Abstract
Body size trends across environmental gradients are widely reported but poorly understood. Here, we investigate contrasting relationships between size (body mass) and depth in the scavenging and predatory demersal ichthyofauna (800–4800 m) of the North-east Atlantic. The mean size of scavenging fish, identified as those regularly attracted to baited cameras, increased significantly with depth, while in non-scavengers there was a significant decline in size. The increase in scavenger size is a consequence of both intra and inter-specific effects. The observation of opposing relationships, in different functional groups, across the same environmental gradient indicates ecological rather than physiological causes. Simple energetic models indicate that the dissimilarity can be explained by different patterns of food distribution. While food availability declines with depth for both groups, the food is likely to be in large, randomly distributed packages for scavengers and as smaller but more evenly distributed items for predators. Larger size in scavengers permits higher swimming speeds, greater endurance as a consequence of larger energy reserves and lower mass specific metabolic rate, factors that are critical to survival on sporadic food items.
Keywords: Northeast Atlantic, deep water, fishes, body size, scavengers, predators
1. Introduction
The size to which an organism grows relates to all aspects of its biology and determining what controls body size is a fundamental question in ecology (Atkinson & Sibly 1997). Distinct trends in body size over environmental gradients have been demonstrated in many taxa (e.g. Bergmann's rule in which endotherm size increases at higher latitudes), but understanding the factors controlling these patterns continues to tax ecologists (e.g. Thiel 1975; Peters 1983; Schmidt-Nielson 1984; Atkinson & Sibly 1997; Rex & Etter 1998). Various hypotheses have been proposed to explain body size patterns including both physiological (e.g. temperature, oxygen uptake: Pauly 1997; Chapelle & Peck 1999) and ecological (e.g. food supply: Thiel 1975) mechanisms. However, the presence of conflicting size patterns in related taxa (e.g. Rex & Etter 1998; Ashton & Feldman 2003) illustrates the complexity of the problem and demonstrates that no single factor can account for all the observed patterns. A pragmatic approach suggests that while physiological responses to physical variables, such as temperature and oxygen, may limit body size for a given taxa, ecological factors will operate within these physiological constraints.
Within the deep marine environment, patterns of changing animal size with depth have been demonstrated in invertebrates (Thiel 1975; Haedrich & Rowe 1977; Rex & Etter 1998; Olabarria & Thurston 2003) and fish (Polloni et al. 1979; Macpherson & Duarte 1991). Early studies of the demersal deep-sea ichthyofauna indicated a general pattern of increased size with depth (Polloni et al. 1979), which came to be known as Heincke's Law (following Heincke's (1913) description of the size of plaice in the North Sea). However, subsequent work demonstrated that the phenomenon was not ubiquitous (Snelgrove & Haedrich 1985), may be an artefact of sampling (Merrett et al. 1991b) and in some regions a decline in size with depth has been reported (Stefanescu et al. 1992).
Since increased depth is accompanied by changes in hydrostatic pressure, light, temperature and food availability (Gage & Tyler 1991), it may be difficult to determine which variable(s) influence size. The observed trends of both increased and decreased size with depth have been associated with the need to maintain a viable population size in the face of reduced overall energy availability (Thiel 1975; Gage 1978), mass-specific changes in metabolic rate (Rex & Etter 1998) and changes in the relative importance of mobility (Haedrich & Rowe 1977). As the importance of each of these factors depends upon the behaviour and ecology of the species involved, it is not surprising that a clear and consistent pattern has not, so far, emerged.
To date, studies of demersal fish have focused on patterns in the whole community or individual species and have not examined trends within functional groups. Here, we examine trawl and camera data on demersal fish, over a very large depth range (800–4800 m), from the Porcupine Seabight (PSB) and Abyssal Plain (NE Atlantic) to examine the relationship between size and depth and consider how it differs between functional groups. Simple energetic models are developed to examine differences in the patterns of body size with depth in scavengers and predators and to consider the consequences of the reduction in food supply with increased depth (Lampitt et al. 1986).
2. Material and methods
Between September 2000 and October 2002 five cruises were undertaken on RRS Discovery to investigate the ichthyofauna of the PSB and Porcupine Abyssal Plain (PAP), SW of Ireland (see Rice et al. (1991, 1994) for details of the study sites). Three cruises were undertaken in the late summer/autumn (250, 255, 262) period and two during spring (252, 266). An additional cruise was undertaken on RRS Challenger in August 1997.
During each cruise the demersal fauna was sampled using a 45-foot (13.7 m) semi-balloon otter trawl (OTSB). The OTSB is shot on twin warps with 120 kg otter boards and transferred to a single warp once the doors have spread (Merrett & Marshall 1981). Haul duration varied with depth, but was typically 30 min (bottom contact) at the shallowest stations increasing to 3 h on the abyssal plain. The trawl was towed at a speed of 2.25–3 knots. Swept area was calculated (during RRS Discovery cruises only) from the nominal wing-spread of the trawl (8.6 m) and the distance of bottom contact.
The catch was sorted immediately. Fishes were identified using published texts (e.g. Whitehead et al. (1984)), measured, weighed and sexed. Body mass was measured to the nearest gram using a Pols motion compensated balance. In certain cases (e.g. damage), fish mass was not recorded and was estimated from significant length–mass regressions calculated from specimens of the same species. Since length measurements vary between species (following ichthyological convention) body mass was used as an indication of size and the log10 of body mass of individual fish was used in subsequent analysis.
Species were classified as scavengers if they were attracted to baited camera deployments in the PSB or on the PAP (Priede et al. 1994; Collins et al. 1999; unpublished data collected during cruises). Bathymetric trends were examined in fish species captured at 10 or more stations and with the total number caught exceeding 100. For species analyses only stations that included three or more individuals from a species were included. Statistical analyses were undertaken using MINITAB (Release 13). For statistical analyses log10 transformations were made to the biomass and abundance data.
Simple energetic models were developed to explore the potential role of food distribution and abundance in influencing different patterns in body size seen in scavenging and non-scavenging fish.
3. Results
(a) Analysis of trawl data
Sixty-one trawl stations were undertaken in the PSB and at PAP (see electronic appendix) which caught 8856 specimens of demersal fish, belonging to 76 species and 29 families. The dominant families were the Macrouridae (13 species; 3246 individuals), Alepocephalidae (16; 297) and Synaphobranchidae (2; 3173) and the 10 most abundant species are listed in table 1. The principal scavenging species (identified from previous (Priede et al. 1994; Collins et al. 1999) and concurrent baited camera studies at the PSB and PAP site were the eels, Synaphobranchus kaupi and Histiobranchus bathybius; the morid, Antimora rostrata; the ophidiid, Spectrunculus grandis; the grenadier Coryphaenoides armatus; the Portuguese shark Centroscymnus coelolepis and the hagfish Myxine ios.
Table 1.
Regression parameters for relationships between size (dependent) and depth (predictor) for scavenging and non-scavenging fish and the 10 most abundant species caught in the Porcupine Seabight.
| species | intercept | slope | N (hauls) | n (fish) | F | p | r2 |
|---|---|---|---|---|---|---|---|
| All fish | 1.386 | 2.65×10−4 | 61 | 8845 | 69.03 | <0.001 | 0.54 |
| Scavengers (mean) | 0.622 | 5.86×10−4 | 58 | 4533 | 165.37 | <0.001 | 0.75 |
| Scavengers (max) | 1.364 | 5.45×10−4 | 58 | 4533 | 46.64 | <0.001 | 0.46 |
| Scavengers (min) | −0.232 | 5.80×10−4 | 58 | 4533 | 106.90 | <0.001 | 0.66 |
| Antimora rostrata (ANR) | −1.567 | 1.88×10−3 | 24 | 304 | 481.73 | <0.001 | 0.96 |
| Coryphaenoides armatus (COA) | 1.328 | 4.04×10−4 | 27 | 248 | 248.3 | <0.001 | 0.88 |
| Synaphobranchus kaupi (SYK) | −0.431 | 1.21×10−3 | 28 | 3094 | 78.33 | <0.001 | 0.75 |
| Non-scavengers | 2.033 | −5.64×10−5 | 61 | 4312 | 4.42 | 0.040 | 0.07 |
| Bathypterois dubius (BPD) | 0.869 | 5.23×10−4 | 11 | 126 | 5.671 | 0.041 | 0.39 |
| Coryphaenoides guntheri (COG) | 0.963 | 4.18×10−4 | 24 | 1165 | 38.73 | <0.001 | 0.64 |
| Coryphaenoides leptolepis (COL) | 2.965 | −3.56×10−4 | 11 | 190 | 9.070 | 0.015 | 0.50 |
| Coryphaenoides rupestris (COR) | 2.049 | 3.20×10−4 | 19 | 439 | 1.045 | 0.321 | 0.06 |
| Halosauropsis machrochir (HAM) | 2.176 | 9.01×10−6 | 16 | 229 | 0.024 | 0.878 | 0.00 |
| Lepidion eques (LEE) | 1.576 | 7.56×10−5 | 17 | 764 | 0.052 | 0.822 | 0.00 |
| Nezumia aequalis (NEA) | 1.192 | 3.50×10−4 | 11 | 261 | 0.884 | 0.372 | 0.09 |
Regression data are also presented for the minimum and maximum size of scavengers. Significant regressions in bold.
Abundance of both scavenging and non-scavenging fish declined significantly with depth (figure 1a) and there was no difference in the slopes (ANCOVA: F=1.53, p=0.129) or elevations (ANCOVA: F=1.98, p=0.163) of the regression lines. The high abundance value of scavengers came from a trawl at 1200 m, which caught 1086 specimens of the eel S. kaupi. In non-scavengers biomass declined with depth (F=141.23; p<0.001), while in scavengers there was a slight, but not significant, increase with depth (F=19.30, p=0.106; figure 1b). The difference between scavenger and non-scavenger biomass at each station (log10 scavenger−log10 non-scavenger) increased significantly with depth (F=66.91, p<0.001). The peak in non-scavenger biomass seen in a trawl at 1541 m included over 150 specimens of the roundnose grenadier (Coryphaenoides rupestris).
Figure 1.
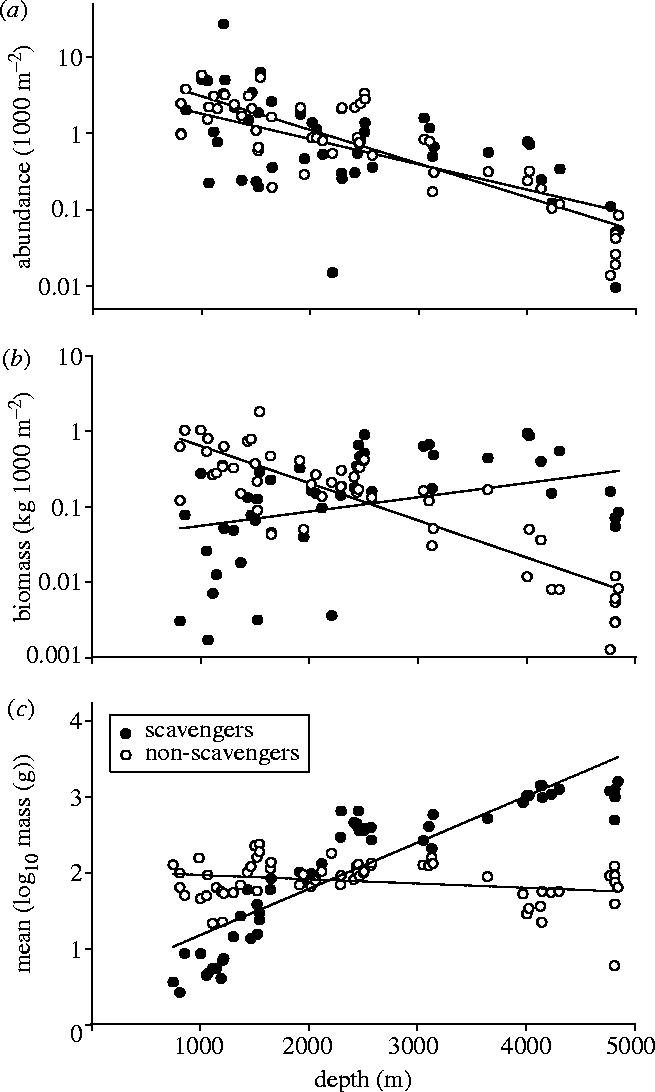
Relationships between (a) abundance, (b) biomass and (c) mean size (log10 body mass) and depth for scavenging and non-scavenging demersal deep-sea fish in the Porcupine Seabight and Abyssal Plain.
The size of scavenging fish increased significantly with increased depth, while the size of non-scavenging fish showed a slight, but significant, decline with increased depth (figure 1c; table 1) and the slopes of the regressions differed significantly (ANCOVA: F=159.01; p<0.001). With increased depth there was a significant increase in both minimum and maximum size of scavenging fish (table 1).
Patterns of size, biomass and abundance were examined in the three dominant species of scavenger (C. armatus; A. rostrata; S. kaupi) (figure 2). In each species, there was a significant increase in size with depth, which can be quantified by the slope of the relationship between the log10 body mass and depth (table 1). Biomass in each species was maximal towards the deeper end of the depth range, where the larger fish occurred, with abundance higher at the shallower end of the range in both C. armatus and S. kaupi.
Figure 2.
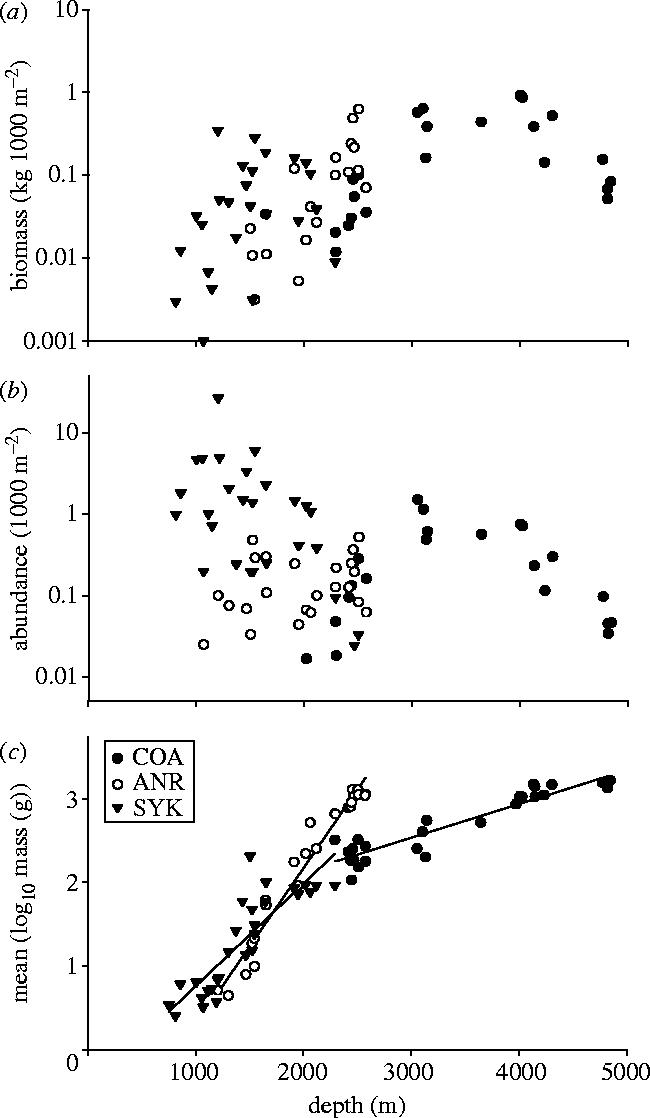
Relationships between (a) abundance, (b) biomass and (c) mean size (log10 body mass) and depth in the three dominant scavenging species (Coryphaenoides armatus (COA), Synaphobranchus kaupi (SYK) and Antimora rostrata (ANR)) in the Porcupine Seabight and Abyssal Plain.
Relationships between size and depth were investigated in the 10 most abundant fish species (including the three dominant scavengers; table 1; figure 3). Among the non-scavenging species, a significant increase in size with depth was detected in Coryphaenoides guntheri and Bathypterois dubius, but the increase in size with depth (slope) was considerably lower than in scavenging species. There was a significant decline in size with depth in Coryphaenoides leptolepis, but no relationship between size and depth in the other species.
Figure 3.
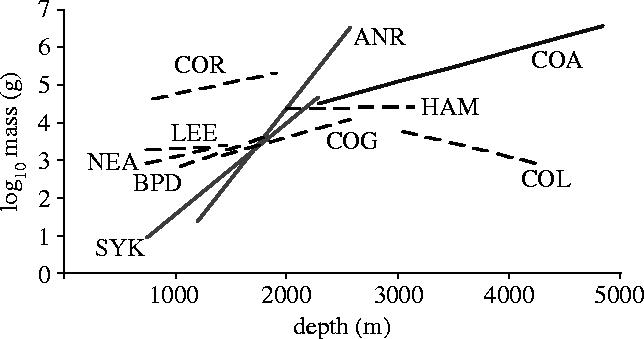
Body mass-depth relationships for the 10 most abundant species of demersal fish caught in the Porcupine Seabight and Abyssal Plain (heavy line=scavengers; broken line=non-scavengers); see table 1 for species codes.
(b) Model development and predictions
(i) Non-scavengers
First we assume that, at a given depth, food is evenly distributed across the sea-floor at energy density e; that a predator travels at speed v, can detect food within a distance d and so sweeps out an area of 2dv. In so doing it uses up energy at its standard metabolic rate R plus an increment due to the added energetic costs associated with foraging S. Hence, the energetic requirement for a positive energy balance is such that
| 3.1 |
which can be rearranged to give
| 3.2 |
We must now consider how the right side of this equation is affected by the mass of the non-scavenger. Following Ruxton & Houston (2004), we assume that the added cost of foraging is a constant multiple (likely to be around 2) of the standard metabolic rate R. Standard metabolism will increase with mass to a power of around 0.75. Since detection distance is likely to be determined principally by the physical properties of the water, it is reasonable to consider that maximum food detection distance is independent of the mass of the fish. The search speed of the fish is likely to increase with mass, but will not do so as rapidly as R; Ruxton & Houston (2004) suggest that it might scale as mass to the power 0.17. Taken together this indicates that the right side of equation (3.2) increases with fish mass (since the numerator increases more rapidly with increasing mass than the denominator). Hence, only fish below some maximum size can achieve a positive energy balance. Further, it is easy to see that, as food density (e) declines, so does this maximum size. Hence, we predict that the maximum size of non-scavenging fish should decline with increasing depth.
(ii) Scavengers
If we assume that, having discovered a food source, the scavenger is able to completely replenish its energy stores, giving it a supply of energy E. It then travels at speed v across the sea-bed, looking for another meal. During this time, it uses energy from its store to fuel both its standard metabolism R and the added cost of foraging S. Assuming that food items are randomly scattered over the sea-bed at density ρ and can be detected if the fish passes within distance d of them, by simple geometric reasoning (see e.g. Gerritsen & Strickler (1977)), the probability of finding another meal before the energy reserves from the last meal are exhausted is given by:
| 3.3 |
If ρ declines with increasing depth, then to keep this probability constant, the term
| 3.4 |
must increase sufficiently to compensate. From our arguments for non-scavengers we expect the denominator of (3.4) to increase with mass to a power of around 0.75 (but certainly less than one). However, it would be reasonable to assume that energy reserves occupy a constant fraction of a fish's body and so increase with mass to the power one (in fact, there is empirical evidence for a power greater than one (Stein & Pearcy 1982)). Search speed is likely to increase with mass and detection distance is likely to be relatively insensitive to mass. Taken together we expect that (3.4) will increase in magnitude for fish of increasing mass. Hence, to maintain a fixed probability of surviving from one meal to the next, in the face of declining food density (declining ρ), demands fish of increasing mass.
Thus, for scavengers we predict that for any energy density, there will be a minimum fish size below which survival from one meal to the next becomes improbable. Further, we expect this minimum size to increase with declining food density and so body mass of scavengers would be expected to increase with increasing depth. As a result, body size in scavengers and non-scavengers is controlled in opposite ways. Scavengers must maintain a minimum body size in order to maintain endurance, while the maximum potential size of predators (non-scavengers) is progressively constrained.
4. Discussion
In contrast to terrestrial and shallow marine habitats, environmental gradients in the deep-sea are temporally very stable (Gage & Tyler 1991), which potentially makes it easier to detect relationships with body size. The data presented here indicate clear differences in the size-depth trends between scavenging and non-scavenging demersal fish. However, before considering explanations for the different trends it is important to understand the limitations of the data. Merrett et al. (1991a,b) demonstrated that the large, active fish of the upper slope of the PSB were able to avoid small trawls, leading to a distinct bias in the data. This study, which used the same gear, is almost certainly subject to the same bias, however, we argue that these fish are, with the exception of the shark C. coelolepis, not scavengers. The failure to catch the larger fish of the upper slope would therefore, result in an under-estimate of the decline in size with depth of the non-scavengers. C. coelolepis is a large scavenger, found at 685–1270 m (Merrett et al. 1991a), which does not fit with the general trend of increased scavenger size with depth presented here. For reasons that are not yet clear, controls on the depth distribution of the Classes Chondrichthyes and Osteichthyes appear to differ, but given the wide anatomical, behavioural and physiological differences between bony and cartilaginous fishes, the differences in distribution are perhaps not surprising. A further potential bias is that size frequency distributions may simply reflect recent recruitment history (see McClain & Rex 2001), however, there was a significant increase in the maximum size of scavengers and within scavenging species there are clear patterns of size increase with depth, indicating recruitment occurs at the shallow end of the depth distribution.
Although a range of physical parameters (light, temperature, pressure) vary between 800 and 4800 m, the fact that opposing size–depth patterns are seen in different functional groups in the same habitat clearly indicates an ecological rather than purely physiological explanation and this is supported by the theoretical predictions. The key difference between scavengers and non-scavengers is the distribution of the available food, which we assume to be uniformly distributed for non-scavengers (Ruhl & Smith 2004), but aggregated into large, sparsely-distributed packages for scavengers (Smith & Baco 2003). The theoretical predictions assume a decline in food availability with depth for both functional groups. Lampitt et al. (1986) showed a logarithmic decline in megabenthos biomass from 800 to 4100 m in the PSB, which is a proxy for predator food availability. The distribution of carrion is not so well described (Stockton & Delacca 1982; Jones et al. 1998; Smith & Baco 2003), but is likely to decline with distance from shore and hence, usually with depth. Consequently, the relative selective pressures for swimming speed, nutrient storage and metabolic economy will differ greatly between scavengers and non-scavengers, even when these species are closely related (note that the four species of Coryphaenoides show positive, negative and no relationship with depth; figure 3). Scavengers are probably able to trade-off a high absolute energy requirement against the ability for faster swimming and greater endurance because of the high food value of carrion. These attributes allow the fish to arrive at carrion before it has been consumed (Haedrich & Rowe 1977) and increase their chances of reaching another meal before starving (Peters 1983). In contrast, predators in such low-food environments must reduce absolute costs to a minimum and, therefore, larger body sizes cannot be sustained. Endurance is not so important, as another food item is likely to be found relatively soon, but as each food item is of lower value they must continually balance energy inputs and outputs.
The data reported here are difficult to compare with other regions, as no other studies have contrasted scavengers and non-scavengers and no study has combined such extensive trawl sampling (and body mass measurements) with the baited camera data required to discriminate between these functional groups. Without these behavioural data it is difficult to know which functional group a fish belongs to. Not all individuals of the same species have the same behaviour, with ecology varying geographically (e.g. the macrourid Chalinura mediterranea and shrimp Acanthephyra eximia are scavengers in the Mediterranean (Jones et al. 2003), but are not attracted to bait in the NE Atlantic) and with ontogeny. Within many scavenging species there is a switch from a generalist diet to scavenging with increased size and that appears to be the case for the three main scavenging species in the PSB. Small individuals of A. rostrata and C. armatus are not attracted to baited cameras at depths, where they are abundant in trawls (Priede et al. 1994; Collins et al. 1999) and this is supported by the limited diet data (Mauchline & Gordon 1984; Martin & Christiansen 1997). Small S. kaupi are attracted to baited cameras (Priede et al. 1994), but diet studies do indicate an increase in dependence on scavenging in larger fish (Gordon & Mauchline 1996). This supports the prediction that at a particular food density, there will be a threshold minimum size necessary to support a scavenging lifestyle. Small individuals may not be able to compete with larger conspecifics as scavengers and hence, occupy a different niche. The model assumes that the scavenging species are obligate scavengers and although Britton & Morton (1994) suggested that there are no obligate scavengers in the marine environment, it has been demonstrated that it is theoretically energetically possible for a mobile fish to be an obligate scavenger (Ruxton & Houston 2004; Ruxton & Bailey 2005). However, we can see no evolutionary pressure that would force a fish to give up the ability to take some live prey, suggesting that a predominant scavenger is more likely than an obligate one.
Intra-specific bigger–deeper trends have been identified in this study and in previous work (e.g. Macpherson & Duarte 1991), in both scavenging and non-scavenging species, but the slopes of the relationships between size and depth are greater in scavenging species. It has been argued that the size–depth pattern should be described as shallower–smaller rather than bigger–deeper (Merrett et al. 1991a), however, our data indicate that, for scavengers in general and for individual scavenging species, both minimum and maximum size increase with depth, indicating a genuine bigger–deeper trend.
The bathymetric succession of scavenging species is probably a consequence of inter-specific competition, with C. armatus unable to compete with the more active A. rostrata (Collins et al. 1999) and with the shallower S. kaupi even more active (Bailey et al. 2005b). Conversely, with increasing depth, the more active fish are unable to find enough food to support their higher metabolism (Bailey et al. 2005b). In a multi-species scavenging assemblage like that in PSB/PAP the larger scavenging fish probably inhabit deeper water to avoid competition with both smaller fish of their own species and large individuals of the ‘shallower’ scavengers. Both of these groups will lack the endurance necessary to survive at greater depth because either their energy stores will be too small or their metabolic rates will be too high. This scenario is similar to that seen in seabirds across a productivity gradient, where competition and energetic constraints mean that larger birds forage in productive areas, with smaller birds in the less productive locations (Ballance et al. 1997).
Our data are predominantly for teleost fish and as noted for elasmobranchs the patterns may differ between taxa. Theoretically the advantages of large size will also apply to invertebrate scavengers and many of the large deep-sea invertebrates are also scavengers (e.g. the scavenging amphipod Eurythenes gryllus and the lithodid crab Neolithodes grimaldii). In the Mediterranean, the decapod shrimp A. eximia is regularly attracted to bait, with larger individuals found at greater depths (Bailey et al. 2005a). Gastropod taxa also show both increases and decreases in size with depth, which may be related to the ecology of the species (Rex & Etter 1998; Olabarria & Thurston 2003).
In this study, we have demonstrated the power of comparing different functional groups to generate hypotheses about the factor or factors influencing variation in individual size with depth and how these hypotheses can be investigated with simple mathematical models. The clear differences in body size patterns with depth in the two functional groups demonstrate that, within the physiological boundaries, the ecology of a species can profoundly influence the optimal size of an organism across an environmental gradient. Understanding the patterns of body size across environmental gradients thus requires an understanding of both the ecology and physiological constraints of the species concerned.
Acknowledgments
The authors would like to thank the officers, crew and UKORS technicians on RRS Discovery cruises 250, 252, 255, 260 and 266. Thanks also to the many people involved with the trawling and catch processing including Louise Allcock, Emma Battle, Dave Billett, Ben Boorman, Amanda Brindley, Camila Henriques, Ian Hudson, Kirsty Kemp, Rob McAllen, Dan Mayor, Nigel and Colleen Merrett, Richard Paterson, Liz White and Ben Wigham. DMB and the fieldwork on which this study was based were supported by a Natural Environment Research Council grant (GR3/12789) to MAC and IGP. DMB is currently supported by a Marie Curie Outgoing International Fellowship (MCOIF-CT-2004-509286). Thanks to Peter Rothery for statistical advice and to Lloyd Peck and two anonymous referees for constructive comments on the manuscript.
Supplementary Material
References
- Ashton K.G, Feldman C.R. Bergmann's rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution. 2003;57:1151–1163. doi: 10.1111/j.0014-3820.2003.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Atkinson D, Sibly R.M. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997;12:235–239. doi: 10.1016/s0169-5347(97)01058-6. 10.1016/S0169-5347(97)01058-6 [DOI] [PubMed] [Google Scholar]
- Bailey D.M, Bagley P.M, Jamieson A.J, Cromarty A, Collins M.A, Tselepides A, Priede I.G. Life in a warm deep sea: routine activity and burst swimming performance of the shrimp Acanthephyra eximia in the abyssal Mediterranean. Mar. Biol. 2005a;146:1199–1206. 10.1007/s00227-004-1525-1 [Google Scholar]
- Bailey D.M, Genard B, Collins M.A, Rees J.F, Unsworth S, Battle E.V, Bagley P.M, Jamieson A.J, Priede I.G. High swimming performance and metabolic activity in the deep-sea eel Synaphobranchus kaupi, revealed by integrated in situ and in vitro measurements. Physiol. Biochem. Zool. 2005b;78:335–346. doi: 10.1086/430042. 10.1086/430042 [DOI] [PubMed] [Google Scholar]
- Ballance L.T, Pitman R.L, Reilly S.B. Seabird community structure along a productivity gradient: importance of competition and energetic constraint. Ecology. 1997;78:1502–1518. [Google Scholar]
- Britton J.C, Morton B. Marine carrion and scavengers. Oceanogr. Mar. Biol. Ann. Rev. 1994;32:369–434. [Google Scholar]
- Chapelle G, Peck L.S. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. 10.1038/20099 [Google Scholar]
- Collins M.A, Priede I.G, Bagley P.N. In situ comparison of activity in two deep-sea scavenging fishes occupying different depth zones. Proc. R. Soc. B. 1999;266:2011–2016. 10.1098/rspb.1999.0879 [Google Scholar]
- Gage J. Animals in deep-sea sediments. Proc. R. Soc. Edin. 1978;76B:77–93. [Google Scholar]
- Gage J.D, Tyler P.A. Cambridge University Press; 1991. Deep sea biology. A natural history of organisms at the deep-sea floor. [Google Scholar]
- Gerritsen J, Strickler J.R. Encounter probabilities and community structure in zooplankton: a mathematical model. J. Fish. Res. Board Can. 1977;34:73–82. [Google Scholar]
- Gordon J.D.M, Mauchline J. The distribution and diet of the dominant slope dwelling eel, Synaphobranchus kaupi, of the Rockall Trough. J. Mar. Biol. Assoc. UK. 1996;76:493–503. [Google Scholar]
- Haedrich R.L, Rowe G.T. Megafaunal biomass in the deep sea. Nature. 1977;269:41–42. [Google Scholar]
- Heincke F. Untersuchungen über die Scholle-Generalbericht I. Schollenfischerei und Schonmassregeln. Vorlaeufige kurze Uebersicht über die wichtigsten Ergebnisse des Berichts. Rapport et Procès-verbaux des Réunions du ICES. 1913;16:1–70. [Google Scholar]
- Jones E.G, Collins M.A, Bagley P.M, Addison S, Priede I.G. The fate of cetacean carcasses in the deep-sea: observations on consumption rates and succession of scavenging species in the abyssal North-east Atlantic. Proc. R. Soc. B. 1998;265:1119–1127. 10.1098/rspb.1998.0407 [Google Scholar]
- Jones E.G, Tselepides A, Bagley P.M, Collins M.A, Priede I.G. Bathymetric distribution of some benthic and benthopelagic species attracted to baited cameras and traps in the Eastern Mediteranean. Mar. Ecol. Prog. Ser. 2003;251:75–86. [Google Scholar]
- Lampitt R.S, Billet D.S.M, Rice A.L. Biomass of the invertebrate megabenthos from 500 to 4100 m in the northeast Atlantic. Mar. Biol. 1986;93:69–81. 10.1007/BF00428656 [Google Scholar]
- Macpherson E, Duarte C.M. Bathymetric trends in demersal fish size: is there a general relationship? Mar. Ecol. Prog. Ser. 1991;71:103–112. [Google Scholar]
- Martin B, Christiansen B. Diets and standing stocks of benthopelagic fishes at two bathymetrically different midoceanic localities in the northeast Atlantic. Deep-Sea Res. 1997;44:541–558. 10.1016/S0967-0637(97)00008-3 [Google Scholar]
- Mauchline J, Gordon J.D.M. Feeding and bathymetric distribution of the gadoid and morid fish of the Rockall Trough. J. Mar. Biol. Assoc. UK. 1984;64:657–665. [Google Scholar]
- McClain C.R, Rex M.A. The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: an application of quantile regression. Mar. Biol. 2001;139:681–685. 10.1007/s002270100617 [Google Scholar]
- Merrett N.R, Marshall N.B. Observations on the ecology of deep-sea bottom-living fishes collected off northwest Africa (08–27° N) Prog. Oceanogr. 1981;9:185–244. 10.1016/0079-6611(80)90002-6 [Google Scholar]
- Merrett N.R, Gordon J.D.M, Stehman M, Haedrich R.L. Deep demersal fish assemblage structure in the Porcupine Seabight (Eastern North Atlantic): Slope sampling by three different trawls compared. J. Mar. Biol. Assoc. UK. 1991a;71:329–358. [Google Scholar]
- Merrett N.R, Haedrich R.L, Gordon J.D.M, Stehman M. Deep demersal fish assemblage structure in the Porcupine Seabight (Eastern North Atlantic): Results of single warp trawling at lower slope to abyssal soundings. J. Mar. Biol. Assoc. UK. 1991b;71:359–373. [Google Scholar]
- Olabarria C, Thurston M.H. Latitudinal and bathymetric trends in body size of the deep-sea gastropod Troschelia berniciensis (King) Mar. Biol. 2003;143:723–730. 10.1007/s00227-003-1116-6 [Google Scholar]
- Pauly D. Geometrical constraints on body size. Trends Ecol. Evol. 1997;12:442. doi: 10.1016/s0169-5347(97)85745-x. 10.1016/S0169-5347(97)85745-X [DOI] [PubMed] [Google Scholar]
- Peters R.H. Cambridge University Press; 1983. The ecological implications of body size. [Google Scholar]
- Polloni P, Haedrich R.L, Rowe G.T, Clifford C.H. The size depth relationship in deep ocean animals. Int. Rev. Ges. Hydrobiol. 1979;64:39–46. [Google Scholar]
- Priede I.G, Bagley P.M, Smith A, Creasey S, Merrett N.R. Scavenging deep demersal fishes of the Porcupine Seabight, North-east Atlantic: Observations by baited camera, trap and trawl. J. Mar. Biol. Assoc. UK. 1994;74:481–498. [Google Scholar]
- Rex M.A, Etter R.J. Bathymetric patterns of body size: implications for deep-sea biodiversity. Deep-Sea Res. II. 1998;45:103–127. 10.1016/S0967-0645(97)00082-9 [Google Scholar]
- Rice A.L, Billet D.S.M, Thurston M.H, Lampitt R.S. The Institute of Oceanographic Sciences Biology programme in the Porcupine Seabight: background and general introduction. J. Mar. Biol. Assoc. UK. 1991;71:281–310. [Google Scholar]
- Rice A.L, Thurston M.H, Bett B.J. The IOSDL DEEPSEAS programme: introduction and photographic evidence for the presence and absence of a seasonal input of phytodetritus at contrasting abyssal sites in the north-eastern Atlantic. Deep-Sea Res. 1994;41:1305–1320. 10.1016/0967-0637(94)90099-X [Google Scholar]
- Ruhl H.A, Smith K.L. Shifts in deep-sea community structure linked to climate and food supply. Science. 2004;305:513–515. doi: 10.1126/science.1099759. 10.1126/science.1099759 [DOI] [PubMed] [Google Scholar]
- Ruxton G.D, Bailey D.M. Searching speeds and the energetic feasibility of an obligate whale-scavenging fish. Deep-Sea Res. I. 2005;52:1536–1541. 10.1016/j.dsr.2005.02.008 [Google Scholar]
- Ruxton G.D, Houston D.C. Energetic feasibility of an obligate marine scavenger. Mar. Ecol. Prog. Ser. 2004;266:59–63. [Google Scholar]
- Schmidt-Nielson K. Cambridge University Press; 1984. Scaling: why is animal size so important. [Google Scholar]
- Smith C.R, Baco A.R. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Ann. Rev. 2003;41:311–354. [Google Scholar]
- Snelgrove P.V.R, Haedrich R.L. Structure of the deep demersal fish fauna off Newfoundland. Mar. Ecol. Prog. Ser. 1985;27:99–107. [Google Scholar]
- Stefanescu C, Rucabado R, Lloris D. Depth–size trends in western Mediterranean demersal deep-sea fishes. Mar. Ecol. Prog. Ser. 1992;81:205–213. [Google Scholar]
- Stein D.L, Pearcy W.G. Aspects of reproduction, early life history and biology of macrourid fishes off Oregon, USA. Deep-Sea Res. 1982;11A:1313–1329. 10.1016/0198-0149(82)90011-5 [Google Scholar]
- Stockton W.L, Delacca T.E. Food falls in the deep-sea: occurrence, quality and significance. Deep-Sea Res. 1982;29:157–169. 10.1016/0198-0149(82)90106-6 [Google Scholar]
- Thiel H. The size structure of the deep-sea benthos. Int. Rev. Ges. Hydrobiol. 1975;60:575–606. [Google Scholar]
- Whitehead P.J.P, Bauchot M.-L, Hureau J.-C, Nielsen J, Tortonese E. UNESCO; Paris: 1984. Fishes of the North-eastern Atlantic and Mediterranean. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


