Abstract
Thyroid hormone signaling during a postnatal period in the mouse is essential for cochlear development and the subsequent onset of hearing. To study the control of this temporal dependency, we investigated the role of iodothyronine deiodinases, which in target tissues convert the prohormone thyroxine into triiodothyronine (T3), the active ligand for the thyroid hormone receptor (TR). Type 2 5′-deiodinase (D2) activity rose dramatically in the mouse cochlea to peak around postnatal day 7 (P7), after which activity declined by P10. This activity peak a few days before the onset of hearing suggests a role for D2 in amplifying local T3 levels at a critical stage of cochlear development. A mouse cochlear D2 cDNA was isolated and demonstrated near identity to rat D2. In situ hybridization localized D2 mRNA in periosteal connective tissue in the modiolus, the cochlear outer capsule and the septal divisions between the turns of the cochlea. Surprisingly, D2 expression in these regions that give rise to the bony labyrinth was complementary to TR expression in the sensory epithelium. Thus, the connective tissue may control deiodination of thyroxine and release of T3 to confer a paracrine-like control of TR activation. These results suggest that temporal and spatial control of ligand availability conferred by D2 provides an unexpectedly important level of regulation of the TR pathways required for cochlear maturation.
Keywords: development, thyroid hormone receptor, deiodinase, cochlea
One of the most sensitive functions controlled by thyroid hormone (TH) is the development of hearing, as is evident from the deafness associated with human congenital hypothyroidism. Experimental hypothyroidism in rodents has revealed that the cochlea is a major site of TH action and has shown that cochlear maturation and the onset of auditory function around postnatal day 14 (P14) require TH during a critical period between the late embryonic stage and the second postnatal week (1–3). This developmental window is strictly delineated, because TH can rescue cochlear morphogenesis and auditory function in hypothyroid mice or rats if provided during early postnatal stages but not after P10–P12. Little is known of the mechanisms that control this temporal regulation.
TH receptors (TR) play a critical role in the auditory system (4). TRα1 and TRβ, encoded by two related genes, are nuclear receptors that act as ligand-dependent transcription factors (5, 6). TRα1 and TRβ are expressed in the cochlea with TRβ mRNA being prominent both in the embryonic otic vesicle and in the postnatal sensory epithelium and spiral ganglion (7–9). TRβ-deficient mice and humans are severely deaf, as assessed by defective auditory-evoked brainstem responses (10, 11), whereas TRα1-deficient mice have a normal auditory-evoked brainstem response (12). Mild hearing defects occur in ≈20% of cases of the human syndrome of resistance to thyroid hormone, which is associated with TRβ point mutations (13).
The control of ligand availability in target tissues constitutes another level of regulation of TR signaling. Thyroxine (T4), the major form of hormone produced by the thyroid gland, must be converted by deiodination into triiodothyronine (T3), the active hormone that binds the TR. In target tissues, T3 levels are regulated by three monodeiodinase isoenzymes that contain the uncommon amino acid selenocysteine (14, 15). Type 1 deiodinase (D1) catalyzes outer (5′-) or inner ring (5-) deiodination of T4, T3, and reverse T3 (rT3). D1 is present in the kidney, liver, and pituitary and thyroid glands, and it is thought to be a main producer of circulating T3. Type 2 5′-deiodinase (D2) converts T4 to T3 and is present primarily in the central nervous system, brown adipose tissue, and pituitary gland, where it is believed to be the principal generator of receptor-bound T3 (16, 17). Type 3 5-deiodinase (D3), abundant in placenta and the central nervous system, converts T4 to rT3 and T3 to 3,3′-diiodothyronine (T2). D3 is considered as an inactivating enzyme because rT3 and T2 lack known biological activity and do not activate the TR.
Previous studies have suggested that deiodinases in target tissues may control local T3 levels in amphibian metamorphosis (18, 19) and rat brain development (20, 21). Therefore, given that the cochlea is particularly TH sensitive, we have characterized deiodinase function in the cochlea and have identified a striking D2 activity peak in the postnatal period before the onset of hearing. Furthermore, D2 mRNA was localized in the connective tissue that gives rise to the bony regions of the cochlea in a pattern that was noncoincident with TR expression in the membranous tissue, suggesting that separation of the cells that generate and respond to T3 provides an unusual means of regulation of TR activation in the cochlea. These findings suggest that D2 serves as an important trigger of the TR-dependent pathways required for maturation of the cochlea by controlling the local availability of T3 during development.
Materials and Methods
Tissue Sampling.
Mice were from the same mixed genetic background (C57BL/6J crossed with 129/Sv) as TRβ-deficient mice (22). Mice were decapitated and the temporal bones dissected out and placed in ice-cold PBS. The cochlear capsule was removed and the soft tissue, including the modiolus, organ of Corti, spiral ligament, and stria vascularis, was taken and immediately frozen on dry ice and stored at −85°C. At stages older than P10, most of the calcifying bone around the cochlea was removed, which unavoidably also removed parts of the lateral regions of the cochlear duct and stria vascularis; internal bony tissue in the modiolus and spiral lamina remained. For deiodinase assays, cochleae were pooled from age-matched litters, each of 5–10 pups. Animal experiments followed approved protocols at Mount Sinai School of Medicine and Georgetown University School of Medicine.
Cochlear D2 cDNA Cloning.
Approximately 250 pairs of P6 cochleae from C57BL/6J mice were pooled, and the total RNA was extracted for poly(A)-enrichment by using two rounds of purification with the poly(A) spin mRNA kit (New England Biolabs). mRNA (8 μg) yielded an unamplified cDNA library of ≈4.0 × 106 plaque-forming units in λZapII phage (Stratagene). A fragment of mouse D2 cDNA (nucleotides 19–696) was amplified from brain RNA by reverse transcription–PCR with primers based on the rat D2 sequence (23) and used as a probe to screen the cochlear library and a brain cDNA library (Stratagene). Positive clones were subjected to automated sequencing.
RNA Analysis and in Situ Hybridization.
Cochleae from ≈200 P8 pups were pooled and total RNA extracted by using Ultraspec reagent (Biotecx Laboratories, Houston). Northern blots were hybridized with mouse D2 and D1 cDNA fragments generated by PCR as probes. For in situ hybridization, pups were anesthetized with CO2 and killed by cervical dislocation. For whole-mount hybridization, cochleae were fixed in 4% paraformaldehyde overnight at 4°C, and then dehydrated through a graded methanol series and stored at −20°C. Before hybridization, the bony labyrinth and septal membranes were removed to expose the spiral lamina and sensory epithelium. For hybridization of sections, P4 pup heads were embedded, frozen, and sectioned horizontally at a 10-μm thickness. Sense and antisense digoxygenin-labeled RNA probes were synthesized from a 1.4-kb mouse D2 cDNA fragment (clone B1; see Results) or from a 0.7-kb D2 PCR fragment (codons 10–696; Fig. 2). A TRβ probe was generated from a full-length mouse TRβ1 cDNA in pGEM7 (24). Probes were hydrolyzed to a length of ≈750 bp before hybridization, which was performed essentially as described (25, 26). After the color reaction, whole-mounted cochleae were embedded, frozen, and sectioned at a 6-μm thickness for further analysis.
Figure 2.
(A) Nucleotide and deduced amino acid sequences of the mouse D2 cDNA. The sequence was closely related to rat D2 and differed at only three amino acids (beginning at nucleotides 304, 316, and 547) (23). The selenocysteine (Se) is encoded by the TGA codon (nucleotides 412–414). An ambiguous TGA codon that may represent a stop codon or potential second selenocysteine is noted by an asterisk at nucleotide 811. The selenocysteine insertion sequence (SECIS) element, that signals readthrough of the internal TGA codon by insertion of a selenocysteine residue, is located in 3′ untranslated sequences not present in these clones (33). (B) Individual cDNA clones isolated from brain (λB1) and cochlear (λC4, C6) libraries, from which the composite sequence above in A was assembled.
Tracers.
[5′-125I]T4, [5′-125I]rT3, and [3′-125I]T3 with specific activities of 40–50 MBq/nmol were obtained (Dupont/NEN). Inner-ring-labeled [3-125I]T3 (specific activity of 1–2 MBq/nmol) was purchased by Formula GmBH (Berlin). Tracers were repurified immediately before use with disposable Sep-Pak C18 cartridges (Waters), yielding a purity of >99% with 125I− as the only contaminant.
Hormone RIAs.
Serum hormone determinations.
Serum samples were pooled from at least three litter-matched animals for the indicated ages between P2 and P42 and stored at −80°C. Total T4 and T3 concentrations were determined as described (27). Total rT3 concentrations were evaluated with RIA by using an antiserum against rT3 (Bio-Trend, Cologne, Germany) with molar crossreactivities of 0.05, 0.03, and 0.012% for T4, T3, and 3,5-T2, respectively; standards were made in hormone-free mouse serum with high purity L-rT3 (Calbiochem). Intra-assay variation was 6.6% and the corresponding interassay variation was < 8%.
Tissue hormone determination.
T4 and T3 concentrations were determined in tissue extracts essentially as described (28). Briefly, pooled cochleae of four to seven age- and litter-matched pups were homogenized in 100% ethanol and centrifuged at 1,500 × g for 20 min at 4°C, and the pellet was reextracted with 1 ml of 100% ethanol. Combined supernatants were purified through Bio-Rad AG1x2 resin columns and iodothyronines eluted with 70% acetic acid, dried by evaporation, and taken up in RIA buffer. The limit of sensitivity was 3.0 and 1.5 pg for T4 and T3, respectively. Samples were assayed in duplicate at two different dilutions. Intra-assay variations were 5.4% for T4 and 6.9% for T3. Results were corrected on the basis of individual recovery data obtained after addition of maximum specific activity [125I]T3 to every sample during the initial extraction process. Recovery was between 80% and 90% for T3. Tissue concentrations are given as pmol of T4 or T3 per mg of DNA. DNA was assayed by using a fluorescent quantification kit (Bio-Rad).
Deiodinase Assays.
Pools of cochleae at the indicated ages were homogenized in 500 μl of ice-cold 0.25 M sucrose/10 mM Hepes, pH 7.0/10 mM DTT and immediately frozen on dry ice. Determination of deiodinase activities is based on the quantification of the release of 125I− from outer- or inner-ring (D3) 125I-labeled substrates (29) in the presence of DTT (Boehringer, Mannheim) and 6-n-propyl-2-thiouracil (PTU, Sigma) as described (30). Protein was assayed by using reagents from Bio-Rad and bovine γ-globulin as the standard.
Results
Characterization of Cochlear D2.
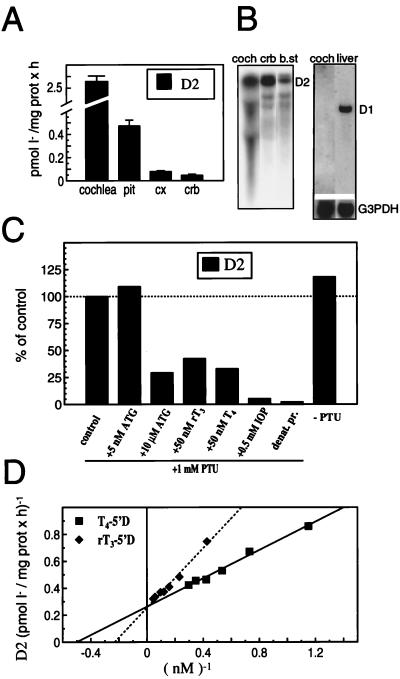
Despite the limited yields of material from mouse cochlea, analysis of cochlear homogenates from P6–P8 pups identified a 5′-deiodinase activity of high specific activity with the characteristics of D2. When compared with adult mouse brain and pituitary, considered as enriched sources of D2, P8 cochlear homogenates had significantly higher activity levels, being 5- and 20-fold higher than in the pituitary and cerebellum, respectively (Fig. 1A). The activity was temperature dependent, linear with time, proportional to protein concentration, and was inactivated by heat (15 min at 56°C), thus confirming its enzymatic nature. Northern blot analysis with a mouse D2 cDNA probe (see below) detected a relatively abundant major mRNA of ≈7 kb in cochlear total RNA (Fig. 1B).
Figure 1.
(A) D2 activity in mouse P8 cochlea, adult pituitary gland (pit), cerebral cortex (cx), and cerebellum (crb); mean of four to six determinations ± SEM. For cochlea D2 assays, samples from five to eight litter-matched pups were pooled. (B) (Left) Northern blot analysis of D2 mRNA in P8 cochlea (coch) total RNA (15 μg), and adult cerebellum (crb) and brainstem (b.st.) poly(A)-selected RNA (8 μg). (Right) D1 mRNA in P8 cochlea total RNA (15 μg) and adult liver poly(A)-selected RNA (6 μg); mRNA sizes: D2, ≈7 kb; D1, ≈1.7 kb; and glyceraldehyde 3P dehydrogenase (G3PDH), ≈1.5 kb. (C) Characterization of D2 activity in P8 cochlear homogenates. Aliquots of the same homogenate were incubated for 60 min at 37°C in a reaction mixture containing 3 nM [125I]T4/1 μM T3/25 mM DTT in the presence and absence of aurothioglucose, PTU, iopanoic acid, or additional T4 and rT3 at the indicated concentration. (D) Double reciprocal Lineweaver–Burk plot of the rate of T4 and rT3 deiodination by cochlea homogenates as a function of T4 or rT3 concentrations in the presence of 25 mM DTT and 1 mM PTU.
The deiodinase activity in the cochlear homogenates was confirmed as being caused by D2 by testing its substrate specificity, cofactor requirement, and inhibitor sensitivity (Fig. 1C). As reported for other tissue sources of D2 (31, 32), cochlear 5′-deiodinase required DTT as a cofactor at mM concentrations; it showed a preference for T4 over rT3 as substrate (Fig. 1D). The activity was insensitive to PTU, a specific inhibitor of D1, and was relatively insensitive to aurothioglucose, a selective inhibitor of D1 but not D2 at low (5 nM) concentrations (14). The activity was largely abolished by 0.5 mM iopanoic acid, an agent that inhibits all three deiodinases. Nanomolar concentrations of T4 and rT3 competitively inhibited the 5′-deiodination of each other, whereas the presence of high levels of T3 in the incubation mixture did not influence the 5′-deiodination of either T4 or rT3, indicating that T3 is not a substrate for the cochlear 5′-deiodinase.
The 5′-deiodination of T4 and rT3 by P8 cochlear homogenates followed simple saturation kinetics and resulted in linear Lineweaver-Burk plots (Fig. 1D). The apparent Km for both substrates was in the nM range (3.1 ± 0.3 nM and 4.4 ± 0.5 nM, for T4 and rT3, respectively; n = 3), with an apparent Vmax of 5.7 ± 1.2 and 4.0 ± 0.9 pmol I− per mg of protein per h for T4 and rT3, respectively, when measured in the presence of 25 mM DTT. By using the Vmax/Km ratio as an indicator of substrate specificity, T4 was the preferred substrate (1.8 vs. 0.9 for T4 vs. rT3).
The presence of D1 activity in the cochlea was also investigated with rT3 as substrate in the presence and absence of PTU that inhibits D1 but not D2 (31). Under these conditions, D1 activity is assessed as the portion of the reaction that is inhibited by PTU. The presence of 1 mM PTU inhibited <10–20% of the 5′-deiodination of rT3 at any postnatal age examined, indicating that the cochlea lacked significant D1 activity. Indeed, no D1 mRNA was detected by Northern blot analysis of cochlear RNA, whereas it was readily detected in liver (Fig. 1B). No D3 activity was detected in cochlear homogenates at any age examined (P2–P60), whereas, as expected, D3 was readily detected in brain as a positive control tissue (data not shown).
Characterization of a Cochlear D2 cDNA.
To confirm the expression of D2 in cochlea and characterize the mouse D2 sequence that has not been previously cloned, a D2 cDNA was isolated from a P6 mouse cochlear cDNA library (Fig. 2). The D2-predicted amino acid sequence was almost identical to that of rat D2 (23), including the conserved region surrounding the selenocysteine residue and the two His residues common to all three deiodinase isoenzymes. Clone C6 contained an initiation codon and diverged at a point corresponding approximately to the recently described junction between the two exons of the gene (33). The 3′ sequence of clone C6 corresponded to the distal 3′ untranslated region and included a poly(A) tail, indicating that this cDNA represented a splice variant. Although the splice points in the D2 gene have not been mapped definitively, the divergence point in clone C6 is probably slightly (33–38 bp) downstream from that indicated by Davey et al. (ref. 33; D. Schneider and D. St. Germain, personal communication), suggesting that an unusual splicing event generated the RNA represented by clone C6.
Developmental Profile of Cochlear D2 Activity.
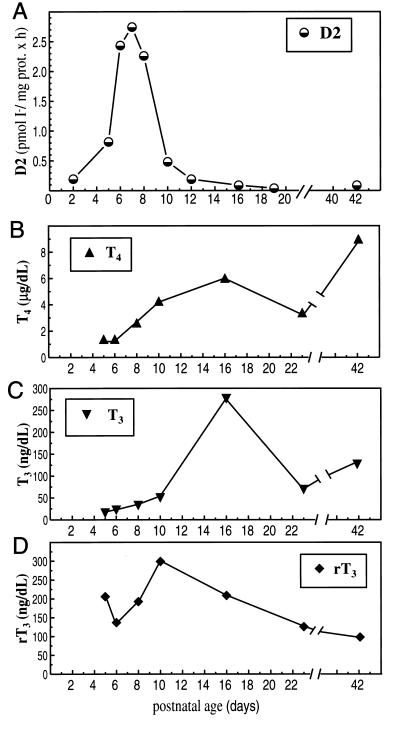
The profile of cochlear D2 activity was determined during postnatal development (Fig. 3A). D2 activity was detectable from P2, the earliest stage studied, and rose dramatically to peak around P7 with activity levels being 14-fold increased over the level at P2. After P8, activity declined sharply and by P12, it had reverted to low levels that were maintained into adulthood.
Figure 3.
(A) Developmental profile of D2 activity in mouse cochlear homogenates. Values are the mean of two to six determinations. For each determination, pools of cochleae (from five to eight litter-matched pups) were incubated in a mixture containing 3.5 nM [125I]T4/25 mM DTT/1 μM T3/1 mM PTU for 75 min at 37°C. (B–D) Developmental profiles of total T4, T3, and rT3 levels in mouse serum. Values are the means of two to five samples, each containing the pooled serum of three to eight litter-matched pups.
The profiles of cochlear D2 activity and circulating levels of TH over the postnatal period were compared (Fig. 3 B–D). Total T4 levels increased between P5 and P16 and declined slightly thereafter, until at P21, after weaning, concentrations began to increase to adult levels. This profile resembled that reported for estimated free T4 levels in mice (34). The total T3 profile paralleled that of T4. Levels of rT3 increased from P5 until P10 and then declined steadily to adult levels. Thus, the postnatal peak of D2 activity occurred when serum levels of T3, the active hormone, were relatively low (e.g., at P8, levels were only ≈25% those of adults) and possibly inadequate for the needs of the developing cochlea.
To assess whether the D2 activity in the cochlea influenced the local levels of T3 and T4, the concentrations of each were determined in serum and cochlear tissue at P2 and P8 (Table 1). Concentrations of T3 and T4 increased in both cochlear extracts and serum between P2 and P8. The 4- to 5-fold increase in T3 content in cochlear extracts was substantially higher than that in serum (2-fold) between P2 and P8, indicating the presence of an increasing T3 concentration gradient in the cochlea versus serum. The means of uptake of T4 and T3 in the cochlea are unknown and could conceivably contribute to this concentration gradient. However, the correlation of the increased cochlear T3 content with the cochlear D2 activity peak around P8 is consistent with a role for D2 in generating the concentration gradient. The high [T3]/[T4] molar ratio (≥ 1) at both P2 and P8 was comparable to that of D2-containing rat brain tissue and was significantly greater than that of non-D2-containing liver and kidney tissue (≤ 0.5) (17). These results are in agreement with a role for cochlear D2 in amplifying local T3 levels by 5′-deiodination of T4.
Table 1.
T3 and T4 concentrations and [T3]/[T4] molar ratios in cochlear tissue and serum of neonatal mice
| Age | Litters, n | Pups, n | Cochlea
|
Serum
|
||||
|---|---|---|---|---|---|---|---|---|
| T3, pmol/mg DNA | T4, pmol/mg DNA | [T3]/[T4] | T3, nmol/liter | T4, nmol/liter | [T3]/[T4] | |||
| P2 | 4 | 21 | 65.3 ± 9.3 | 57.4 ± 12.0 | 1.14 | 0.32 ± .07 | 7.1 ± 1.0 | 0.05 |
| P8 | 3 | 15 | 284.6 ± 47.3* | 161.8 ± 45 | 1.76 | 0.70 ± 0.1* | 33.7 ± 5.6* | 0.02 |
Values are the means ± SEM of three to four separate determinations, each representing the pooled cochlear tissue or pooled serum of four to seven litter-matched pups of the indicated ages.
*P < 0.05 vs. postnatal day 2 (P2).
Localization of D2 mRNA in the Cochlea.
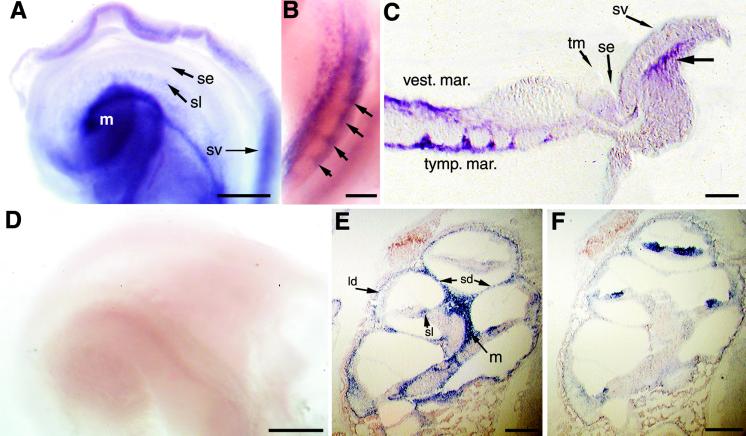
D2 mRNA was localized in the cochlea by in situ hybridization. D2 was predominantly detected in the periosteal connective tissue that gives rise to the bony labyrinth (Fig. 4). Analysis of P5 or P8 cochlear whole mounts detected D2 signals in the connective tissue that borders the lateral portions of the cochlear duct including in regions adjacent to the stria vascularis, and in the modiolus and both the vestibular and tympanic margins of the developing spiral lamina, that will develop into the lip of bone adjacent to the sensory epithelium (Fig. 4C). In the spiral lamina, expression was observed in two distinct parallel lines between which a periodic laddering occurred (Fig. 4 B and C). This pattern may correlate with the formation of the habenula perforata, a series of foramina, or pores, in the spiral lamina through which dendritic and axonal projections extend to and from the inner and outer hair cells.
Figure 4.
In situ hybridization analysis of D2 mRNA in mouse cochlea. (A) Whole-mount hybridization of P5 cochlea with D2 antisense probe, showing the surface of the basilar membrane viewed from the apex of the cochlea. Most of the surrounding bony labyrinth and Reissner's membrane were removed before hybridization. D2 was detected in the modiolus (m), spiral lamina (sl), and the connective tissue adjacent to the stria vascularis (sv). No specific signal was detected in the sensory epithelium (se). (B) The side view of the basal turn of a P8 cochlea after whole-mount hybridization, showing D2 expression in parallel bands in the vestibular and tympanic margins of the spiral lamina. Note between these bands the periodic ladder of signal (arrows) that may correlate with the formation of the habenula perforata. (C) Section of a P5 cochlea that had been subjected to whole-mount hybridization. D2 was detected in periosteal tissue at the vestibular (vest.) and tympanic margins (tymp. mar.) of the spiral lamina and in connective tissue (bold arrow) adjacent to the stria vascularis (sv); se, sensory epithelium; tm, tectorial membrane. (D) Whole-mount hybridization of P5 cochlea by using a D2 sense probe. (E and F) Hybridization of sections of P4 cochlea with D2 (E) and TRβ (F) antisense probes. D2 was detected in the lateral region of the cochlear duct (ld), the septal divisions (sd) between the scala tympani and scala vestibuli, and in the osseous spiral lamina (sl) and modiolus. TRβ was detected in the sensory epithelium. No specific signals were detected by using the D2 sense probe (not shown). [Bars = 100 μm (A and D), 50 μm (B), 50 μm (C), and 150 μm (E and F).]
Hybridization of sections of the cochlea yielded corresponding results. D2 signals were detected in the connective tissue of the temporal bone, in particular in the septal division between the scala vestibuli and the scala tympani, as well as in connective tissue in the modiolus (Fig. 4E). No specific hybridization was detected in the sensory epithelium. The pattern of D2 expression was nonoverlapping with that of TRβ which was predominantly in the sensory epithelium (Fig. 4F), as described in the rat (7). TRα1 mRNA has been localized in the sensory epithelium and spiral ganglion (7, 8). Thus, the pattern of D2 expression was largely complementary to that of both TRβ and TRα1.
The D2 distribution was confirmed by determination of D2 activity in homogenates of subdissected portions of the cochlea. In cochleae from P7 pups, activity was detected in the modiolus and spiral lamina (2.8 pmol of I− per mg protein per h) and separately, in the outer capsule and attached spiral ligament (2.7 pmol I− per mg protein per h).
Discussion
TH is essential for a postnatal phase of cochlear maturation before the onset of hearing. This temporal TH dependency may be delineated by several factors, including the expression of the TR genes in the cochlea (7, 11) and the increasing serum levels of T4 and T3 during development (Fig. 3). The present study demonstrates that D2, which converts T4 into T3, the ligand for the TR, exhibits a marked activity peak in the cochlea around P7, and suggests that D2 is likely to play a key role as a temporal trigger of the TR pathways required for cochlear development.
The remarkably abrupt peak of D2 expression and the relatively high T3 content in the P8 cochlea indicate a role for D2 in amplifying the local levels of T3 at a critical phase of development. A plausible role for D2 may be to uncouple the program of cochlear development from what may otherwise be inadequate circulating levels of T3 in the early postnatal period (Fig. 3). Increased T3 levels in the cochlea at this time may be required for the many maturational events that depend on TH, as indicated in hypothyroid animals: Hypothyroidism causes deformity of the tectorial membrane, poor differentiation of hair cells (1, 35), defective synapse formation (3), and impaired myelination (9). Also, deletion of TRβ in mice retards expression of a potassium conductance in inner hair cells (12). These processes presumably rely on a timed network of T3-activated transcription, which may be facilitated by D2.
Such a role for cochlear D2 is further supported in rats made mildly hypothyroid with the antithyroid agent PTU or polychlorinated biphenyls, environmental pollutants that bear structural similarity to TH (36, 37). These treatments reduce serum levels of T4 but not T3, because of protective responses that maintain serum levels of T3 (36). Nonetheless, auditory deficits arose, supporting the view that circulating T3 is an inadequate source of hormone for the developing cochlea and that most of the T3 in this tissue must be derived locally from T4 by D2.
In agreement with a role for D2 in the timing of cochlear development, deiodinases have been proposed to coordinate other TH-dependent developmental processes. The deiodinase inhibitor iopanoic acid impairs limb growth during metamorphosis in Rana catesbeiana tadpoles (18). Also, transgenic overexpression of D3, which inactivates T3, arrests gill and tail resorption in Xenopus laevis tadpoles (19). Increases in D2 activity have been observed in the developing rat brain (20), including in central auditory pathways, where, as in the cochlea, it has been proposed that circulating T3 is not an adequate source of hormone (38).
D2 mRNA was localized in the connective tissue that gives rise to the bony labyrinth, including in the modiolus, the septal divisions of the cochlea, lateral regions of the cochlear duct, and periodically along the spiral lamina, possibly correlating with the habenula perforata, through which dendritic and axonal projections connect with the hair cells. Surprisingly, D2 expression was complementary to rather than coincident with that of TRβ or TRα1, which are mainly expressed in the sensory epithelium and spiral ganglion (Fig. 4F) (7, 8). This pattern suggests that D2-containing cells in the connective tissue take up T4 from the circulatory system, convert T4 to T3, and then release T3 to responsive cells in the sensory epithelium and spiral ganglion. This invokes a local release of T3 to stimulate nearby TR-expressing cells, suggestive of a paracrine rather than endocrine mode of signaling in the cochlea. This model resembles the recently proposed model in the rat brain where D2 is mainly located in astrocytes but not in the neurons that are the primary T3-responsive cells (39). These findings suggest that cellular communication, mediated by cells that control deiodination and local transport of different forms of TH, provides an unexpectedly important level of control of TR activation.
Finally, it is known that inductive signaling between the ectodermal epithelia and mesenchyme influences the early formation of the inner ear (40). Growth factors produced in the otic epithelium stimulate chondrogenesis in the mesenchyme that forms the cartilaginous otic capsule in the mouse embryo (41). In this context, it is interesting to consider the release of T3 from the bony labyrinth as an inductive signal that stimulates maturation of the membranous labyrinth during postnatal stages. Thus, the bony labyrinth may serve a functional as well as a structural support role in the maturing cochlea.
Acknowledgments
We thank Dr. Zhendong Wang for assistance with the cochlea dissections, Igor Lisoukov for animal husbandry, and Dr. Lily Ng for comments on the manuscript. This work was supported in part by National Institutes of Health Grant DC-03441, the Deafness Research Foundation, and the Human Frontiers Science Program (to D.F.), and National Institutes of Health Grant DC 02949 and an Independent Investigator Award K02 from the National Institute on Deafness and Other Communication Diseases (to M.W.K.). A.C.B. was supported by the Spanish Ministry of Culture and Education Grant 97 PF 00679951.
Abbreviations
- TH
thyroid hormone
- T4
L-thyroxine
- T3
L-triiodothyronine
- TR
thyroid hormone receptor
- D2
type 2 iodothyronine deiodinase
- D1
type 1 iodothyronine deiodinase
- D3
type 3 iodothyronine deiodinase
- P
postnatal day
- rT3
reverse T3
- PTU
6-n-propyl-2-thiouracil
Footnotes
References
- 1.Deol M S. J Med Genet. 1973;10:235–242. doi: 10.1136/jmg.10.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hébert R, Langlois J-M, Dussault J H. Dev Brain Res. 1985;23:161–170. doi: 10.1016/0165-3806(85)90037-9. [DOI] [PubMed] [Google Scholar]
- 3.Uziel A, Pujol R, Legrand C, Legrand J. Dev Brain Res. 1983;7:295–301. doi: 10.1016/0165-3806(83)90186-4. [DOI] [PubMed] [Google Scholar]
- 4.Forrest D. J Clin Endocrinol Metab. 1996;81:2764–2767. doi: 10.1210/jcem.81.8.8768825. [DOI] [PubMed] [Google Scholar]
- 5.Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B. Nature (London) 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger C, Thompson C C, Ong E S, Lebo R, Gruol D J, Evans R M. Nature (London) 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 7.Bradley D J, Towle H C, Young W S., III Proc Natl Acad Sci USA. 1994;91:439–443. doi: 10.1073/pnas.91.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauterman J, ten Cate W-J F. Hear Res. 1997;107:23–28. doi: 10.1016/s0378-5955(97)00014-2. [DOI] [PubMed] [Google Scholar]
- 9.Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner H-P, Zimmermann U. Development (Cambridge, UK) 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- 10.Refetoff S, DeWind L T, DeGroot L J. J Clin Endocrinol Metab. 1967;27:279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- 11.Forrest D, Erway L C, Ng L, Altschuler R, Curran T. Nat Genet. 1996;13:354–357. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- 12.Rüsch A, Erway L, Oliver D, Vennström B, Forrest D. Proc Natl Acad Sci USA. 1998;95:15758–15762. doi: 10.1073/pnas.95.26.15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brucker-Davis F, Skarulis M C, Pikus A, Ishizawar D, Mastroianni M-A, Koby M, Weintraub B D. J Clin Endocrinol Metab. 1996;81:2768–2772. doi: 10.1210/jcem.81.8.8768826. [DOI] [PubMed] [Google Scholar]
- 14.St. Germain D, Galton V. Thyroid. 1997;7:655–668. doi: 10.1089/thy.1997.7.655. [DOI] [PubMed] [Google Scholar]
- 15.Larsen P. Biochem Soc Trans. 1997;25:588–592. doi: 10.1042/bst0250588. [DOI] [PubMed] [Google Scholar]
- 16.Crantz F, Silva J, Larsen P. Endocrinology. 1982;110:367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- 17.Van Doorn J, Roelfsema F, Van der Heide D. Endocrinology. 1985;117:1201–1208. doi: 10.1210/endo-117-3-1201. [DOI] [PubMed] [Google Scholar]
- 18.Becker K, Stephens K, Davey J, Schneider M, Galton V. Endocrinology. 1997;138:2989–2997. doi: 10.1210/endo.138.7.5272. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Marsh-Armstrong N, Brown D. Proc Natl Acad Sci USA. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan M, Yaskoski K. J Clin Invest. 1981;67:1208–1214. doi: 10.1172/JCI110136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obregon M, Ruiz de Oña C, Calvo R, Escobar del Rey F, Morreale de Escobar G. Endocrinology. 1991;129:2663–2673. doi: 10.1210/endo-129-5-2663. [DOI] [PubMed] [Google Scholar]
- 22.Forrest D, Hanebuth E, Smeyne R J, Everds N, Stewart C L, Wehner J M, Curran T. EMBO J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 23.Croteau W, Davey J, Galton V, St. Germain D. J Clin Invest. 1996;98:405–417. doi: 10.1172/JCI118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng L, Forrest D, Haugen B R, Wood W M, Curran T. Mol Endocrinol. 1995;9:1202–1213. doi: 10.1210/mend.9.9.7491112. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson D, Nieto M. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- 26.Lanford P, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley M. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 27.Campos-Barros A, Erway L C, Krezel W, Curran T, Kastner P, Chambon P, Forrest D. NeuroReport. 1998;9:2933–2937. doi: 10.1097/00001756-199809140-00003. [DOI] [PubMed] [Google Scholar]
- 28.Morreale de Escobar G P R, Obregon M J, Escobar del Rey F. Endocrinology. 1985;117:1890–1900. doi: 10.1210/endo-117-5-1890. [DOI] [PubMed] [Google Scholar]
- 29.Leonard J, Rosenberg I. Endocrinology. 1980;107:1376–1383. doi: 10.1210/endo-107-5-1376. [DOI] [PubMed] [Google Scholar]
- 30.Campos-Barros A, Meinhold H, Stula M, Köhler R, Eravci M, Putzien O, Baumgartner A. J Pharmacol Exp Ther. 1994;268:1143–1152. [PubMed] [Google Scholar]
- 31.Visser T, Leonard J, Kaplan M, Larsen P. Proc Natl Acad Sci USA. 1982;79:5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campos-Barros A, Hoell T, Musa A, Sampaolo S, Stoltenberg G, Pinna G, Eravci M, Meinhold H, Baumgartner A. J Clin Endocrinol Metab. 1996;81:2179–2185. doi: 10.1210/jcem.81.6.8964848. [DOI] [PubMed] [Google Scholar]
- 33.Davey J, Schneider M, Becker K, Galton V. Endocrinology. 1999;140:1022–1025. doi: 10.1210/endo.140.2.6678. [DOI] [PubMed] [Google Scholar]
- 34.Seyfried T, Glaser G, Yu R. Science. 1979;205:598–600. doi: 10.1126/science.451624. [DOI] [PubMed] [Google Scholar]
- 35.O'Malley B W, Li D, Turner D S. Hear Res. 1995;88:181–189. doi: 10.1016/0378-5955(95)00111-g. [DOI] [PubMed] [Google Scholar]
- 36.Goldey E S, Kehn L S, Rehnberg G L, Crofton K M. Toxicol Appl Pharmacol. 1995;135:67–76. doi: 10.1006/taap.1995.1209. [DOI] [PubMed] [Google Scholar]
- 37.Goldey E S, Kehn L S, Lau C, Rehnberg G L, Crofton K M. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- 38.Guadaño-Ferraz A, Escámez M, Rausell E, Bernal J. J Neurosci. 1999;19:3430–3439. doi: 10.1523/JNEUROSCI.19-09-03430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guadaño-Ferraz A, Obregón M, St. Germain D, Bernal J. Proc Natl Acad Sci USA. 1997;94:10391–10396. doi: 10.1073/pnas.94.19.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fritzsch B, Barald K, Lomax M. In: Development of the Auditory System. Rubel E, Popper A, Fay R, editors. New York: Springer; 1996. pp. 80–145. [Google Scholar]
- 41.Frenz D, Liu W, Williams J, Hatcher V, Galinovic-Schwartz V, Flanders K, Van De Water T. Development (Cambridge, UK) 1994;120:415–424. doi: 10.1242/dev.120.2.415. [DOI] [PubMed] [Google Scholar]