR activation in response to direct AR stimulation. Expression of Timp-1 and Timp-3 carrying a C-terminal vesicular stomatitis virus (VSV) tag was confirmed by immunoblotting total lysates with anti-VSV antibodies (Figure 5C).
Fig. 5. Expression of TACE in HNSCC cell lines and effect of Timp-1 and Timp-3 expression on the EGFR transactivation signal. (A) TACE was immunoprecipitated from lysates of HNSCC cells with monoclonal TACE antibody. HEK-293 cells transfected with human TACE cDNA served as positive control. (B) Timp-3, but not Timp-1, inhibits EGFR signal transactivation. SCC-9 cells were infected with retrovirus encoding human Timp-1 or Timp-3. EGFR activation was determined by immunoblot after stimulation with agonists as indicated. (C) Expression of Timp-1 and TIMP-3 carrying a C-terminal VSV tag was confirmed by immunoblotting total cell lysates with anti-VSV antibody.
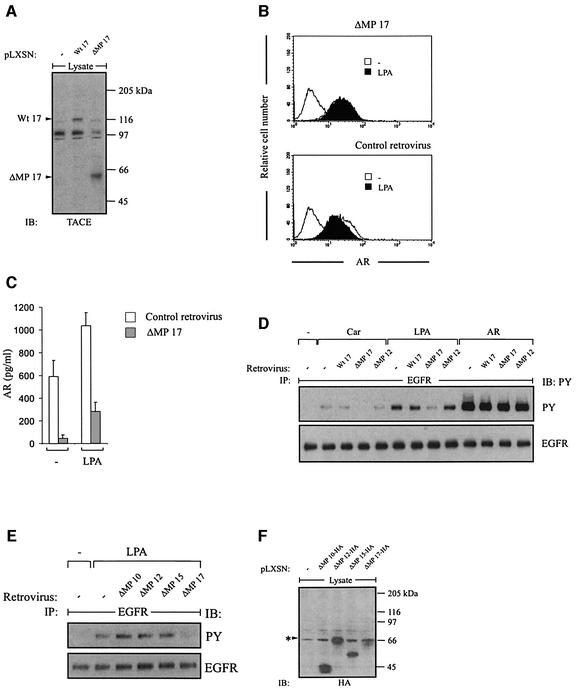
Furthermore, ectopic expression of dominant-negative TACE (ΔMP 17), which lacks the pro- and metalloprotease domain (Solomon et al., 1999; Figure 6A), suppressed GPCR-induced proAR cleavage (Figure 6B), release of mature AR (Figure 6C) and EGFR signal transactivation in SCC-9 cells (Figure 6D). In contrast, neither dominant-negative mutants of ADAM10 (Lemjabbar and Basbaum, 2002) and ADAM12 (Asakura et al., 2002), which have been shown to be involved in GPCR-triggered proHB-EGF processing, nor an analogous ADAM15 mutant or wild-type TACE affected the GPCR-induced responses (Figure 6D and E). Expression controls of haemagglutinin (HA)-tagged ADAM constructs are shown in Figure 6F.
Fig. 6. Dominant-negative TACE suppresses GPCR-induced AR release and EGFR signal transactivation. (A) Expression of wild-type (Wt 17) and dominant-negative TACE (ΔMP 17) in SCC-9 cells after retroviral gene transfer. Total lysates were immunoblotted with polyclonal anti-TACE antibody. (B) Dominant-negative TACE (ΔMP 17) abrogates LPA-induced proAR cleavage and (C) AR release into cell culture medium as determined by flow cytometric analysis and AR ELISA, respectively. (D) Effect of wild-type (Wt 17), dominant-negative TACE (ΔMP 17) and dominant-negative ADAM12 (ΔMP 12) on GPCR-stimulated EGFR signal transactivation. (E) Effect of dominant-negative ADAM10 (ΔMP 10), ADAM12 (ΔMP 12), ADAM15 (ΔMP 15) and TACE (ΔMP 17) on GPCR-stimulated EGFR signal transactivation. (F) Expression of dominant-negative ADAM mutants carrying a C-terminal HA tag in SCC-9 cells after retroviral gene transfer. Total lysates were immunoblotted with anti-HA antibody.
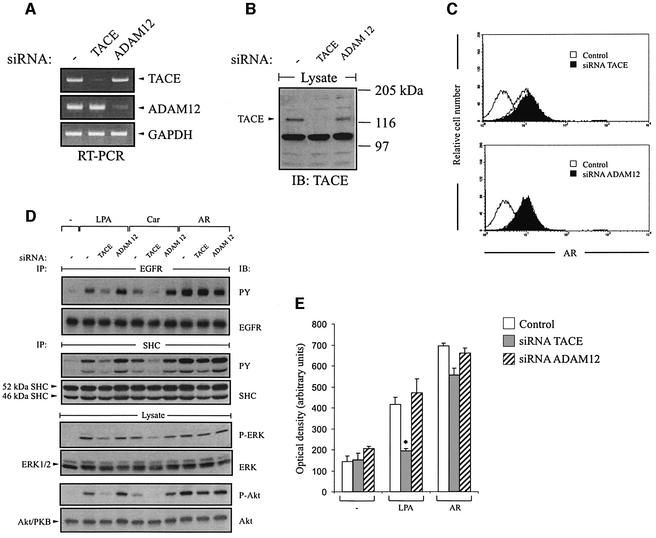
To verify independently the specific requirement for TACE for the EGFR transactivation pathway in HNSCC, we blocked the endogenous expression of TACE and ADAM12 by RNA interference. Suppression of TACE expression was monitored by RT–PCR (Figure 7A) and western blot analysis of total cell lysates following siRNA transfection (Figure 7B). Interestingly, siRNA-directed inhibition of TACE resulted in the accumulation of proAR at the cell surface of SCC-9 cells (Figure 7C), supporting the view that TACE is involved in basal proAR ecto domain processing. In addition, TACE siRNA specifically suppressed GPCR-induced EGFR, SHC, ERK/MAPK and Akt/PKB activation (Figure 7D) without significantly alter ing the signalling events evoked by direct AR stimulation. Finally, we evaluated the effect of TACE siRNA on the migratory behaviour of SCC-9 cells, and found that knock-down of TACE completely prevented chemotactic migration of SCC-9 cells in response to LPA (Figure 7E). Together, these results establish a novel effector function for TACE in GPCR signalling and demonstrate a critical role for TACE in the regulation of AR-dependent proliferation and migration of squamous cell carcinoma cells.
Fig. 7. TACE siRNA inhibits EGFR signal transmission and cell migration by GPCR agonists. (A and B) TACE siRNA blocks endogenous TACE expression. SCC-9 cells were transfected with TACE or ADAM12 siRNA. Gene expression was analysed by (A) RT–PCR or (B) immunoblot with polyclonal anti-TACE antibody. (C) Knockdown of TACE results in accumulation of proAR at the cell surface. siRNA-transfected SCC-9 cells were analysed for AR cell surface content by FACS. (D) EGFR signal transmission upon GPCR activation requires TACE. SCC-9 cells were transfected with siRNA and stimulated with agonists as indicated. Activation of EGFR, SHC, ERK and Akt was determined as described above. (E) Squamous cancer cell motility in response to LPA depends on TACE. siRNA-transfected SCC-9 cells were treated with LPA or AR and analysed in a transwell migration assay.
Discussion
Increasing experimental evidence supports the concept that the EGFR functions as a central integrator of diverse GPCR signals, which are thereby funnelled to downstream pathways (reviewed in Carpenter, 1999; Gschwind et al., 2001; Marinissen and Gutkind, 2001). Here we present data that add new information towards the full understanding of the GPCR/EGFR transactivation mechanism that appears to be a major driving force in the oncogenic process in cancer cells. We show that TACE-dependent AR release is functionally relevant for EGFR activation, downstream adaptor protein recruitment, activation of the ERK/MAPK pathway, phosphorylation of Akt/PKB, induction of cell proliferation and migration by GPCR agonists.
Previously, Brown et al. (2001) reported on metalloprotease-dependent shedding of proAR in Madin–Darby canine kidney cells by several non-physiological stimuli such as TPA and calcium ionophore. This is the first demonstration that transmembrane proAR is cleaved in response to physiologically abundant GPCR ligands. Furthermore, it was demonstrated here that proAR cleavage in head and neck cancer cells following LPA treatment was attenuated by PTX and completely blocked by batimastat (Figure 2B). A similar result was obtained on the level of EGFR tyrosine phosphorylation (Figure 1, lower panel), suggesting that GPCR-mediated proAR shedding may be directly involved in the EGFR transactivation pathway. This hypothesis was supported further by the finding that silencing of endogenous proAR gene expression by siRNA prevented EGFR activation and cell migration in response to GPCR agonists (Figure 3). Moreover, function-perturbing anti-AR antibodies and heparin abrogated the EGFR transactivation signal and downstream mitogenic responses by LPA (Figure 4). In these experiments, the potential involvement of HB-EGF and TGF-α was excluded. The growth-promoting signalling events are accompanied by phosphorylation of the survival mediator Akt/PKB via PI3K downstream of the EGFR (Figure 4C and D). Recently, AR was shown to be a potent inhibitor of apoptosis induced by serum deprivation in non-small cell lung cancer cell lines (Hurbin et al., 2002), suggesting that AR can provide survival signals for cancer cells derived from different forms of squamous cell carcinoma. In the current experiments using anti-AR neutralizing antibodies and heparin, however, no complete blockade of LPA-induced ERK/MAPK activation, DNA synthesis and transwell migration was observed. This could be due to limitations in inhibitor potency or to limited access of the inhibitors to the growth factor embedded in the heparan sulfate proteoglycan matrix.
Despite the expression of a variety of EGF-like growth factors in HNSCC cells (Figure 2A) and some functional redundancy within the EGFR ligand family in developmental processes (Luetteke et al., 1999), the results of this study demonstrate that AR is required specifically for LPA- and carbachol-induced EGFR transactivation and downstream signalling events (Figures 3 and 4). However, what is the (patho-)physiological function of proHB-EGF and proTGF-α in head and neck cancer cells? Previous studies showed that basal EGFR and ERK1/2 activities as well as cell proliferation are significantly reduced by batimastat and AG1478 (Gschwind et al., 2002; O-Charoenrat et al., 2002), arguing for the existence of autocrine EGFR activation loops that require metalloprotease activity for EGFR ligand shedding. Furthermore, anti-TGF-α neutralizing antibodies reduced proliferation of SCC-9 and FaDu cells (Solorzano et al., 1997), and TGF-α antisense therapy recently has been shown to inhibit HNSCC tumour growth in nude mice (Endo et al., 2000). Collectively, these studies demonstrate a critical role for TGF-α in autocrine stimulation of the EGFR resulting in sustained proliferation of HNSCC cells, while our current data establish a function for AR in GPCR-induced cellular responses.
The enzymes implicated in shedding of EGF-like growth factor precursors belong to the ADAM family of zinc-dependent proteinases, which are widely expessed in many tissues. The finding that the EGFR transactivation signal in HNSCC cells is sensitive to Timp-3 but not Timp-1 (Figure 5B) is in accordance with the inhibitor spectrum of recombinant TACE in vitro (Amour et al., 1998). Moreover, Sunnarborg et al. (2002) recently have proposed a role for TACE in constitutive ectodomain cleavage of proAR, proHB-EGF and proTGF-α. They showed that reintroduction of TACE into TACE-deficient taceΔZn/ΔZn EC-2 murine fibroblasts resulted in increased basal shedding of the co-transfected growth factor precursors.
Our current data identify a novel biological function for the metalloprotease TACE in GPCR signalling since expression of a dominant-negative TACE mutant blocked cell surface proAR cleavage, release of mature AR and EGFR tyrosine phosphorylation by LPA and carbachol (Figure 6). The critical involvement of TACE in the GPCR-induced EGFR transactivation pathway in HNSCC cells was confirmed in an independent experimental approach using RNA interference. Inhibition of endogenous TACE expression similarly resulted in blockade of the transactivation signal and EGFR downstream signalling (Figure 7). Other mechanisms, in which HB-EGF- dependent transactivation of the EGFR is mediated by ADAM10 in lung epithelial cells (Lemjabbar and Basbaum, 2002) and COS-7 cells (Yan et al., 2002) or by ADAM12 in cardiomyocytes (Asakura et al., 2002), have been described. The involvement of these metalloproteases in EGFR signal transactivation in HNSCC cells, however, was excluded in this study (Figures 6D and E, and 7D).
How TACE is activated by heterotrimeric G proteins currently is not known. Although ERK has been shown to bind to and phosphorylate the cytoplasmic domain of TACE at Thr735 in response to TPA stimulation (Diaz-Rodriguez et al., 2002), GPCR-induced AR release and EGFR tyrosine phosphorylation are insensitive to MEK inhibitors in HNSCC cells (unpublished observation), suggesting ERK1/2 not to be involved upstream of the EGFR. Another report revealed that TACE must be expressed with its membrane-anchoring domain for TPA-stimulated shedding of TNF, p75 TNFR and interleukin (IL)-1R-II, but that the cytoplasmic domain of TACE is not required for the shedding of these substrates (Reddy et al., 2000). Future studies will therefore have to focus on the question of whether the cytoplasmic domain of TACE and other ADAM proteases is involved in GPCR-induced shedding of EGF-like ligands.
Recent reports identified TGF-α as an element in signal transmission from GPCRs to the EGFR in gastric epithelia (Pai et al., 2002) and T-84 cells (McCole et al., 2002), suggesting that at least three (HB-EGF, AR and TGF-α) of the eight known EGF-like growth factors can be mediators of EGFR signal transactivation. Although TACE-deficient murine fibroblasts show partial defects in constitutive and 4-aminophenylmercuric acetate-induced TGF-α release (Peschon et al., 1998; Merlos-Suarez et al., 2001; Sunnarborg et al., 2002), the identity of the ADAM(s) responsible for proTGF-α cleavage by GPCR ligands in the cell systems mentioned above is still not known.
A possible explanation for our finding that proTGF-α and proHB-EGF are not cleaved by TACE in SCC-9 cells in response to short-term LPA stimulation might be that pre-formed signalling complexes consisting of LPA receptors, TACE, proAR and the EGFR are present at the cell membrane. Likewise, in a cell type-specific fashion, proHB-EGF and proTGF-α might be located in the vicinity of other ADAM family members such as ADAM10 and ADAM12, which have been shown to cleave proHB-EGF in response to bombesin (Yan et al., 2002) and phenylephrine (Asakura et al., 2002), respectively. Interestingly, co-immunoprecipitation studies by Maudsley et al. (2000) demonstrated that the β2-adrenergic receptor physically interacts with the ‘transactivated’ EGFR in COS-7 cells. Although experimental proof of this ‘signalling complex’ hypothesis will have to await further investigations, the existence of such macromolecular signalling units would also account for signal specificity by different GPCRs. Accordingly, Izumi et al. (1998) have demonstrated recently that in Vero-H cells, TPA induces proHB-EGF shedding via ADAM9, while LPA-induced proHB-EGF cleavage in the same cell line is independent of ADAM9 (Umata et al., 2001). These data suggest that different stimuli can induce proteolytic cleavage of an individual growth factor precursor via different metalloproteases in one cell system. Therefore, an important issue of future studies will be to determine what defines the choice of either ADAM9, ADAM10/HB-EGF, ADAM12/HB-EGF or TACE/AR in signal transmission of GPCRs to the EGFR in different cell types. While our experimental results represent compelling evidence for the relevance of widely abundant GPCR ligands, TACE and AR in cancer cell migration and proliferation, the role of TMPS pathways for EGFR signal transactivation in physiological processes remains to be elucidated.
Materials and methods
Cell culture, plasmids and retroviral infections
All cell lines (American Type Culture Collection, Manassas, VA) were routinely grown according to the supplier’s instructions. Transfections of HEK-293 cells were carried out by calcium phosphate co-precipitation as previously described (Prenzel et al., 1999). Anti-AR, anti-HB-EGF neutralizing antibodies (R&D Systems, Minneapolis, MN), PTX, heparin, wortmannin, LY294002 (Sigma, St Louis, MO), marimastat (BB2516; Sugen Inc., South San Francisco, CA) and batimastat (BB94; British Biotech, Oxford, UK) were added to serum-starved cells before the respective growth factor.
Full-length cDNAs encoding ADAM10, 12, 15 and 17, as well as TIMP-1 and TIMP-3, were amplified by PCR from a human placenta cDNA library and subcloned into pcDNA3 (Invitrogen, Carlsbad, CA) and pLXSN vectors (Clontech, Palo Alto, CA). For virus production, dominant-negative protease constructs lacking the pro- and metalloprotease domains were generated as described before (Solomon et al., 1999; Asakura et al., 2002). All protease constructs included a C-terminal HA tag, detectable with an anti-HA monoclonal antibody (Babco, Richmond, CA). TIMP constructs included a C-terminal VSV tag, detectable with an anti-VSV monoclonal antibody (Roche, Mannheim, Germany). The amphotropic packaging cell line Phoenix was transfected with pLXSN retroviral expression plasmids by the calcium phosphate/chloroquine method as described previously (Kinsella and Nolan, 1996). At 24 h after transfection, the viral supernatant was collected and used to infect subconfluent SCC-9 cells (5 × 104 cells/6-well plate).
Protein analysis
Cells were lysed and proteins immunoprecipitated as described before (Gschwind et al., 2002). Following SDS–PAGE, proteins were transferred to a nitrocellulose membrane. Western blots were performed according to standard methods. The antibodies against human EGFR (108.1) and SHC (Prenzel et al., 1999), as well as a GST–Grb2 fusion protein (Daub et al., 1996), have been characterized before. Phosphotyrosine was detected with the 4G10 monoclonal antibody (UBI, Lake Placid, NY). Polyclonal anti-phospho-p44/p42 (Thr202/Tyr204) MAPK antibody and anti-phospho-Akt (Ser473) antibody were purchased from New England Biolabs (Beverly, MA). Polyclonal anti-Akt1/2 and anti-ERK2 antibody were from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-TACE antibodies were from Chemicon (Harrow, UK).
Flow cytometric analysis
Fluorescence-activated cell sorting (FACS) analysis was performed as described before (Prenzel et al., 1999). In brief, cells were seeded, grown for 20 h, and in some cases retrovirally infected as indicated. Upon serum starvation for 24 h, cells were treated with inhibitors and growth factors as indicated. After collection, cells were stained with ectodomain-specific antibodies against HB-EGF, AR (R&D Systems) or TGF-α (Oncogene, Boston, MA) for 45 min. After washing with phosphate-buffered saline (PBS), cells were incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies for 15 min and washed again with PBS. Cells were analysed on a Becton Dickinson FACScalibur flow cytometer.
AR ELISA
SCC-9 cells were seeded into 12-well plates (3.2 × 104 cells/well) and incubated for 18 h. Upon serum deprivation for 24 h, cells were subjected to pre-incubation with batimastat (10 µM) for 20 min and stimulated as indicated in the figure legends. Cell culture medium was collected and, after addition of phenylmethylsulfonyl fluoride (PMSF; 1 mM), was pre-cleared by centrifugation (10 min; 13 000 r.p.m.). Samples were transferred to antibody-coated plates.
The concentration of free AR was determined by sandwich ELISA (R&D Systems) using monoclonal anti-AR capture antibody (MAB262) and biotinylated polyclonal detection antibody (BAF262). Plate preparation and assay procedure were performed according to the manufacturer’s recommendations using tetramethyl benzidine as a substrate. The absorbance at 455 nm was read with a reference wavelength of 650 nm using an ELISA plate reader. AR concentrations for each sample were calculated after generating a standard curve using the dilution series of human recombinant AR protein.
For statistical analysis, Student’s t-test was used to compare data between two groups. Values are expressed as mean ± SD of at least triplicate samples. P < 0.05 was considered statistically significant.
RNA interference and RT–PCR analysis
Transfection of 21 nucleotide siRNA duplexes (Dharmacon Research, Lafayette, CO) for targeting endogenous genes was carried out using Oligofectamine (Invitrogen) and 4.2 µg of siRNA duplex per 6-well plate as previously described (Elbashir et al., 2001). Transfected SCC-9 cells were serum starved and assayed 4 days after transfection. The highest efficiencies in silencing target genes were obtained by using mixtures of siRNA duplexes targeting different regions of the gene of interest. Sequences of siRNA used were CCACAAAUACCUGGCUATAdTdT, AAAUCCAUGUAAUGCAGAAdTdT (AR); GUGAAGUUGGGCAU GACUAdTdT, UACAAGGACUUCUGCAUCCdTdT (HB-EGF); AAC ACUGUGAGUGGUGCCGdTdT, GAAGCAGGCCAUCACCGCCTdT (TGFα); AGUUUGCUUGGCACACCUUdTdT, AGUAAGGCCCAGG AGUGUUdTdT, CAUAGAGCCACUUUGGAGAdTdT (TACE); CCU CGCUGCAAAGAAUGUGdTdT, GACCUUGATACGACUGCUGdT dT (ADAM12); CGUACGCGGAAUACUUCGAdTdT (control, GL2).
Specific silencing of targeted genes was confirmed by western blot (TACE) and RT–PCR analysis. RNA isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) was reverse transcribed using AMV reverse transcriptase (Roche, Mannheim, Germany). PuReTaq Ready-To-Go PCR Beads (Amersham Biosciences, Piscataway, NJ) were used for PCR amplification. Primers (Sigma Ark, Steinheim, Germany) were proAR, 5′-tGGTGCTGTCGCTCTTGATA-3′ and 5′-GCCAGGTATTTGTGG TTCGT-3′; proHB-EGF, 5′-TTATCCTCCAAGCCACAAGC-3′ and 5′-TGACCAGCAGACAGACAGATG-3′; proTGF-α, 5′-TGTTCGCTCT GGGTATTGTG-3′ and 5′-ACTGTTTCTGAGTGGCAGCA-3′; TACE, 5′-CGCATTCTCAAGTCTCCACA-3′ and 5′-TATTTCCCTCCCTGG TCCTC-3′; and ADAM12, 5′-CAGTTTCACGGAAACCCACT-3′ and 5′-GACCAGAACACGTGCTGAGA-3′. PCR products were subjected to electrophoresis on a 2.5% agarose gel and DNA was visualized by ethidium bromide staining.
Proliferation and migration assays
For the [3H]thymidine incorporation assay (Daub et al., 1996), SCC-15 cells were seeded into 12-well plates at 3 × 104 cells/well. Upon serum deprivation for 48 h, cells were subjected to pre-incubation and stimulation as indicated. After 18 h, cells were pulse-labelled with [3H]thymidine (1 µCi/ml) for 4 h, and thymidine incorporation was measured by trichloroacetic acid precipitation and subsequent liquid scintillation counting.
Analysis of cell motility was performed as described before (Gschwind et al., 2002) using a modified Boyden chamber. At 24 h after transfection with siRNAs, SCC-9 cells were seeded into polycarbonate membrane inserts (6.5 mm diameter and 8 µm pore size) in 24-transwell dishes at 1 × 105 cells/well in the presence or absence of agonist. The lower chamber was filled with standard medium without fetal calf serum containing 10 µg/ml fibronectin as chemoattractant. Cells were permitted to migrate for 36 h. Following incubation, non-migrated cells were removed from the upper surface of the membranes. The cells that had migrated to the lower surface were fixed and stained with crystal violet. The stained cells were solubilized in 10% acetic acid, and the absorbance at 570 nm was measured in a micro-plate reader.
Acknowledgments
Acknowledgements
We thank R.Black and his laboratory staff for their pioneering collaboration and advice, I.Sures for critical revision of the text, T.Knyazeva for cDNA, and R.Abraham for his advice with cDNA arrays. O.M.F. is the recipient of a Boehringer Ingelheim Fonds PhD scholarship.
References
- Amour A. et al. (1998) TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett., 435, 39–44. [DOI] [PubMed] [Google Scholar]
- Amour A., Knight,C.G., Webster,A., Slocombe,P.M., Stephens,P.E., Knauper,V., Docherty,A.J. and Murphy,G. (2000) The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett., 473, 275–279. [DOI] [PubMed] [Google Scholar]
- Asakura M. et al. (2002) Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med., 8, 35–40. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D. and Hall,A. (2000) Ras and Rho GTPases: a family reunion. Cell, 103, 227–238. [DOI] [PubMed] [Google Scholar]
- Brown C.L., Coffey,R.J. and Dempsey,P.J. (2001) The proamphiregulin cytoplasmic domain is required for basolateral sorting, but is not essential for constitutive or stimulus-induced processing in polarized Madin–Darby canine kidney cells. J. Biol. Chem., 276, 29538–29549. [DOI] [PubMed] [Google Scholar]
- Carpenter G. (1999) Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J. Cell Biol., 146, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. (2000) EGF receptor transactivation mediated by the proteolytic production of EGF-like agonists. Sci. STKE, 2000, PE1. [DOI] [PubMed] [Google Scholar]
- Cook P.W., Mattox,P.A., Keeble,W.W., Pittelkow,M.R., Plowman,G.D., Shoyab,M., Adelman,J.P. and Shipley,G.D. (1991) A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol. Cell. Biol., 11, 2547–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H., Weiss,F.U., Wallasch,C. and Ullrich,A. (1996) Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature, 379, 557–560. [DOI] [PubMed] [Google Scholar]
- Daub H., Wallasch,C., Lankenau,A., Herrlich,A. and Ullrich,A. (1997) Signal characteristics of G protein-transactivated EGF receptor. EMBO J., 16, 7032–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rodriguez E., Montero,J.C., Esparis-Ogando,A., Yuste,L. and Pandiella,A. (2002) Extracellular signal-regulated kinase phosphorylates tumor necrosis factor α-converting enzyme at threonine 735: a potential role in regulated shedding. Mol. Biol. Cell, 13, 2031–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S. et al. (1998) Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem., 273, 8890–8896. [DOI] [PubMed] [Google Scholar]
- Eguchi S., Dempsey,P.J., Frank,G.D., Motley,E.D. and Inagami,T. (2001) Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J. Biol. Chem., 276, 7957–7962. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- Endo S. et al. (2000) TGF-α antisense gene therapy inhibits head and neck squamous cell carcinoma growth in vivo. Gene Ther., 7, 1906–1914. [DOI] [PubMed] [Google Scholar]
- Faure M., Voyno-Yasenetskaya,T.A. and Bourne,H.R. (1994) cAMP and βγ subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem., 269, 7851–7854. [PubMed] [Google Scholar]
- Fujiyama S. et al. (2001) Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ. Res., 88, 22–29. [DOI] [PubMed] [Google Scholar]
- Gschwind A., Zwick,E., Prenzel,N., Leserer,M. and Ullrich,A. (2001) Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene, 20, 1594–1600. [DOI] [PubMed] [Google Scholar]
- Gschwind A., Prenzel,N. and Ullrich,A. (2002) Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res., 62, 6329–6336. [PubMed] [Google Scholar]
- Hartmann D. et al. (2002) The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for α-secretase activity in fibroblasts. Hum. Mol. Genet., 11, 2615–2624. [DOI] [PubMed] [Google Scholar]
- Hurbin A., Dubrez,L., Coll,J.L. and Favrot,M.C. (2002) Inhibition of apoptosis by amphiregulin via an insulin-like growth factor-1 receptor-dependent pathway in non-small cell lung cancer cell lines. J. Biol. Chem., 277, 49127–49133. [DOI] [PubMed] [Google Scholar]
- Huyer G., Liu,S., Kelly,J., Moffat,J., Payette,P., Kennedy,B., Tsaprailis,G., Gresser,M.J. and Ramachandran,C. (1997) Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem., 272, 843–851. [DOI] [PubMed] [Google Scholar]
- Izumi Y. et al. (1998) A metalloprotease-disintegrin, MDC9/meltrin-γ/ADAM9 and PKCδ are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J., 17, 7260–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keates S., Sougioultzis,S., Keates,A.C., Zhao,D., Peek,R.M.,Jr, Shaw,L.M. and Kelly,C.P. (2001) cag+Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J. Biol. Chem., 276, 48127–48134. [DOI] [PubMed] [Google Scholar]
- Kinsella T.M. and Nolan,G.P. (1996) Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther., 7, 1405–1413. [DOI] [PubMed] [Google Scholar]
- Kodama H. et al. (2002) Role of EGF receptor and Pyk2 in endothelin-1-induced ERK activation in rat cardiomyocytes. J. Mol. Cell. Cardiol., 34, 139–150. [DOI] [PubMed] [Google Scholar]
- Lemjabbar H. and Basbaum,C. (2002) Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med., 8, 41–46. [DOI] [PubMed] [Google Scholar]
- Luetteke N.C., Qiu,T.H., Fenton,S.E., Troyer,K.L., Riedel,R.F., Chang,A. and Lee,D.C. (1999) Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development, 126, 2739–2750. [DOI] [PubMed] [Google Scholar]
- Marinissen M.J. and Gutkind,J.S. (2001) G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci., 22, 368–376. [DOI] [PubMed] [Google Scholar]
- Massagué J. and Pandiella,A. (1993) Membrane-anchored growth factors. Annu. Rev. Biochem., 62, 515–541. [DOI] [PubMed] [Google Scholar]
- Mateo C., Moreno,E., Amour,K., Lombardero,J., Harris,W. and Perez,R. (1997) Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology, 3, 71–81. [DOI] [PubMed] [Google Scholar]
- Maudsley S., Pierce,K.L., Zamah,A.M., Miller,W.E., Ahn,S., Daaka,Y., Lefkowitz,R.J. and Luttrell,L.M. (2000) The β(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J. Biol. Chem., 275, 9572–9580. [DOI] [PubMed] [Google Scholar]
- McCole D.F., Keely,S.J., Coffey,R.J. and Barrett,K.E. (2002) Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-α. J. Biol. Chem., 277, 42603–42612. [DOI] [PubMed] [Google Scholar]
- Merlos-Suarez A., Ruiz-Paz,S., Baselga,J. and Arribas,J. (2001) Metalloprotease-dependent protransforming growth factor-α ectodomain shedding in the absence of tumor necrosis factor-α-converting enzyme. J. Biol. Chem., 276, 48510–4857. [DOI] [PubMed] [Google Scholar]
- O-Charoenrat P., Rhys-Evans,P. and Eccles,S. (2000) Expression and regulation of c-ERBB ligands in human head and neck squamous carcinoma cells. Int. J. Cancer, 88, 759–765. [DOI] [PubMed] [Google Scholar]
- O-Charoenrat P., Rhys-Evans,P. and Eccles,S. (2002) A synthetic matrix metalloproteinase inhibitor prevents squamous carcinoma cell proliferation by interfering with epidermal growth factor receptor autocrine loops. Int. J. Cancer, 100, 527–533. [DOI] [PubMed] [Google Scholar]
- Pai R., Soreghan,B., Szabo,I.L., Pavelka,M., Baatar,D. and Tarnawski,A.S. (2002) Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med., 8, 289–293. [DOI] [PubMed] [Google Scholar]
- Peschon J.J. et al. (1998) An essential role for ectodomain shedding in mammalian development. Science, 282, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Prenzel N., Zwick,E., Daub,H., Leserer,M., Abraham,R., Wallasch,C. and Ullrich,A. (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature, 402, 884–888. [DOI] [PubMed] [Google Scholar]
- Reddy P., Slack,J.L., Davis,R., Cerretti,D.P., Kozlosky,C.J., Blanton,R.A., Shows,D., Peschon,J.J. and Black,R.A. (2000) Functional analysis of the domain structure of tumor necrosis factor-α converting enzyme. J. Biol. Chem., 275, 14608–14614. [DOI] [PubMed] [Google Scholar]
- Sautin Y.Y., Crawford,J.M. and Svetlov,S.I. (2001) Enhancement of survival by LPA via Erk1/Erk2 and PI 3-kinase/Akt pathways in a murine hepatocyte cell line. Am. J. Physiol. Cell Physiol., 281, C2010–C2019. [DOI] [PubMed] [Google Scholar]
- Schlondorff J., Becherer,J.D. and Blobel,C.P. (2000) Intracellular maturation and localization of the tumour necrosis factor α convertase (TACE). Biochem. J., 347, 131–138. [PMC free article] [PubMed] [Google Scholar]
- Solomon K.A., Pesti,N., Wu,G. and Newton,R.C. (1999) Cutting edge: a dominant negative form of TNF-α converting enzyme inhibits proTNF and TNFRII secretion. J. Immunol., 163, 4105–4108. [PubMed] [Google Scholar]
- Solorzano C.C. et al. (1997) Inhibition of transforming growth factor α stimulation of human squamous cell carcinoma of the head and neck with anti-TGF-α antibodies and tyrphostin. Ann. Surg. Oncol., 4, 670–684. [DOI] [PubMed] [Google Scholar]
- Sunnarborg S.W. et al. (2002) Tumor necrosis factor-α converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem., 277, 12838–12845. [DOI] [PubMed] [Google Scholar]
- Umata T. et al. (2001) A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J. Biol. Chem., 276, 30475–30482. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Cai,H., Brodie,T.A., Higashyama,S., Manova,K., Ludwig,T. and Blobel,C.P. (2002) Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol. Cell. Biol., 22, 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Shirakabe,K. and Werb,Z. (2002) The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J. Cell Biol., 158, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick E., Daub,H., Aoki,N., Yamaguchi-Aoki,Y., Tinhofer,I., Maly,K. and Ullrich,A. (1997) Critical role of calcium-dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. J. Biol. Chem., 272, 24767–24770. [DOI] [PubMed] [Google Scholar]