Abstract
The theoretical foundation of sexually antagonistic coevolution is that females suffer a net fitness cost through their interactions with males. The empirical prediction is that direct costs to female lifetime fecundity will exceed indirect benefits despite a possible increase in the genetic quality of offspring. Although direct costs of males have been repeatedly shown, to date no study has comprehensively tested whether females are compensated for this direct harm through indirect benefits. Here we use experimental evolution to show that a mutation giving Drosophila melanogaster females nearly complete resistance to the direct costs of male courtship and remating, but which also excluded almost all indirect benefits, is strongly favoured by selection. We estimated the selection coefficient favouring the resistance allele to be +20%. These results demonstrate that any indirect benefits that females accrued were not sufficient to counter-balance the direct costs of males, and reinforce a large body of past studies by verifying interlocus sexual conflict in this model system.
Keywords: cost of males, indirect benefits, male harm, sexual conflict, sexually antagonistic coevolution
1. Introduction
Recently the empirical foundation of sexually antagonistic coevolution has been called into question: namely, that females suffer a net cost through their interactions with males. Conflicts between the sexes over traits, such as the number of sexual partners or the amount of parental care, are an integral part of all models of sexual selection (Bateman 1948; Trivers 1972). Sexually antagonistic coevolution, however, is driven by interlocus sexual conflict in which allelic substitution at one locus, expressed in males, reduces the fitness of females, and counter-adaptation at a second locus, expressed in females, reduces this harm while simultaneously creating selection for a new allele at the first locus (Dawkins 1976; Parker 1979; Rice & Holland 1997). In this model of sexual coevolution, males evolve traits that reduce the fitness of females and females evolve resistance to this harm.
The fact that males of some species harm females has been well established in a diverse range of taxa (McKinney et al. 1983; Crudgington & Siva-Jothy 2000; Hosken et al. 2001; Stutt & Siva-Jothy 2001; Arnqvist & Rowe 2002), and has been most extensively examined in laboratory populations of Drosophila melanogaster (Cohet & David 1976; Partridge et al. 1987; Rice 1996; Partridge & Hurst 1998; Holland & Rice 1999; Rice 2000; Chapman 2001), where it has been shown that males directly harm females during both courtship (Partridge & Fowler 1990) and mating (Fowler & Partridge 1989; Chapman et al. 1993; Chapman et al. 1995). However, it has been debated whether or not establishing a direct reduction in female lifetime fecundity is the same as demonstrating net harm to females, since there is the possibility that indirect benefits (i.e. sexy sons (Fisher 1930; Kirkpatrick & Ryan 1991) or good genes (Arnqvist 1992; Andersson 1994; Møller & Alatalo 1999)) could compensate for the direct costs of males (Parker 1979; Arnqvist 1992; Andrés & Morrow 2003; Cordero & Eberhard 2003). For these observations to truly demonstrate net harm, the direct costs of males must be shown to be larger than the indirect benefits females may gain from such an interaction. Were the indirect benefits to equal or exceed the direct costs, then, by definition, there would be no net interlocus sexual conflict (Parker 1979; Andrés & Morrow 2003; Cordero & Eberhard 2003). Therefore, to conclusively demonstrate interlocus sexual conflict, a multigenerational experiment is necessary in order to allow females to accrue possible indirect benefits (Cordero & Eberhard 2003). Although several authors have suggested that indirect benefits are unlikely to be large enough to compensate for the direct costs (Cameron et al. 2003; Chapman et al. 2003), to date no one has comprehensively tested this assertion (but see Head et al. 2005; Orteiza et al. in press).
In both the field and laboratory, D. melanogaster males persistently court non-virgin females and most male–female interactions occur in this context. Persistent male courtship and remating of non-virgin females have well documented direct costs to females (Cohet & David 1976; Partridge et al. 1987; Fowler & Partridge 1989; Partridge & Fowler 1990; Chapman et al. 1993; Chapman et al. 1995; Rice 1996; Holland & Rice 1999). In the study described here, we tested whether or not indirect benefits compensate females for these direct costs, and thereby preclude sexually antagonistic coevolution. Our experiments were not designed to test the general models of sexual selection, via good genes or sexy sons. Instead, they addressed a more specific hypothesis that non-virgin females can recoup the direct costs of persistent male courtship and remating by trading-up with males of superior genetic quality. In order for remating to allow females to recoup the direct costs of interacting with males, there must be heritable genetic variation for male fitness and females must remate with males of superior genetic quality compared to their primary mates.
The most direct way to assess the existence of sexually antagonistic coevolution would be to trace the deterministic fate of a new mutation which gives females resistance to the direct cost of male-induced harm, while also preventing indirect benefits from accruing. If such a mutation failed to accumulate, for reasons other than stochastic loss, then this would provide evidence that indirect benefits more than compensate for direct harm (on balance, when averaging across the entire genome), and sexually antagonistic coevolution would not be supported at the level of net interactions between the sexes. But if the mutation accumulated, then the inter-sexual arms race would be directly observed and this would provide compelling evidence for net conflict between the sexes (i.e. direct costs exceed indirect benefits). In the experiments described here, we trace the fate of such a new mutation that gives non-virgin females resistance to the direct costs of their interactions with males. The resistance allele that we studied protected females from the direct costs of males, but simultaneously impeded, to the same degree, females from gaining indirect benefits from males. An example of such an allele, and the specific context we sought to emulate in the laboratory, would be one where females, after mating, either produced a male-specific pheromone or stopped producing a female-specific pheromone, thus eliminating, or greatly reducing, their attractiveness to males. In this case, these ‘disguised’ or ‘masked’ females would experience considerably less male-induced harm, via courtship and remating, but would also be denied, to the same degree, the opportunity to ‘trade-up’ and remate with a more attractive or genetically superior male. Such an allele would only increase toward fixation if direct costs outweighed indirect benefits, demonstrating that interlocus sexual conflict was operating on balance across the genome. In this case, the costs and benefits of male–female interactions would not need to be individually quantified because their net combined effect would be assessed by the net selection on the resistance allele.
Here we analysed the fate of such a mutation and thereby tested the hypothesis that interlocus sexual conflict was operating overall. We benefited from the considerable power of the D. melanogaster model system, since we could experimentally make a simple, arbitrary genetic marker (influencing eye-colour) emulate a new female resistance allele, and thereby trace the evolutionary fate of such a mutation. We also benefited from the availability of a large outbred population of D. melanogaster that had adapted to the laboratory environment for over 320 generations. The trade-off between direct costs of males and indirect benefits gained through remating could be assessed in this population because it harboured high levels of genetic variation for both net fitness and male fertilization success (Chippindale et al. 2001), while manifesting sizeable direct harm to females from their interactions with males (Linder & Rice 2005; Orteiza et al. in press).
Experimental evolution was used to trace the fate of a mutation that protected females from the cost of interacting with males, but also, to the same degree, reduced the opportunity for indirect benefits (see detailed description below). More specifically, females expressing the resistance mutation experienced nearly an order of magnitude less exposure to persistent male-courtship, but they consequently had the same reduction in the opportunity to gain indirect benefits via remating. These trade-offs are identical to those that would be experienced by females expressing the resistance gene described above. To emulate this new female resistance mutation, we introduced a dominant marker allele (red eye-colour) into two treatments of D. melanogaster and traced its fate. In the first, experimental treatment we applied artificial selection on the marker to make it behave as if it were a new female resistance mutation. In the control treatment, we applied no experimental selection, but allowed pleiotropic natural/sexual selection alone to act on the marker. Then, by tracing the change in gene frequency in the experimental treatment, after adjusting for any pleiotropic selection on the marker itself, as measured in the control, we were able to estimate the direction and strength of selection acting on the resistance allele. The fate of the resistance allele (accumulation or decline) unambiguously determines whether or not interlocus sexual conflict is operating in the population (on balance, across the entire genome), and the estimated selection coefficient estimates the genome-wide magnitude of interlocus sexual conflict.
2. Material and methods
(a) Base population
LHM is a large outbred population that has adapted to laboratory conditions for over 320 generations, and is reared on a two week, non-overlapping generation cycle (for a detailed description see Rice et al. 2005). During culture, flies are sequentially transferred to three consecutive vials each generation. On day 0, eggs are laid in a first set of 56 ‘juvenile competition’ vials. Offspring remain in this first set of vials throughout the larval, pupal, and early adult stages, at a density of 150–200 per vial. On day 12, adult flies are mixed among vials and 1792 randomly selected flies are transferred to a second set of 56 ‘male–female interaction’ vials (16 pairs/vial; also called ‘adult competition vials’ in some of our other publications), for 2 days. During this time, females compete for a limited supply of live-yeast (10 mg), which strongly influences their fecundity (see Electronic Appendix A), and males compete to fertilize females. Eighteen hours before the end of the two week life cycle, adult flies are transferred to a third set of 56 ‘oviposition’ vials, without live-yeast. Eggs produced during this time are reduced to 150–200 per vial and used to begin the next generation (ca 9800 eggs). Throughout the culture process adult mortality is negligible. Experiments described below closely match the timing of events, culture medium, and densities used during the normal culturing of LHM. Consequently, lifetime reproductive success is measured under the environmental conditions that closely match those to which the flies were adapted.
(b) Experimental design
The artificial selection experiment was composed of two treatments (experimental and control), each replicated five times. The five replicates were carried out in two blocks—two replicates in one and three in the other. To dissociate the red-eyed marker allele (bw+, from the LHM base population), which would be made to emulate a resistance allele in the experimental treatment, from the influence of its genetic background, we needed to carryout extensive backcrossing prior to starting the experiment. Accordingly, we backcrossed the dominant red eye-colour allele (bw+), 23 times, into a replica of the LHM base population of flies that was homozygous for a recessive brown eye-colour allele (bw, Rice et al. 2005 for a more complete description of this LHM-bw population).
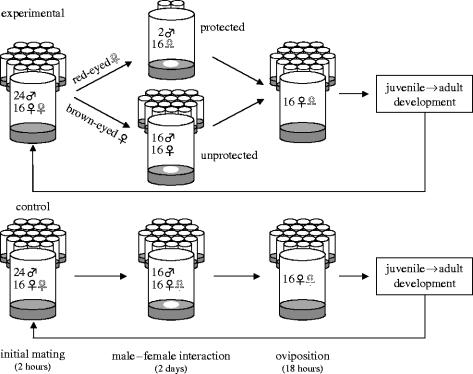
The schematic in figure 1 summarizes the experimental design. To begin each replicate, we first collected the males and virgin females needed for the matings that would start each generation. Twenty vials of virgin females (16/vial) were collected, under light CO2 anaesthesia (<60 s), from ‘juvenile competition’ vials, on day 9 of their 14-day cycle. In the same way, 20 vials of males (24/vial) were collected on day 11. For the first generation, the starting frequency of the resistance allele was set at 7.5%, contained exclusively in heterozygous (bw/bw+) flies. After the first generation, the frequency of the resistance allele was not experimentally controlled. The initial matings that began each generation were made on day 12 of the 14 day generation cycle. At this time, 24 males were combined with 16 virgin females without anaesthesia, for a period of 2 h (figure 1; left). Separate control experiments have shown that virtually all females mate during this time (96.8±1.1% (mean±s.e.); see Electronic Appendix B), while remating rarely occurs (Rice 1996; Holland & Rice 1999). At the end of the initial mating bout, all 20 vials of flies were mixed and re-sorted into 20 ‘male–female interaction’ vials (figure 1, centre), each containing a limited amount of live-yeast (10 mg). It is during this male–female interaction stage that the experimental and control treatments diverged: females expressing the red-eye marker experienced a low sex ratio (2 males per vial containing 16 females) and brown-eyed females experienced the normal 1 : 1 sex ratio (16 males and 16 females).
Figure 1.
Experimental evolution makes a red eye-colour marker evolve as if it were a dominant allele conferring resistance to harm from males. Experimental and control flies were treated identically throughout the experiment, with the exception of the ‘male–female interaction’ stage. In the experimental treatment, females expressing the dominant resistance allele (red eye-colour marker) competed for a limiting resource (live-yeast depicted as a white oval, (Electronic Appendix A)) in an environment ‘protected’ from males, whereas brown-eyed females, not expressing this allele, experienced an ‘unprotected’ environment. Males were randomly assigned to these two environments irrespective of eye-colour. In the control treatment both types of female were placed in an unprotected environment, so that selection acted only on the pleiotropic effects of the resistance allele. The protected environment is depicted by three of the twenty vials that comprise the experimental treatment. This represents the initial frequency of resistant females, however after the first generation, the number of vials in the protected environment was determined by the observed frequency of resistant females.
More specifically, experimental female flies were sorted into male–female interaction vials based on eye-colour (figure 1; top centre). Groups of 16 brown-eyed (bw/bw) females were housed for 2 days with 16 randomly selected males (with respect to eye colour) in an ‘unprotected’ environment (to which the base population was previously adapted). The brown-eyed females in the unprotected environment were not sheltered from the direct costs of males, but were unhampered in their ability to accrue indirect benefits through remating (which occurred at high frequency, see Electronic Appendix C). However, groups of 16 red-eyed (resistant; bw/bw+ and bw+/bw+) females were housed with only two randomly selected males (again, with respect to eye-colour) in a ‘protected’ environment. The red-eyed females experienced greatly reduced exposure to persistent male courtship, which consequently impeded the opportunities to obtain indirect benefits via remating by the same amount. The inclusion of the two males in the protected environment guarded against the possibility that a female had not mated during the initial mating period and would, therefore, suffer a severe fitness cost not associated with carrying the resistance allele.
In the control treatment, the protocol was identical to the experimental treatment except that the eye colour marker did not influence the level of persistent male courtship experienced by females during the 2 day male–female interaction phase of their life cycle. More specifically, flies were sorted into male–female interaction vials in groups of 16 females and 16 males, irrespective of eye-colour (figure 1, centre bottom). This control treatment was run to account for any changes in resistance allele frequency due to pleiotropic natural/sexual selection. By running this control, we could subtract any changes in the resistance allele frequency due to pleiotropic natural/sexual selection on the red eye-colour allele within the experimental populations, leaving only changes due to artificial selection. These measures allowed us to calculate the net selection coefficient for the resistance allele itself, and hence quantify the level of net interlocus sexual conflict in this model system.
A complication that arises in the experimental treatment is that the number of brown- and red-eyed females was not always divisible by 16 (the number of females in the male–female interactions vials), so that incompletely filled vials were created in some generations. To retain a constant female density in all vials, when the numbers of either brown-eyed or red-eyed females were not divisible by 16, ‘surrogate’ females of the opposite eye-colour (collected from the progeny of the previous generation for such a contingency) were added to complete the vial of 16 females. This procedure ensured that all females experienced the appropriate competitive environment, and all surrogate females were discarded immediately before females laid the eggs that produced the next generation.
After the 2 day male–female interaction stage, all flies were mixed and groups of 16 randomly selected females were placed in ‘oviposition’ vials for 18 h (figure 1; right). All remaining males were then scored and tallied by eye-colour and discarded.
After oviposition, females were tallied by eye-colour, discarded, and the number of eggs per vial reduced to approximately 150. Offspring remained in these 20 oviposition vials throughout the larval, pupal, and early adult stages (i.e. these vials then became the ‘juvenile competition’ vials of the next generation; figure 1; left). Given that the strength of selection on a dominant allele is maximal at a frequency of 0.333 (Hendrick 1983), we decided a priori to terminate the experiment prior to either treatment reaching this value to prevent allele frequencies from converging due to an unbalanced response to selection. All flies were maintained in 25 °C incubators on a 12 : 12 light : dark cycle.
The frequency of the dominant red allele was experimentally controlled to be 7.5% at the start of each experiment (see above). Thereafter, in all generations except the last, allele frequencies were estimated by counting the numbers of red and brown eyed flies in the random sample of 840 flies (520 males+320 females) used during the initial matings at the beginning of each generation. In the last generation of each experiment, all progeny were counted. Frequency of the recessive brown allele was estimated as the square-root of the proportion of brown-eyed flies, and the frequency of the red allele as the complement of this value. Resistance allele frequencies at the end of the experiment were arcsine square-root transformed before analysis via ANOVA. Block was initially included in the analysis, but was non-significant (F2,9=2.3071, P=0.1726) and was, therefore, excluded from the analysis, resulting in an unpaired t-test between the final allele frequencies of the five experimental and five control treatments. Statistical analysis was done using Jmp version 5.1.1.
3. Results
After five generations of selection, the frequency of the resistance allele was significantly greater in the experimental treatment than in the control treatment (t=6.67, p=0.0002; d.f.=8; figure 2a). Although pleiotropic natural/sexual selection did favour the red-eye marker allele in the controls (selection only favoured red-eyed males, while the fitness of red- and brown-eyed females did not differ; for discussion see Electronic Appendix D), this selection was acting in both treatments and cannot account for the difference between them. Therefore, the observed positive difference between the two treatments represents the advantage of the resistance allele due to our experimental selection on female resistance alone (figure 2b). The fact that the resistance allele accumulated, after adjustment for pleiotropic selection on the marker, demonstrates interlocus conflict in this model system. Moreover, we estimate that the net selective advantage of females being resistant to persistent male courtship and mating attempts is large (20.38%, table 1). This large selection coefficient indicates substantial net interlocus conflict between the sexes that is not compensated by indirect benefits.
Figure 2.
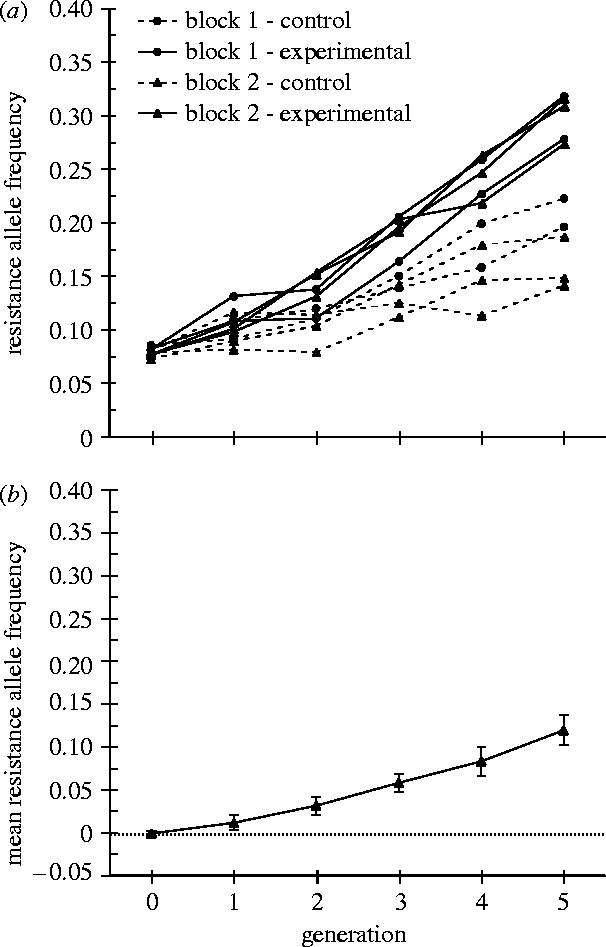
Temporal change in frequency of the resistance allele in experimental and control lines. In all replicates the frequency of the resistance allele was initially 0.075. (a) Values for the individual replicates of control and experimental lines from the two blocks of the experiment. (b) Mean frequency (±standard error) of the resistance allele in the experimental lines after adjustment for the pleiotropic effects of natural/sexual selection (mean experimental minus mean control).
Table 1.
Selection coefficients for the resistance allele.
| generation | selection coefficient (s) |
|---|---|
| 0→1 | — |
| 1→2 | 0.2083 |
| 2→3 | 0.2325 |
| 3→4 | 0.1669 |
| 4→5 | 0.2074 |
| mean s | 0.2038 |
| standard error | 0.0136 |
| upper (95%) confidence bound | 0.2470 |
| lower (95%) confidence bound | 0.1605 |
Selection coefficients (s) were calculated, after subtracting the mean of the control from the mean of the experimental treatment, from one generation to the next using s=((p′−p)/p)/(1−p′). Where p′ is the frequency of the resistance allele in the current generation and p is the frequency in the previous generation. s for generations 0→1 was 0.1774, but was not included because indirect benefits can only act through grandchildren (i.e. from generation 2 onwards).
4. Discussion
The main result of our experiments, that the net direct costs of male-induced harm outweigh any potential indirect benefits gained through remating, is straightforward. This finding confirms the operation of interlocus sexual conflict in the D. melanogaster model system that has been reported from many studies over the last 30 years (e.g. Cohet & David 1976; Fowler & Partridge 1989; Partridge & Fowler 1990; Chapman et al. 1995; Rice 1996; Rice & Holland 1997; Partridge & Hurst 1998; Holland & Rice 1999; Rice 2000; Chapman 2001; Chippindale et al. 2001; Wigby & Chapman 2004; Linder & Rice 2005). We next discuss three details of the experimental design with regards to density effects, the genome-wide scale of sexual conflict, and the cost of female resistance, that could influence the interpretation of our experiments.
In order to vary the level of male-induced harm to females, our experimental treatment varied the density of males (but not females). Changes in density of males could influence females in two ways. The first is by directly influencing the intensity of male–female interactions (Wigby & Chapman 2004), and second is by ‘non-directly’ influencing their limiting resource, live-yeast (see Electronic Appendix A). The first effect was the main way males conferred direct harm on females and our experimental design relied on this effect, since we wanted to experimentally influence the level of male-induced harm that females experienced (or were protected from). We tested for the second effect in two additional control experiments that quantified the effects of males on the availability of the limited supply of live-yeast, and the effects of density over and beyond its influence on the supply of live-yeast. Importantly, we found that the presence of males produced no measurable effect on the supply of live-yeast (through consumption or fouling; see Electronic Appendix E) and that variation in the density of flies had no effect other than its influence on competition for live-yeast among females (see Electronic Appendix A). Therefore, our experimental protocol did not give resistant females an unintentional advantage over non-resistant females, and if anything, was conservative (see Electronic Appendix A).
Our study tested for interlocus sexual conflict when summing across the entire genome, and therefore summing across all male–female interactions. In doing so, this study demonstrated that the direct harm that males impose upon females is not compensated for by the production of offspring with higher genetic quality after remating, through either the good genes or sexy sons mechanisms. It is important to reiterate that our study does not rule out the operation of models of sexual selection through either good genes or sexy sons, but only that the indirect benefits gained through remating are not sufficient to compensate for male-induced harm. As a consequence, interlocus sexual conflict is currently operating, on balance, in our laboratory population of D. melanogaster.
Nonetheless, because the genome is likely to be a mosaic of many pairs (or small groups) of interacting loci that influence interactions between the sexes, indirect benefits may compensate for direct costs in the context of particular pairs of interacting loci, despite the fact that this compensation does not occur on balance when summing across the entire genome. This possibility can only be addressed by studying individual pairs of loci that mediate interactions between the sexes. However, even when indirect benefits compensate for direct harm to females, in the context of a particular pair of interacting loci, a form of sexually antagonistic coevolution is still possible. This could occur when an adaptive allelic substitution at a locus expressed in males interacts with one or more loci expressed in females in a way that produces both costs and benefits in females, but the correlation between these two effects, in females, can be genetically disentangled. Under these circumstances, females receive a net benefit from the interlocus interaction with males, but selection, nonetheless, acts to reduce the costs. When an adaptation at one or more loci expressed in females reduces costs, but also reduces the efficacy of male mating and/or fertilization success, then this will select for a counter-adaptation at the male-expressed locus and potentially drive sexually antagonistic coevolution.
Finally, naturally occurring resistance in females is likely to involve a cost, which we did not impose. However, in order for a cost to prevent a naturally occurring resistance allele from accumulating, it would have to exceed the 20% fitness advantage we observed for the artificially resistant females. Interestingly, our estimate for the fitness advantage of the resistance allele closely matches estimates of the direct cost of males to non-virgin females in our laboratory population (e.g. ca 16%, Linder & Rice 2005 and Orteiza et al. in press, to 20%, unpublished data, pooled across many experiments). This agreement between our study, where females were continuously exposed to two males, and those of Linder & Rice (2005) and Orteiza et al. (in press), where females had no continuous exposure to males, indicates that the protected environment did, indeed, virtually eliminate male-induced harm. The large advantage of the resistance allele indicates that at least some allelic forms of female resistance would have a net selective advantage and contribute to interlocus antagonistic coevolution between the sexes. The large selection coefficient we observed for a new female resistance mutation begs the question: why have such resistance alleles not accumulated in the past and reduced the male-induced harm to females? We think that the answer lies in the dynamic nature of the inter-sexual arms race. Males are continuously selected to exploit the higher parental investment of females in their offspring. Males are, therefore, expected to lead in the coevolutionary arms race, since females can only respond to, rather than anticipate, new male adaptations that harm them. As a consequence, males will typically be ahead of females in the arms race, as indicated in our study, despite continual counter-adaptation by females.
Our finding of interlocus sexual conflict is only directly applicable to the D. melanogaster laboratory model system, nonetheless, it demonstrates the substantial potential for sexually antagonistic coevolution in nature. Females in natural populations of D. melanogaster are also persistently courted by males who patrol the resources females need for reproduction (Markow 1988) and they frequently carry stored sperm from multiple males (Harshman & Clark 1998; Imhof et al. 1998). These conditions have the potential to promote sexually antagonistic coevolution in natural populations. Last, the experimental protocol described here could be applied to a number of additional taxa, and may be valuable in distinguishing between putative interlocus sexual conflict and actual interlocus sexual conflict.
Acknowledgments
We thank J. Linder and N. Orteiza for their assistance with supplementary control experiments. We also thank U. Friberg, B. Kuijper, L. Rowe, C. Stewart and T. Tregenza for comments on the work. This work was supported by two grants from the US National Sciences Foundation (DEB-0128780 and DEB-0410112).
Supplementary Material
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andrés J.A, Morrow E.H. The origin of interlocus sexual conflict: is sex-linkage important? J. Evol. Biol. 2003;16:219–223. doi: 10.1046/j.1420-9101.2003.00525.x. 10.1046/j.1420-9101.2003.00525.x [DOI] [PubMed] [Google Scholar]
- Arnqvist G. Precopulatory fighting in a waterstrider: inter-sexual conflict or mate assessment? Anim. Behav. 1992;43:559–567. 10.1016/0003-3472(92)90079-O [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Cameron E, Day T, Rowe L. Sexual conflict and indirect benefits. J. Evol. Biol. 2003;16:1055–1060. doi: 10.1046/j.1420-9101.2003.00584.x. 10.1046/j.1420-9101.2003.00584.x [DOI] [PubMed] [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. 10.1046/j.1365-2540.2001.00961.x [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Chapman T, Hutchings J, Partridge L. No reduction in the cost of mating for Drosophila melanogaster females mating with spermless males. Proc. R. Soc. B. 1993;253:211–217. doi: 10.1098/rspb.1993.0105. [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle L.F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chippindale A.K, Gibson J.R, Rice W.R. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:1671–1675. doi: 10.1073/pnas.041378098. 10.1073/pnas.041378098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohet Y.A, David J.R. Deleterious effects of copulation in Drosophila females as a function of growth temperature of both sexes. Experientia. 1976;32:696–697. doi: 10.1007/BF01919838. 10.1007/BF01919838 [DOI] [PubMed] [Google Scholar]
- Cordero C, Eberhard W.G. Female choice of antagonistic male adaptations: a critical review of current research. J. Evol. Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. 10.1046/j.1420-9101.2003.00506.x [DOI] [PubMed] [Google Scholar]
- Crudgington H.S, Siva-Jothy M.T. Genital damage, kicking and early death. Nature. 2000;407:855–856. doi: 10.1038/35038154. 10.1038/35038154 [DOI] [PubMed] [Google Scholar]
- Dawkins R. Oxford University Press; Oxford, UK: 1976. The selfish gene. [Google Scholar]
- Fisher R.A. Clarendon Press; Oxford: 1930. The genetical theory of natural selection. [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruitflies. Nature. 1989;338:760–761. 10.1038/338760a0 [Google Scholar]
- Harshman L.G, Clark A.G. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998;52:1334–1341. doi: 10.1111/j.1558-5646.1998.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Head M.L, Hunt J, Jennions M.D, Brooks R. The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol. 2005;3:289–294. doi: 10.1371/journal.pbio.0030033. 10.1371/journal.pbio.0030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick P.W. Science Books International; Boston: 1983. Genetics of populations. [Google Scholar]
- Holland B, Rice W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. 10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D.J, Garner T.W.J, Ward P.I. Sexual conflict selects for male and female reproductive characters. Curr. Biol. 2001;11:489–493. doi: 10.1016/s0960-9822(01)00146-4. 10.1016/S0960-9822(01)00146-4 [DOI] [PubMed] [Google Scholar]
- Imhof M, Harr B, Brem G, Sclotterer C. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol. Ecol. 1998;7:915–917. doi: 10.1046/j.1365-294x.1998.00382.x. 10.1046/j.1365-294x.1998.00382.x [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Ryan M.J. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. 10.1038/350033a0 [Google Scholar]
- Linder J, Rice W.R. Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 2005;18:568–575. doi: 10.1111/j.1420-9101.2004.00872.x. 10.1111/j.1420-9101.2004.00872.x [DOI] [PubMed] [Google Scholar]
- Markow T.A. Reproductive behaviour of Drosophila melanogaster and D. nigrospiracula in the field and in the laboratory. J. Comp. Psychol. 1988;102:169–173. doi: 10.1037/0735-7036.102.2.169. 10.1037//0735-7036.102.2.169 [DOI] [PubMed] [Google Scholar]
- McKinney F, Derrickson S.R, Mineau P. Forced copulation in waterfowl. Behaviour. 1983;86:250–294. [Google Scholar]
- Møller A.P, Alatalo R.V. Good-genes effects in sexual selection. Proc. R. Soc. B. 1999;266:85–91. 10.1098/rspb.1999.0607 [Google Scholar]
- Orteiza, N., Linder J. E., Rice, W. R. In press. Sexy sons from remating do not recoup the direct costs of harmful male interactions in the Drosophila melanogaster laboratory model system. J. Evol. Biol (10.1111/j.1420-9101.2005.00923.x) [DOI] [PubMed]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; London: 1979. pp. 123–166. [Google Scholar]
- Partridge L, Fowler K. Non-mating costs of exposure to males in female Drosophila melanogaster. J. Insect Physiol. 1990;36:419–425. 10.1016/0022-1910(90)90059-O [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. Insect Physiol. 1987;33:745–749. 10.1016/0022-1910(87)90060-6 [Google Scholar]
- Partridge L, Hurst L.D. Sex and conflict. Science. 1998;281:2003–2008. doi: 10.1126/science.281.5385.2003. 10.1126/science.281.5385.2003 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. 10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Dangerous liaisons. Proc. Natl Acad. Sci. USA. 2000;97:12953–12955. doi: 10.1073/pnas.97.24.12953. 10.1073/pnas.97.24.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific Red Queen. Behav. Ecol. Sociobiol. 1997;41:1–10. 10.1007/s002650050357 [Google Scholar]
- Rice W.R, Linder J.E, Friberg U, Lew T.A, Morrow E.H, Stewart A.D. Inter-locus antagonistic coevolution as an engine of speciation: assessment with hemiclonal analysis. Proc. Natl Acad. Sci. USA. 2005;102(Suppl.1):6527–6534. doi: 10.1073/pnas.0501889102. 10.1073/pnas.0501889102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutt A.D, Siva-Jothy M.T. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc. Natl Acad. Sci. USA. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. 10.1073/pnas.101440698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man, 1871–1971. Heinemann; London: 1972. pp. 136–179. [Google Scholar]
- Wigby S, Chapman T. Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution. 2004;58:1028–1037. doi: 10.1111/j.0014-3820.2004.tb00436.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.