Abstract
Across large parts of the world, wildlife has to coexist with human activity in highly modified and fragmented landscapes. Combining concepts from population viability analysis and spatial reserve design, this study develops efficient quantitative methods for identifying conservation core areas at large, even national or continental scales. The proposed methods emphasize long-term population persistence, are applicable to both fragmented and natural landscape structures, and produce a hierarchical zonation of regional conservation priority. The methods are applied to both observational data for threatened butterflies at the scale of Britain and modelled probability of occurrence surfaces for indicator species in part of Australia. In both cases, priority landscapes important for conservation management are identified.
Keywords: connectivity, reserve selection, site selection algorithm, conservation planning, landscape zonation
1. Introduction
Steep past (Groombridge 1992; Gaston et al. 2003; Thomas et al. 2004b) and projected (Brooks et al. 1997; Sala et al. 2000; Thomas et al. 2004a) declines in biodiversity highlight the need to develop conservation strategies for regions that have already been substantially modified by human activities. In these areas, traditional conservation, namely the protection of untransformed landscapes as large individual reserves, is difficult to apply. Yet, the biodiversity value of modified landscapes and of archipelagos of small habitat fragments can still be high (Jongman & Pungetti 2004), and human activities (e.g. low intensity farming) can be compatible with the maintenance of biodiversity within these regions (Tucker & Evans 2004).
Conservation strategies for fragmented or modified regions need to prioritize areas where populations are most likely to persist in the long-term (Margules & Pressey 2000; Cabeza & Moilanen 2001): usually where a given species' habitats are common, of high quality, and close together (Hanski 1998; Hanski & Ovaskainen 2000). Whilst this qualitative message is widely accepted, quantitative multi-species applications to identify priority landscapes at the spatial scale of entire countries have been limited: for most species in most landscapes, insufficient ecological data, population parameters or habitat distribution information are available to allow the application of simulation modelling (Sjögren-Gulve & Ebenhard 2000) or calculation of the capacity of the landscape to support populations (Hanski & Ovaskainen 2000). The challenge we address here is to develop multi-species landscape-scale conservation planning methods that target population persistence but have data-requirements that do not preclude their use in the real world.
We calculate population connectivity surfaces of individual species (correlated with the likelihood that populations will persist; Hanski 1998; Hanski & Ovaskainen 2000), and use these as a basis for zoning (prioritizing) landscapes for multi-species conservation. Highly connected landscapes are areas where species are normally most widely distributed (at multiple scales, Kunin 1998), have the highest actual and effective (genetic) population sizes, are least likely to become extinct, and where they also have the greatest likelihood of colonizing fresh habitat that is created either naturally (e.g. through succession) or by human intervention (e.g. by restoration). For those regions and taxa for which observational data are sparse, connectivity surfaces can be calculated from modelled probability of occurrence surfaces for each species (Guisan & Zimmermann 2000): regions with high levels of predicted occurrence are expected to have highest carrying capacities and lowest extinction rates. In summary, ecological, economic and logistic requirements demand that high connectivity be maintained for biodiversity priority areas (Hanski 1998; Debinski & Holt 2000; Gaston et al. 2002; Possingham et al. 2000).
We apply our methods to two different scenarios. Our first application concerns landscape prioritization for butterfly conservation in Britain based on connectivity surfaces calculated directly from observational data for 57 (excluding re-introduced and vagrant) species (Asher et al. 2001). Our second application concerns conservation planning in the Hunter Valley Central Coast (HCC) region of eastern Australia. This example utilises modelled probability of occurrence surfaces for seven priority fauna species (Wintle et al. 2005). In Britain, essentially all habitats have been modified and distributions of species reflect land management by humans, as well as natural processes. In contrast, the HCC region is comprised of a few large contiguous natural forest areas and many remnant forest fragments with high conservation value in an urban–agricultural matrix of little conservation value. Local conservation planning aims to ameliorate the impacts of urban and agricultural expansion, the primary threat to species persistence in the HCC region.
2. Material and methods
(a) Distribution data
For the British butterflies we use a data set of 1.55 million butterfly location×species observations made during 1995–1999 by approximately 10 000 amateur naturalists. The records were available through the Butterflies for the New Millennium project (Asher et al. 2001). All records were converted to presence/absence in 1 km2 of the Ordnance Survey National Grid.
The HCC prioritization is based on probabilities of occurrence predicted by statistical habitat models (120×140 km2 area at a 100 m grid cell resolution) for seven priority species in the HCC region (Wintle et al. in press): the koala (Phascolarctos cinereus), the tiger quoll (Dasyurus maculatus), the squirrel glider (Petaurus norfolcensis), the yellow-bellied glider (Petaurus australis), the masked owl (Tyto novaehollandiae), the powerful owl (Ninox strenua), and the sooty owl (Tyto tenbricosa). The HCC Regional Environmental Management Strategy has commissioned the development of these models expressly for the purpose of conservation planning.
(b) The Zonation algorithm
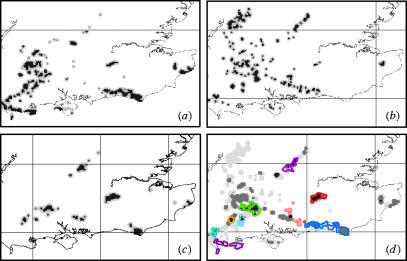
Because the distributions of different species are likely to overlap only partially (figure 1a–c), efficient landscape prioritization requires the identification of areas that support high connectivity for many species simultaneously (figure 1d). Our approach is the Zonation algorithm, which starts from the full landscape, and then iteratively discards locations (grid cells) of lowest value from the edge of the remaining area, thus maintaining a high degree of structural connectivity in the remaining habitat. The order of cell removal gives a landscape zoning with most important areas remaining last. Limiting cell removal to the edge of the remaining area is very important because it allows the identification of a nested sequence of aggregated landscape structures with the high priority core areas of species distributions remaining until the last, and previously removed areas showing as lower priority buffer zones. A nested zoning can easily be visualized and interpreted for the purpose of conservation planning. Other currently used methods for generating aggregated spatial reserve designs do not produce nested solutions (see Cabeza et al. 2004). Edge removal also has the advantage of reducing computation times by orders of magnitude (because only some cells are candidates for removal), making it possible to apply Zonation to extremely large landscapes with high spatial resolution and allowing rigorous sensitivity analysis within practical time-frames.
Figure 1.
Example connectivity surfaces for three butterfly species, (a) Polyommatus bellargus, (b) Hamearis lucina and (c) Hesperia comma in south-east England. Grey and black indicate areas having connectivity more than 0.1% and more than 1%, respectively, of the maximum for each species. (d) Areas of overlap of the connectivity surfaces shown in panels (a–c): black, dark grey and light grey indicate overlap for 3, 2 and 1 species, respectively. Outlines for the management landscapes for these species derived using the Zonation algorithm (below) are shown with colours. (d) Demonstrates how conservation recommendations obtained by graphically delineating overlap areas of species distributions may differ from a more detailed numerical analysis of the same situation: in these three-species cases, zonation does select some areas where all three species are present, but it also selects some two- and one-species areas where individual species have particularly high connectivity. Lines show 100 km OS National Grid.
The following algorithm description is in terms of the proportion of the distribution of species j in cell i, qij; , where pij is any measure of the abundance of species j in cell i. For the butterflies of Britain we used pij=Iij (connectivity, below), but pij could as well be a modelled probability of occurrence (as for HCC), or some measure of abundance, density, vegetation cover or ecosystem type etc.
The rate of loss of value is minimized using measure
| 2.1 |
in which wj is weight of species j, and ci is cost of site i. At each step, δi is calculated for all sites at the edge of the remaining area, and the site having smallest δi is removed. Candidates for removal must have at least one 8-neighbour cell that already has been removed. Initially, edge is defined as cells either at the borders of the study area or as cells neighbouring missing data. The order of removal is recorded to allow identification of the landscape zoning. Importantly, equation (2.1) calculates cell value as the maximum biological value over all species rather than as the sum of species-specific value. Utilising such a sum could result in the replacement of cells having high value to a particular species by groups of cells with low to moderate value for several other species (thus allowing core areas of species occurring in otherwise species-poor areas to be wiped out). This is not satisfactory if the aim is to assure the retention of some high quality areas for all species.
The important feature of equation (2.1) is term Qj(S)=Σi∈Sqij, which is the proportion of the original distribution of species j located in the set of remaining cells S. Essentially, when part of the distribution of a species is lost; the importance of remaining habitat for that species increases as Qj(S) decreases. This ensures that core areas of individual species are retained even if they occur in species-poor regions, and prevents the early removal of core areas of even the initially widespread species. The last sites to be removed would be those sites having strong occurrences of high priority (high weight) species.
In other applications of Zonation, one might use a pre-processing step to discard low quality (urban etc.) locations from the landscape to provide extra starting points (edge) for the iterative cell removal process. For this purpose, sites can be ranked using and a given fraction of sites with lowest values can then be removed. For both of the present analyses inclusion of an initial removal of 10–20% of the landscape made negligible difference for final results and therefore this step was omitted.
(c) Identification of priority landscapes
We classified spatially separate blocks of land (having multiple grid cells) present in a priority zone (top 10% of area for the butterflies and top 20% for the indicator species at the HCC) into priority landscapes. A block was joined to a landscape if it was near enough and similar enough in faunal composition to any other block in the same landscape. For the butterflies our distance requirement was less than 10 km, which corresponds to the approximate maximum colonization distance of intermediate mobility species (Thomas et al. 2001). For the indicator species the distance criterion was 5 km. Our faunal dissimilarity measure between two separate blocks of land A and B is d(A, B)=J−1Σj|cAj−cBj|. Here cAj is a relative density rank for species j in block A, and J is the number of species having either cAj>0 or cBj>0. We define cAj with respect to log intervals around the average density of the species in the selected landscape. Let mSj=E[qij]i∈S be the mean density of species j calculated over all cells i included in the selected landscape S. We define cAj=0 (species practically absent), if E[qij]i∈A<0.01mSj. Respectively, cAj gets values 1 (rare), 2, 3, 4 or 5 (relatively very abundant) corresponding with intervals [0.01mSj, 0.1mSj), [0.1mSj, mSj), [mSj, 10mSj), [10mSj, 100mSj), and more than 100mj. In the analysis we required d(A, B)≤0.3, meaning that if blocks A and B are to go into the same landscape, then at most three species out of 10 can have a one order of magnitude difference in the occurrence rates between sites A and B. Note that a rank of cAj=0 is given to all blocks A having mAj<0.01mSj, because otherwise the far tails of connectivity distributions would identify artefactual differences between blocks of land from which species are in fact absent.
In addition to the distance conditions, we required a priority landscape to contain some core area that is retained in the cell removal process until a very late stage. This reflects our desire to build the priority landscapes around the very best core areas available. For the butterflies we used the top 0.5% fraction, and for the indicator species we used the top 5% fraction, which reflects the fact that the indicator species are comparatively widespread and do not have very distinct small core areas.
(d) Connectivity computations
Although zonation can be used with any kind of distribution data, the use of connectivity distributions has special meaning: connectivity computations increase the importance of the habitat matrix between known populations or suitable habitat. This makes it possible to identify potentially important dispersal and buffer habitats for patchy or fragmented species distributions. Our use of connectivity is comparable to the utilisation density distribution in home-range analyses: i.e. assuming that species use the landscape at the locations of observations and also around them (Worton 1989).
In the case of British butterflies connectivity is a species-based measures of landscape utilisation, which could be calculated without exact knowledge of species' habitat requirements and population dynamical parameters. The connectivity (see Moilanen & Nieminen 2002) for species j in grid cell i was calculated as where dik is the distance between cells i and k, and Akj is the size of the source population k. For the butterflies Akj was 1, 2 and 3, respectively, for a maximum of 1, 2–9 and more than 9 individuals sighted/visit. These categories correspond to those used by butterfly recorders in data collection for Asher et al. (2001), and we use the ordinal scale as a robust measure reflecting the likelihood of the site having a local breeding population. Parameter αj gives the dispersal capacity of each species. Values of α corresponding to yearly butterfly movements range from 0.25 for the most dispersive species (10% of individuals disperse more than 9.2 km), to 3 (10% move more than 0.76 km), based on prior knowledge of relative dispersal capacities (Cowley et al. 2001). Because we are concerned about population connectivity in a more than 1 year time scale, we (heuristically) divided these values by two (doubling the dispersal distances) to reflect the importance of infrequent long-distance movements to population dynamics in a longer time scale. For efficiency reasons, connectivity computations (Iij, above) for large landscapes should implemented via the use of the fast Fourier transform algorithm (Brewster & Allen 1997).
For the HCC, the connectivity computation was applied to the probability of occurrence surfaces (described by Wintle et al. in press) to identify areas where many cells with high predicted probabilities of occurrence are concentrated: such areas are expected to contain contiguous or connected populations, where persistence is most likely. In contrast, isolated sites achieve low connectivity, such that isolated sites with high local quality lose relative value. Note that this kind of a computation is most relevant when working at a high spatial resolution, where even a good quality region is likely to be a mosaic of high quality sites interspersed with less optimal habitat. (For coarse-resolution analyses, where most connectivity occurs within single grid cells, Zonation might be applied to unconverted probability of occurrence surfaces.) Values of α for the species were 1.5 (squirrel glider), 1.0 (koala, yellow-footed glider), 0.75 (tiger quoll), 0.5 (masked owl, sooty owl) and 0.25 (powerful owl).
3. Results
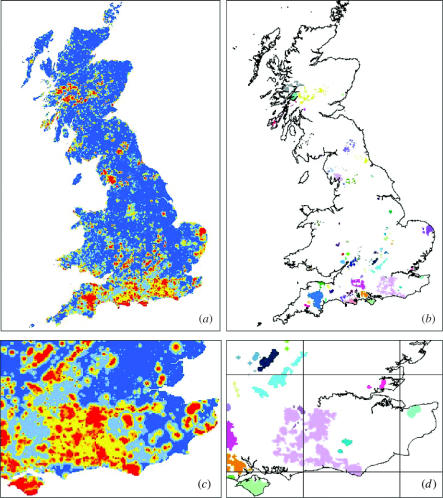
Figure 2a shows a landscape prioritization (zoning) for British butterflies, based on species-specific surfaces of population connectivity. Most high priority landscapes are species-rich regions in southern England, which contain core areas of the distributions of many rare species. Nonetheless, high priority areas are also found in Scotland, where the core areas of five northern species occur. The most important 10% of area (figure 2a) included 3868 separate (usually multi-cell) blocks of land, which demonstrates a high degree of fragmentation of distributions of threatened species in modified habitats at this scale. Some of these small fragments have populations that are populations that are dynamically connected and, for conservation purposes, should be managed as part of the same landscape. To identify such landscapes, we grouped together blocks of land to reflect their natural biological affinities and proximity to one another (figure 2b, see §2). This procedure identified 75 landscapes that cover 4.9% of the British land surface, and could logically be identified as single landscapes with respect to long-term population dynamics and conservation management. This method provides a means by which conservation planning for British butterflies can move forward from the current focus on sites for the rarest and most threatened species (e.g. the UK Biodiversity Action Plan, UK Biodiversity Steering Group 1995), to the conservation of landscapes that will maintain the entire butterfly fauna.
Figure 2.
(a) Landscape prioritization zones for British butterflies. Colour-scale from low to high priority (cumulative percent of landscape removed when the focal cell is removed): dark blue 0–60%, light blue 60–80%, yellow 80–90%, orange 90–95% and red 95–100%. (b) Priority landscape groupings based on the top 10% zone. Each landscape (shown by a colour) contains blocks of land that are close together, similar in species composition, and contain a core area present late in the cell removal process (see §2). (c) and (d) show partial enlargements of (a) and (b). Lines in (d) show 100 km OS National Grid.
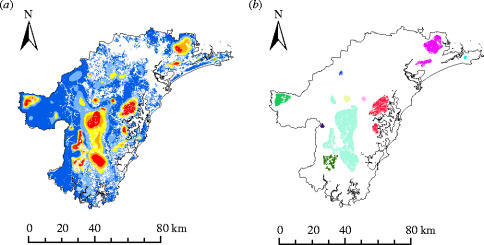
Figure 3 shows a landscape prioritization for detailed regional planning at a high spatial resolution in the HCC region, based on connectivity surfaces derived from habitat models (Wintle et al. in press). A hierarchy of solutions with significant habitat aggregation is found both for the UK butterflies and the Hunter valley even though they are based on different data at different spatial scales. The connectivity computation makes a great difference to the small-scale spatial pattern of the recommended reserve area of HCC: a 20% solution based directly on probability of occurrence includes 3915 often closely spaced distinct blocks of land (separate analysis, not shown). In contrast, calculations based on connectivity produce only 22 compact and well connected blocks (figure 3), which are well suited as starting points for local conservation planning.
Figure 3.
(a) Landscape prioritization for the indicator species in Hunter Valley, eastern Australia. Colour-scale as in figure 2a. (b) Priority landscape groupings based on the top 20% zone (see §2).
We assessed the use of weights in Zonation for the British butterflies. We classified species as habitat specialists or wider-countryside species (Asher et al. 2001) and weighted them as 10 and 1, respectively: the specialists have declined (Warren et al. 2001) whereas the wider-countryside species survive in a multitude of rural and urban landscapes outside protected areas. Figure 4a,b demonstrate the success of our approach: more than 90% of the original summed connectivity of high and medium priority (Asher et al. 2001) species is retained in a small fraction (less than 10%) of the landscape identified as high priority zones in our analysis. Low priority species lose more of their distribution (figure 4c) because they have much larger initial distributions (figure 4d). A higher fraction is retained for habitat specialists than for wider countryside species, as expected due to the weighting of species (figure 4b,c). Even following a high proportional loss of connectivity, the low priority species still retain higher absolute levels of connectivity within reserve areas than the habitat specialists (figure 4d). The situation is different in the Hunter Valley, where the top 20% fraction of the remaining forest (12.9% of the total land surface) includes only more than 25% of the distributions of all indicator species. Because the indicators have wide but mostly non-overlapping distributions, essentially none of the remaining forest cover can be lost without some predicted loss of biological value. Nonetheless, the core area of each species is covered by our solution (figure 3).
Figure 4.
(a–c) Proportion of original distribution (connectivity) retained for each of the 57 British butterfly species as a function of proportion of landscape remaining as lower priority zones are removed. Habitat specialists (weight 10) and wider countryside species (weight 1) are shown with solid and dashed lines, respectively. (a) High priority species. (b) Medium priority species. (c) Low priority species. (d) Relationship between amount of connectivity for species in the full original landscape and the proportion retained in the top 5% landscape: shown for high (triangles), medium (circles) and low (squares) priority species, and for habitat specialists (filled symbols) and wider countryside species (empty).
Sensitivity analyses reveal that our recommended solutions are not overly dependent on dispersal abilities assumed in connectivity computations or species weights used in Zonation. Doubling or halving dispersal distances (for UK and HCC) caused the identity of ca 15% of cells in the top 10% zone to change. For the butterflies of Britain, 74.3% of the grid cells in the top 10% of our base solution (figure 2a) were also included when all species received equal weights, the latter giving more emphasis to regions with strong occurrences of common species. The corresponding value was 98.7% when only habitat specialist species were used in the analysis, indicating that the habitat specialists are strongly driving the zoning when using our default 10 : 1 weighting.
4. Discussion
In Britain's highly fragmented landscape, conservation effort must be closely targeted and the zoning approach provides a suitable framework. For the British butterflies, zone 1 (figure 2a; top 5% of area) represents regions where targeted conservation of semi-natural or natural vegetation is most important for the long-term maintenance of the threatened butterfly fauna. Zone 2 (5–10%) might suggest areas where strong environmental input into land use planning would be helpful (in addition to the protection of key habitats). In other cases, zone 2 would represent areas of regional, more than national, priority. Elsewhere, conservation would principally be mediated through policies related to sustainable land use (keeping common species common and maintaining ecosystem services), although these areas may be important for other groups of animals or plants (see Prendergast et al. 1993). In the HCC at least 20% of the remaining forest could be retained (figure 3a): regions between the recommended priority core areas would be natural targets for landscape ecological planning including the establishment and maintenance of connecting corridors.
The primary purpose of the proposed method, in most cases, will not be to propose a detailed reserve structure, but to identify landscapes that could be subjected to more detailed planning. Our approach produces a hierarchy of priorities, using connectivity derived either from raw distribution data or from modelled probability of occurrence as the basis for generating the aggregation of priority areas. Our methods conceptually draw on landscape-scale population studies that deal with persistence (MacArthur & Wilson 1967; Levin 1974; Hanski 1998; Hanski & Ovaskainen 2000), as well as reserve selection approaches, that deal with complementary representation of species (see Margules & Pressey 2000; Cabeza & Moilanen 2001; Cabeza et al. 2004; Williams et al. 2004 for reviews). The use of connectivity-based distributions in Zonation is a practical means of incorporating elements of both approaches. The methods can be applied to very large data sets containing thousands of species in multi-million element landscapes. They can also be applied to undisturbed as well as human-dominated regions of the world.
Acknowledgments
We thank the many thousand recorders who contributed to the British butterfly distribution data set and the HCC Regional Environmental Management Strategy. I. Hanski, M. Cabeza and A. van Teeffelen kindly commented on the manuscript. This study was funded by the Academy of Finland, NERC/UKPopNet, and the Countryside Council for Wales.
References
- Asher J, Warren M, Fox R, Harding P, Jeffcoate G, Jeffcoate S, Greatorex-Davies N, Robert E. Oxford University Press; 2001. The millennium atlas of butterflies in Britain and Ireland. [Google Scholar]
- Brewster C.C, Allen J.C. Spatiotemporal model for studying insect dynamics in large-scale cropping systems. Environ. Entomol. 1997;26:473–482. [Google Scholar]
- Brooks T.M, Pimm S.L, Collar N.J. Deforestation predicts the number of threatened birds in insular Southeast Asia. Conserv. Biol. 1997;11:382–394. [Google Scholar]
- Cabeza M, Moilanen A. Design of reserve networks and the persistence of biodiversity. Trends Ecol. Evol. 2001;16:242–248. doi: 10.1016/s0169-5347(01)02125-5. [DOI] [PubMed] [Google Scholar]
- Cabeza M, Moilanen A, Possingham H.P. Metapopulation dynamics and reserve network design. In: Hanski I, Gaggiotti O, editors. Ecology, genetics and evolution of metapopulations. Academic Press; London: 2004. pp. 541–564. [Google Scholar]
- Cowley M.J.R, et al. Density-distribution relationships in British butterflies. I. The effect of mobility and spatial scale. J. Anim. Ecol. 2001;70:410–425. [Google Scholar]
- Debinski D.M, Holt R.D. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 2000;14:342–355. [Google Scholar]
- Gaston K.J, Pressey R.L, Margules C.R. Persistence and vulnerability, retaining biodiversity in the landscape and in the protected areas. J. Biosci. 2002;27:361–384. doi: 10.1007/BF02704966. [DOI] [PubMed] [Google Scholar]
- Gaston K.J, Blackburn T.M, Goldewijk K.K. Habitat conversion and global avian biodiversity loss. Proc. R. Soc. B. 2003;270:1293–1300. doi: 10.1098/rspb.2002.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groombridge B. Chapman & Hall; London: 1992. Global biodiversity. [Google Scholar]
- Guisan A, Zimmermann N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000;135:147–186. [Google Scholar]
- Hanski I. Metapopulation dynamics. Nature. 1998;396:41–49. [Google Scholar]
- Hanski I, Ovaskainen O. The metapopulation capacity of a fragmented landscape. Nature. 2000;404:756–758. doi: 10.1038/35008063. [DOI] [PubMed] [Google Scholar]
- Jongman R, Pungetti G, editors. Ecological networks and greenways: concept, design and implementation. Cambridge University Press; 2004. [Google Scholar]
- Kunin W.E. Extrapolating species abundance across spatial scales. Science. 1998;281:1513–1515. doi: 10.1126/science.281.5382.1513. [DOI] [PubMed] [Google Scholar]
- Levin S.A. Dispersion and population interactions. Am. Nat. 1974;108:207–228. [Google Scholar]
- MacArthur R.H, Wilson E.O. Princeton University Press; 1967. The theory of island biogeography. [Google Scholar]
- Margules C.R, Pressey R.L. Systematic conservation planning. Nature. 2000;405:243–253. doi: 10.1038/35012251. [DOI] [PubMed] [Google Scholar]
- Moilanen A, Nieminen M. Simple connectivity measures in spatial ecology. Ecology. 2002;84:1131–1145. [Google Scholar]
- Possingham H.P, Ball I.R, Andelman S. Mathematical methods for identifying representative reserve networks. In: Ferson S, Burgman M, editors. Quantitative methods for conservation biology. Springer; New York: 2000. pp. 291–305. [Google Scholar]
- Prendergast J.R, Quinn R.M, Lawton J.H, Eversham B.C, Gibbons D.W. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature. 1993;365:335–337. [Google Scholar]
- Sala O.E, et al. Biodiversity—global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Sjögren-Gulve P, Ebenhard T, editors. The use of population viability analysis in conservation planning. Ecological bulletin. vol. 48. Munksgaard International Publishers; Copenhagen: 2000. [Google Scholar]
- Thomas C.D, Bodsworth E.J, Wilson R.J, Simmons A.D, Davies Z.G, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004a;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Thomas J.A, Telfer M.G, Roy D.B, Preston C.D, Greenwood J.J.D, Asher J, Fox R, Clarke R.T, Lawton J.H. Comparative losses of British butterflies, birds and plants, and the global extinction crisis. Science. 2004b;303:1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- Tucker G.M, Evans M.I, editors. Habitats for birds in Europe: a conservation strategy for the wider environment. Birdlife International; Cambridge: 2004. [Google Scholar]
- UK Biodiversity Steering Group . HMSO; London: 1995. Biodiversity: the UK Steering Group report—meeting the Rio Challenge, vol. 1. [Google Scholar]
- Warren M.S, et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. [DOI] [PubMed] [Google Scholar]
- Williams J, ReVelle C.S, Levin S.A. Using mathematical optimization models to design nature reserves. Front. Ecol. Environ. 2004;2:98–105. [Google Scholar]
- Wintle, B. A., Elith, J. & Potts, J. 2005 Fauna habitat modelling and mapping: a case study in the Lower Hunter Central Coast region of NSW. Aust. Ecol.30 (In press.)
- Worton B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70:164–168. [Google Scholar]