Abstract
Body size is one of the most important traits of organisms and allows predictions of an individual's morphology, physiology, behaviour and life history. However, explaining the evolution of complex traits such as body size is difficult because a plethora of other traits influence body size. Here I review what we know about the evolution of body size in a group of island reptiles and try to generalize about the mechanisms that shape body size. Galapagos marine iguanas occupy all 13 larger islands in this Pacific archipelago and have maximum island body weights between 900 and 12 000 g. The distribution of body sizes does not match mitochondrial clades, indicating that body size evolves independently of genetic relatedness. Marine iguanas lack intra- and inter-specific food competition and predators are not size-specific, discounting these factors as selective agents influencing body size. Instead I hypothesize that body size reflects the trade-offs between sexual and natural selection. We found that sexual selection continuously favours larger body sizes. Large males establish display territories and some gain over-proportional reproductive success in the iguanas' mating aggregations. Females select males based on size and activity and are thus responsible for the observed mating skew. However, large individuals are strongly selected against during El Niño-related famines when dietary algae disappear from the intertidal foraging areas. We showed that differences in algae sward (‘pasture’) heights and thermal constraints on large size are causally responsible for differences in maximum body size among populations. I hypothesize that body size in many animal species reflects a trade-off between foraging constraints and sexual selection and suggest that future research could focus on physiological and genetic mechanisms determining body size in wild animals. Furthermore, evolutionary stable body size distributions within populations should be analysed to better understand selection pressures on individual body size.
Keywords: reptile, scaling, digestion, thermoregulation, diving, island rule
1. Introduction
Evolution of complex traits in nature is notoriously difficult to explain and has posed significant heuristic and theoretical problems for ecologists (Darwin 1883; Bonner 1984; West-Eberhard 2003). Here I attempt to account for the evolution of one such complex trait, body size, using an intra-specific analysis. Body size is one of the most important characteristics of organisms because practically all other morphological, physiological, behavioural or life history traits scale with body size (Peters 1983; Schmidt-Nielsen 1984; Reiss 1989; Perrin 1998). At the same time these ubiquitous interrelationships make body size one of the most difficult traits to explain because it is unclear what specific factors cause body size to evolve in one or the other direction. Even the most general trends in body size are still debated in some detail, for example Bergmann's rule stating that endotherms increase in size towards higher latitudes, Cope's rule suggesting that animals generally evolve towards larger body sizes, or the island rule showing that most mammals larger than ca 100 g decrease towards smaller sizes on islands (Meiri & Dayan 2003; Brown & Maurer 1989).
What appears to be generally accepted, and what I assume here also is that body size is an adaptation to the specific life history of an animal (Harvey & Purvis 1999). I will, therefore, focus on trade-offs between the selection pressures of both natural and sexual selection and determine how potential trade-offs influence body size in a system of island reptiles, the marine iguanas of the Galapagos archipelago (Darwin 1883; Carpenter 1966). I will evaluate four other hypotheses that have been used to explain inter-specific differences in body size in addition to, or as non-exclusive alternatives for, natural and sexual selection trade-offs. The problem with these hypotheses is that they often do not address the mechanisms causing body size differences between animals. I will highlight these problems and suggest alternative, mechanistic explanations. As the first hypothesis, variation in size-specific predation pressure could affect the body size of animals (Case & Schwaner, 1993; Boback 2003). The second hypothesis is that animals from different populations or of different species could have different body sizes because they live in habitats that differ in total area (Burness et al. 2001). Generally, the more extensive an area a species occupies, the larger its body size is predicted to be. Third, the average productivity of resources in the area an animal occupies could influence its body size (Case 1976; Madsen & Shine 1993). Fourth, the variability of resources and the variability in their productivity could determine body size, particularly if animals are not buffered against sudden or long-term changes in productivity (Case 1976). As these hypotheses argue on varying levels of ultimate or mechanistic understanding of natural phenomena, I will attempt to bring them to the smallest common mechanistic denominator in the context of the Galapagos marine iguanas system and thereby hope to resolve the relationships between them (Wikelski & Romero 2003).
The Galapagos islands have proven to be a showcase of evolution largely because they provide repeated natural experiments on changing selection pressures (Grant 1986). Cyclic El Niño Southern Oscillations of varying magnitudes perturb the natural systems in Galapagos just enough to provide deep insights into selection and evolution in action. El Niños are also ultimately important for marine iguanas as they almost completely wipe out their food supply by covering the intertidal rocks with poorly digestible algae (Laurie 1989) and dramatically increase environmental temperatures.
2. Assumptions and background: natural history of Galapagos marine iguanas
It is becoming clear that body size even in supposedly continuously growing animals such as reptiles is limited; thus the analysis of phenotypic expressions of body size has validity in Galapagos marine iguanas. Furthermore, body size has a strong genetic component in many reptiles (Tracy 1999; Wikelski & Romero 2003), making selection for and inheritance of body size possible. Stamps & Andrews (1992) showed that for comparisons between populations one can use the largest individual(s) per sample and achieve highly reliable estimates of asymptotic body sizes. This is important for marine iguanas where island populations differ in body mass by more than one order of magnitude. The maximum body mass on southwestern Isabela island is more than 12 kg, whereas the heaviest individuals on Genovesa island in the northeastern part of the archipelago only reach ca 900 g (Wikelski & Trillmich 1997). Furthermore, two factors that often confound the analysis of body size are largely absent: There is no aggressive competition within iguanas in the foraging areas, i.e. no individual can exclude others from food resources but individuals are in scramble competition with each other. Second, inter-specific competition between iguanas and other (at least terrestrial) species does not appear to be prevalent. Marine iguanas gather algae in the intertidal flats that are exposed at low tide, or by diving (Wikelski & Trillmich 1994), using specialized hindgut bacteria to digest algae cell walls (Mackie et al. 2003). The algae harvested by marine iguanas are not eaten by other vertebrates, however Sally-lightfoot crabs (Grapsus grapsus) and other invertebrates may eat as much as 8% of algae otherwise available to iguanas (Wikelski, unpublished data). Given how low the inter-specific competition is, I will not consider it for this paper. Most individuals of each population (95%) forage every second day in the intertidal areas as soon as high tides recede, while only the largest individuals also dive for food (mostly males, ∼5% of individuals), at minimum diving body weights of 400–5000 g, depending on which island iguanas are from (Hobson 1965; Wikelski & Hau 1995). The main reason seems to be that the largest individuals in each population are out-competed by the smaller individuals in the scramble competition for food (see below). The phylogenetic history of the various marine iguana island populations is well described (Rassmann et al. 1997) but does not match the distributions of body sizes across the archipelago. In fact, the smallest and the largest individuals both occur within the ‘northern clade’, which connects Fernandina island to Genovesa island into a common lineage and contrasts it against the central and the southern clade. The most likely hypothesis emanating from this genetic data set is that body size is a fast evolving trait in Galapagos marine iguanas that cannot be predicted by evolutionary changes in the mitochondrial DNA. Thus, an ecological analysis of selection on body size is warranted.
3. Methods
We investigated marine iguana population dynamics and ecology on Santa Fe island continuously since 1981 (Laurie 1989) and on Genovesa from 1990 to 2000, usually staying more than 3 months on the islands from October to January each year. Individuals were marked for their life times and recaptured annually and painted for observations. On both islands we also conducted various eco-physiological studies ranging from temperature adaptions to cold and hot climate to stomach washings, energy expenditure and hindgut microbiology. The composite knowledge now allows us to qualitatively and quantitatively test the various hypotheses put forward to explain body size evolution.
(a) Hypothesis 1: predation explains differences in body size
Several marine iguana island population of various body sizes have no predators on adult animals (Curio 1965). Therefore, it seems likely that predation is not a very pervasive force in the evolution of marine iguana body sizes, or at least does not influence marine iguana body size in a systematic way. In fact, on Santa Fe island, in a random set of 38 predation events by Galapagos hawks (Buteo galapagoensis) on nesting females recorded in 4 years, we did not see a selection differential on body size different from zero (M. Wikelski, unpublished data). However, there are no quantitative data yet to determine whether in general very large size protects marine iguanas against some natural predators like hawks (cf. Boersma 1984). Predation on adult males is negligible and yet there are major differences in adult male size within populations, enforcing the argument that predation is not causing body size differences in marine iguanas. Nevertheless, predation can be a powerful selective pressure on organism size, such as in lizards that can escape snakes by growing large (Snell et al. 1988). Such a scenario may also apply for marine iguana hatchlings. Unfortunately, the influence of predators on hatchling size is largely unexplored. It seems possible that survival of large hatchlings from large eggs is an important selection pressure for large size in females (see below).
(b) Hypothesis 2: island area explains body size differences
Marine iguanas occupy all of the 14 larger and many of the smaller islands in the archipelago. At first sight (figure 1), 10 of the populations for which we have detailed data show increasing body sizes with increasing island areas. However, when investigating the historic records of the California Academy of Sciences (VanDenburgh & Slevin 1913) we found that the maximum body size increased on all islands within the past century, a fact somewhat inconsistent with the island area argument per se. Instead we argued that an increase in overall ambient temperatures on land, important for the rate of warming-up for feeding, could be responsible for the island-wide increase in marine iguana body sizes, independent of island area (see below and Wikelski & Romero 2003). To resolve whether the positive relationship between area and size is causal or just spurious I will try to investigate covariates of island size on a mechanistic level.
Figure 1.
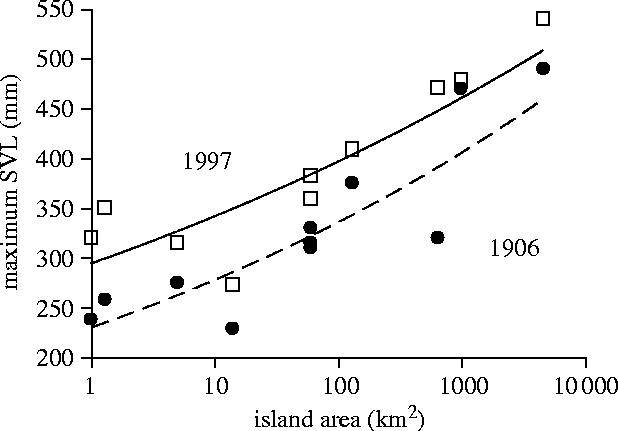
The island area in Galapagos appears to predict the maximum snout-to-vent length (SVL) of marine iguanas. Only populations are shown for which measurements existed in 1906 (filled circles) and 1997 (open squares). The lines represent best fit quadratic regressions.
(c) Hypotheses 3 and 4: mean resource productivity or its variability explains differences in maximum body size
In the Galapagos archipelago, the larger islands are generally more exposed to the up welling and nutrient-rich currents from the southwest, and thus have larger standing crops as measured by algae sward heights (figure 2; cf. Illius & Gordon 1992). Longer swards also represent higher productivity (Scheibling & Wikelski, unpublished data). Maximum island body size of marine iguanas is tightly linked to the size of the standing crop in the intertidal areas. However, such a relationship only holds for the non-El Niño periods. During El Niños the upwelling stops and the intertidal rocks barely offer grazable algae swards (figure 2, open squares). Could the variability of environmental productivity explain body size, with higher variability in productivity being associated with larger body sizes? Again, as for island area, it is unclear what the exact mechanisms for such a relationship would be. Therefore, in the following I will concentrate on a detailed mechanistic analysis of selection pressures (Arnold 1983). I also want to stress that hypotheses 3 and 4 are not necessarily different from hypothesis 5, but represent a subset of hypotheses relating to natural selection only.
Figure 2.
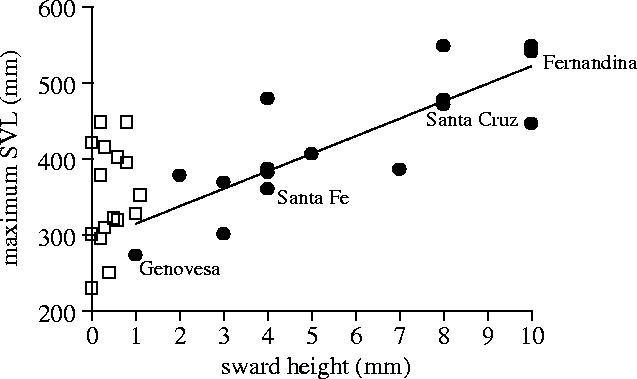
The algae sward height as measured in the intertidal zone where adult marine iguanas forage predicts maximum snout-to-vent length (SVL) of marine iguanas. Note that in this graph, small islands (Genovesa) also have small body sizes and large islands (Fernandina) have large body sizes (compare with figure 1). Filled circles show non-El Niño data (1998) and open squares show El Niño data (1999). The line represents a linear regression.
(d) Hypothesis 5: body size is set at the interplay between natural and sexual selection
For this hypothesis to work we assume that sexual selection drives animals towards larger body sizes whereas natural selection either caps body size at a certain value, for example when the carrying capacity of an environment is reached (Arnold & Wade 1984). Similarly, whenever bad environmental conditions arise such as during El Niños, we predict massive starvation particularly of large animals. Such a prediction is counterintuitive because large individuals are expected to have lower per gram metabolic expenses and thus generally can endure starvation longer (Perrin 1998). However, if the large size itself prevents efficient energy intake and utilization, natural selection should act against larger animals. Below I will employ a mechanistic approach toward understanding body size evolution.
(i) Evidence for natural selection against large body size in marine iguanas
When following size cohorts during El Niño events we repeatedly observed strong natural selection against the largest sized individuals in each population (figure 3; Laurie & Brown 1990a,b). Interestingly, for similar sized males and females we found no significant differences in their probability to survive an El Niño. The same was true for adult animals regardless of their relative age, i.e. young and old adults of the same body size suffered similarly during an El Niño (Wikelski & Trillmich 1997). However, because males on each island are larger than females, males are the first to die whenever bad conditions cause deterioration in the iguanas' food supply. Whereas females often survive medium El Niño events with 70–80% probability, the largest males usually do not have higher odds than 20–50% of making it into the next year. The survival probabilities also differ markedly between islands. Overall, medium-sized animals in each population survive well, but the largest animals suffer independent of their absolute size. For example, all individuals with a snout-to-vent length of more than 250 mm died on Genovesa island during the 1991 El Niño whereas almost 90% of similar sized individual survived on Santa Fe where they are medium sized relative to all marine iguana populations (figure 4; Wikelski & Trillmich 1997). The low survival probabilities of small animals (young of the year) in all populations are most likely related to their inferior foraging abilities, in particular to their relatively low strength of holding onto the slippery intertidal substrate (Wikelski & Trillmich 1994).
Figure 3.
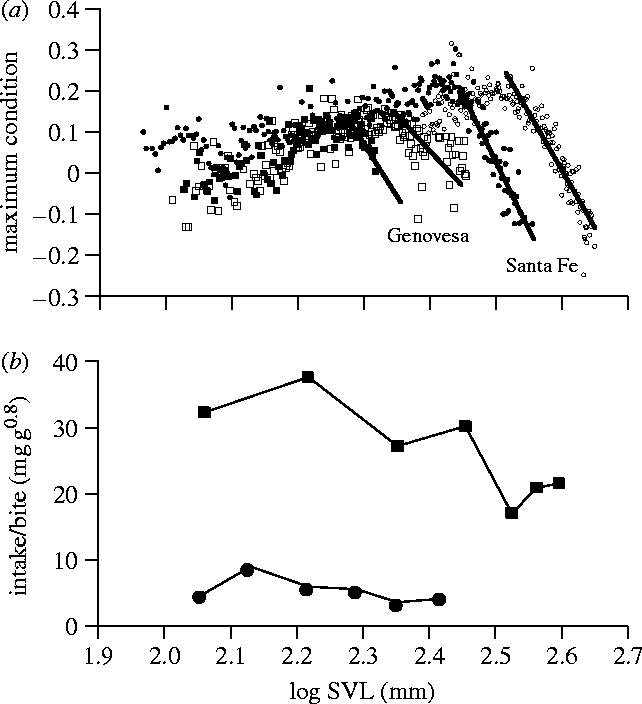
(a) The maximum ever measured body condition for each mm body size for marine iguanas on two islands, Genovesa and Santa Fe. Circles show data for Santa Fe, squares data for Genovesa—open symbols are males and closed symbols, females. The lines represent regression lines that start at the deflection point when maximum body conditions start to decline (above a threshold body size). (b) The intake per bite, corrected for the metabolic body mass generally decreases towards larger body size, but no decrease can be detected within the largest males of each island. To calculate mass-specfic intake we assume an allometric scaling of energy expenditure of 0.8. Redrawn from Wikelski & Trillmich (1997).
Figure 4.
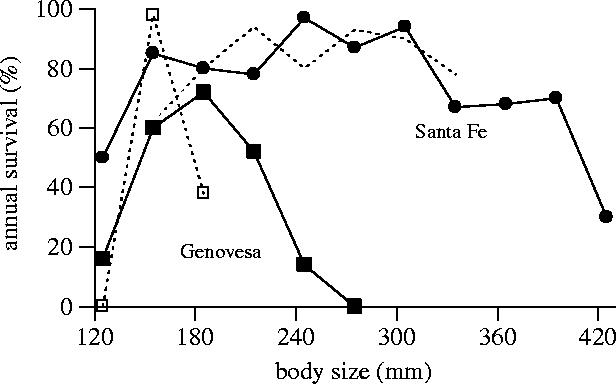
Annual survival estimates as determined from mark–recapture data on Genovesa and Santa Fe islands from February 1991 to March 1992. Survival data are estimates from Jolly–Seber survival models. Circles show data for Santa Fe, squares data for Genovesa, filled symbols are males, open symbols females. Redrawn from Wikelski & Trillmich (1997).
(ii) Mechanistic explanations for natural selection against large body size
To investigate the mechanisms underlying size-related mortalities we first determined the maximum body condition that was reached by individuals of a given size throughout the study period (10–20 years). Body conditions were determined as the residuals of a global double logarithmic regression of weight against size, but other condition measures gave similar results. While the maximum body condition is relatively stable over most of the body size range on each island, we found that above a certain body size threshold, body conditions invariably drop down. Maximum body conditions in females decrease at smaller body sizes compared to males (figure 3a). Again we found strong differences in the absolute body sizes above which maximum body conditions drop between islands. While this decrease in body condition indicated some constraints on large animals, it was unclear why body conditions should drop at a lower body size for females compared to males (see below).
To understand low body condition of large animals mechanistically, we decided to relate the metabolizable energy that individuals could gather during their foraging trips to their energy expenditure as measured via doubly labelled water studies in free-living animals on three islands (Nagy & Shoemaker 1984; Wikelski et al. 1997; Drent et al. 1999). Metabolizable intake was calculated from stomach washings of individuals (energy intake) and corrected for the digestive efficiency we found for digesting algae (70%; Wikelski et al. 1993). The results from this analysis were encouraging (figure 5). First, energy intake had a lower slope than energy expenditure, a fact that invariably leads to a break-even point and to a negative energy balance for larger animals (at least during the season we measured). Again, the absolute body size level of this break-even point differed between islands, being higher in Santa Fe. Second, the residual levels of energy intake and energy expenditure were higher on Santa Fe than on Genovesa, supporting the fact that individuals on Santa Fe achieve higher growth rates at a given body size. In fact, high growth rates are often associated with large overall size (Charnov 1993) and the mechanistic explanation we found for marine iguanas could apply generally. Third, we found that energy intake was lower during El Niño conditions, thus the break-even point and the sustainable body size was lower as well. This El Niño estimate is conservative because the high ambient temperatures during El Niños should increase field metabolic rate (FMR). However, even this energy analysis still left us with the question of why only large males but not females would suffer mortalities under deteriorating environmental conditions.
Figure 5.
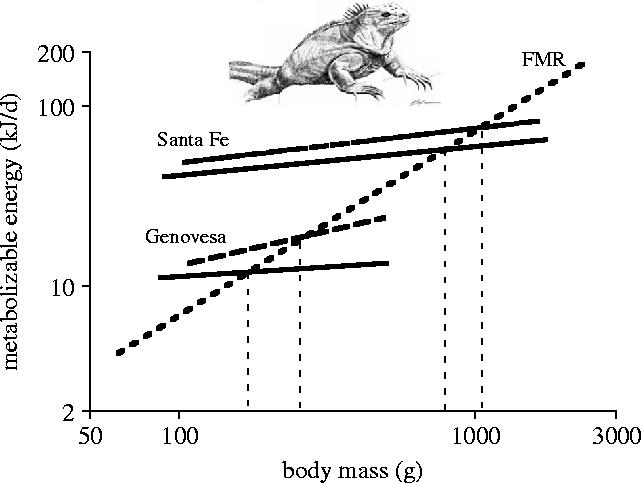
Food intake (dashed and solid lines) of marine iguanas from two islands (Genovesa, Santa Fe) differs dramatically, both between islands and between seasons. Iguanas on Santa Fe have much higher food intakes than Genovesa iguanas. Food intake is lower during El Niño periods (solid line; 1991/92) compared to a ‘normal’ year (dashed line, 1992/93). The regression lines from food intake cross the regression line from energy expenditure (field metabolic rate, FMR) at much lower body sizes in Genovesa compared to Santa Fe. The predicted maximum body mass is much lower on Genovesa than on Santa Fe, and lower during El Niños than during normal years. Regressions lines only are shown here for graphical clarity. Redrawn from Wikelski et al. (1997).
We subsequently hypothesized that high mortality and relatively low energy intake in large animals was caused by a declining foraging efficiency with increasing size. In a detailed analysis of the assimilated energy intake per bite we documented a generally decreasing trend in efficiency towards larger animals. However, the largest animals of each island did not show the predicted strong decrease in foraging efficiency that would correspond to their decreasing maximum body condition (figure 3b). Note that foraging efficiency was much lower overall on Genovesa island where animals reach much smaller absolute body sizes.
The only remaining option for performance of the largest animals to be worse than that of smaller animals was related to thermal constraints. Indeed we found in both measurements and in model simulations that the largest animals cannot sufficiently warm up for foraging in the cold intertidal areas particularly when low tides are in the early morning. Large animals also have problems warming up sufficiently for overnight digestion after late-afternoon foraging trips (Wikelski & Carbone 2004; cf. Buttemer & Dawson 1993). Such a thermal constraint on body size becomes more obvious when considering that sea surface temperatures in Galapagos can be as low as 11 °C during upwellings. The largest animals are generally foraging in the lowest intertidal areas (closest to the water) and thus are submerged by waves most of the time and cool down rapidly (Trillmich & Trillmich 1986). Furthermore, large animals are unable to warm up fast between foraging bouts because of their large size. Small animals, in contrast, can quickly run into the intertidal, eat algae with a high bite rate and return to safe and warm resting spots above the tide line. Small animals make up to eight foraging and rewarming trips per day (Wikelski & Trillmich 1994). The only minor remedy for thermal size constraints is for large animals to arrive at the intertidal foraging sites early, a trait that has a selective advantage during El Niño conditions (Wikelski & Hau 1995). The reason why large animals need to be early is that they are out-competed by small animals in the foraging scramble competition. Marine iguanas can achieve a proper timing of their foraging trips using an endogenous tidal foraging clock that is synchronized with the tides (Woodley et al. 2003).
Finally, we combined the information on size-specific energy intake in relation to algae sward height with environmental temperatures into a simulation model that is based on first principles of thermodynamics (Wikelski & Carbone 2004). We used two island populations to parameterize this model and then predicted the maximum body weights of marine iguanas across the archipelago. Subsequently, we tested the body size predictions with empirical data. Most measured body sizes fitted the predicted body sizes well, in fact observed body sizes showed no significant differences from predicted body sizes except for one population (figure 6). Marine iguanas on North Seymour island were much larger than predicted from the algae sward heights and environmental temperatures they experienced. Interestingly, North Seymour was the only place where some individuals supplemented their marine diet with land plants (Wikelski & Wrege 2000). Eating on land circumvented the time constraint for foraging in the intertidal areas, which are only exposed for a short period every day (low tide). Thus, land plant foraging in this population could be considered a key innovation that allowed some animals to break the evolutionary rules. However, during the subsequent El Niño the area where North Seymour animals foraged on land got flooded and all land plants temporarily disappeared. As predicted, these over-sized individuals were the first to die during the El Niño. We suggested that land plant eating was a local foraging tradition and would resurface once the local residents reached an upper-size limit (Wikelski & Wrege 2000). In fact, recent reports from the Galapagos seem to support this prediction. Overall, the availability of food of high quality appears to be of such pervasive importance for marine iguanas that they precisely time their one annual reproductive season with minute changes in food quality (Rubenstein & Wikelski 2003).
Figure 6.
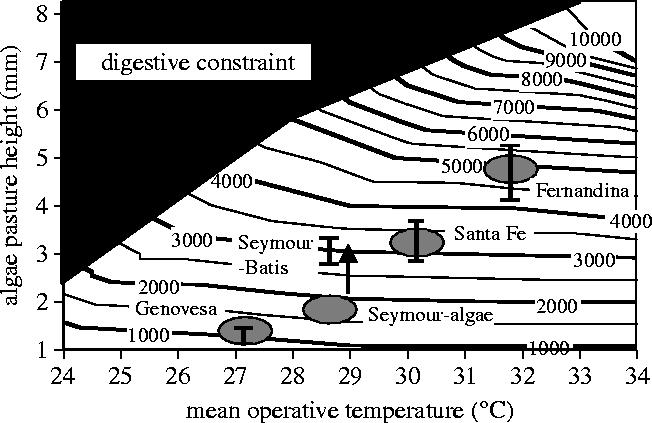
Mean standard operative temperature and mean algae pasture height predict the maximum body mass of marine iguanas in the Galapagos archipelago. Isocline lines indicate maximum body mass in grams. The grey ovals indicate the range of average values predicted from the measurement of environmental parameters in the field, while the vertical bars show the range of maximum body masses found in the wild. The black area to the left top of the graph identifies the range of environmental conditions when the digestive system of the iguanas cannot cope any more with maximum amount of ingested food, such as when the environmental temperature falls too much or when the algae pasture height increases too much. Redrawn from Wikelski & Carbone (2004).
(iii) Evidence for sexual selection pushing for larger body sizes in marine iguanas
Why should marine iguanas grow to sizes at which natural selection will wipe them out as soon as environmental conditions deteriorate? For male marine iguanas on all islands, the answer appears to be that larger animals gain a disproportionally large number of copulations (figure 7). During the mating season, marine iguana males congregate in mating arenas that are hotspots of female traffic during the non-breeding season (Trillmich 1983; Wikelski et al. 1996). Within these hotspots, high quality males (hotshots) establish display territories and are joined by inferior males who attempt to access the spillover females from the hotshots' territories (Partecke et al. 2002). Inferior (generally smaller sized) males can become satellites roaming around territories, or sneakers who are indistinguishable by morphology from females. We showed that early-season behavioural interactions most likely cause androgen hormone surges in successful combatants, which in turn feeds back to make them into males of higher status (Wikelski et al. 2005). The advantage of such a system is that even males that were sneakers in one year can become territorial in the next year if in the meantime El Niño killed off all large males (as happened during the 1991 El Niño on Genovesa island). What is important for the evolution of body size is that in every case large body size is at a sexually selected advantage. The best males in each mating arena gained upwards of 20 copulations per seasons, translating into 30–40 offspring. The only successful alternative mating tactic for small males was to prepare ejaculate in penis pouches, jump upon females and rapidly insert the hemipenis, thus transferring sperm immediately even without ejaculation (Wikelski & Bäurle 1996).
Figure 7.
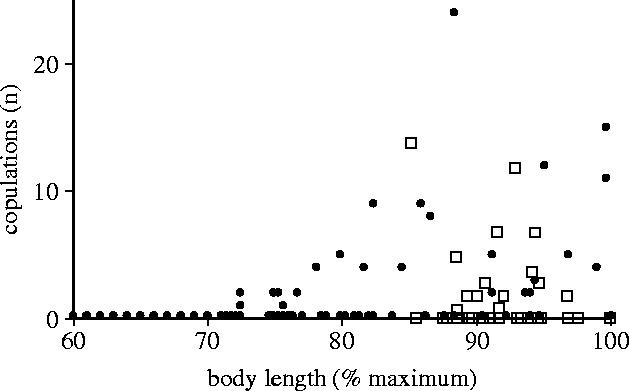
On both Santa Fe and Genovesa island, males of large body size relative to the rest of the population achieve high numbers of copulation. Filled circles show data for Santa Fe and open squares show data for Genovesa.
We know fewer details about potential actions of sexual selection on size of females. What is clear, however, is that larger females have larger clutch masses and larger egg sizes, resulting in larger hatchlings that have better survival chances (figure 8; Wikelski & Romero 2003). Furthermore, it may be easier for large females compared to smaller ones to select among the various displaying males to mate with. Female marine iguanas make deliberate movements onto territories of males and most probably select the individual for copulation that is displaying most vigorously in a female's subjective judgment (Wikelski et al. 2001). However, while choosing among males, females often have to move and reposition themselves to fend off approaches by overeager males. Thus, choice of mating partners appears to be costly for females, indicated mechanistically by both weight loss and increased energy expenditure during the mate choice period (Butler et al. 2002; Vitousek et al. unpublished data). We expect that smaller females will have a harder time rejecting copulation attempts by males, thus providing another advantage for large female size. Larger females also have advantages during the egg-laying period, when hundreds of them gather in sandy areas to dig nest holes and subsequently guard laying sites against other females (Trillmich 1983). Females engage in fierce damaging fights around nest sites during which they appear to rapidly convert testosterone into oestradiol, which they then presumably use to boost their fighting ability (Rubenstein & Wikelski in press).
Figure 8.
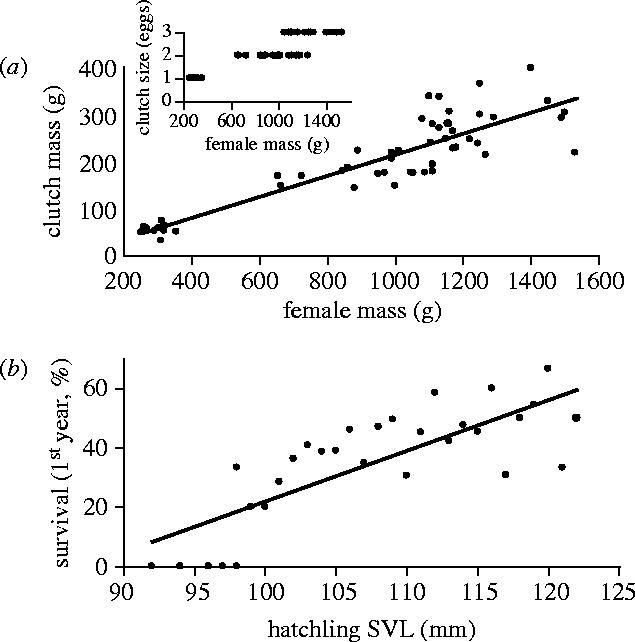
(a) Clutch mass (top panel) and clutch size (inset) increase with female mass. All females below 400 g body mass are from Genovesa island, the remainder from Santa Fe. (b) The survival of young marine iguanas depends on their initial size at hatching (Santa Fe iguanas only). Each data point represents the average survival per mm SVL for a cohort of 1285 marine iguanas hatched in 1988. Redrawn from Wikelski & Romero (2003).
In general it appears that for both males and females we find consistent and often strong sexual selection differentials towards large body size.
(iv) Mechanisms to ameliorate problems with large body size
Ideally, marine iguanas should have large sizes during the mating season because of sexual selection and small sizes during the non-reproductive season because of natural selection. This was generally considered impossible in vertebrates, but nevertheless iguanas appear to have physiological mechanisms to decrease as well as increase their body size. When bad environmental conditions hit, marine iguanas first lose weight. As soon as individuals reach a critically low body condition, they start secreting glucocorticoid stress hormones (Romero & Wikelski 2001). It appears that at this point individuals also start to decrease in overall body length, most likely including skeletal size (Wikelski & Thom 2000). Body length changes could be incidental by-products of stress hormone secretion, but could also be an adaptation selected because smaller animals survive better. We found that individuals that shrank more had a higher survival rate during an El Niño. It seems likely that other organisms (Hofmann et al. 1999; but not all; Madsen & Shine 2001; Luiselli 2005) have similar ways of changing their body size in relation to bad environmental conditions, but in many instances this may remain undetected.
(e) A hypothetical scenario for the evolution of body size in marine iguanas
When the ancestors of marine iguanas arrived in the Galapagos between 10 and 15 million years ago from South America (Christie et al. 1992; Rassmann 1997), they presumably found plentiful food supply in the intertidal areas, but not on land. Foraging was then only possible during low tide for a limited amount of time per day. Larger sized animals were favoured because of their thermal intertia (Bartholomew 1966) and their stronger ability to hold onto rocks in the intertidal against the force of waves. Population densities increased at suitable foraging and egg-laying sites and soon males occupied hotspots of female traffic during the reproductive period. As females chose to copulate with the large males only, sexual selection towards larger male size increased, thus also rendering alternative mating tactics an option for size-disadvantaged males. Initially, females only had to grow to sizes that allowed the production of hatchlings large enough to survive (Wikelski & Trillmich 1997). Subsequently, sexual selection favoured larger female size due to their lowered costs of mate choice and higher competitive abilities at crowded nesting sites. The difference in maximum size between males and females, sexual size dimorphism, is best explained by the females' need to export material into eggs. On the other hand, large body size is selected against by mortality selection during El Niño events when food becomes scarce. Individuals that are too large for the prevailing conditions try to ameliorate their situation by shrinking in body length, but can regrow when good conditions reappear.
Some of the ideas proposed in the above paragraphs are so far only circumstantially supported by data. Ideally, one should conduct reciprocal transplant experiments to truly understand trade-offs for animals of different sizes in different populations (Niewiarowski & Roosenburg 1993). To demonstrate whether sexual size dimorphism is caused by differences in resource export one could prevent females from laying eggs and see whether they reach the same size as males. In any case, the key future project will be to build demographic models from individual-based life history data for many island populations and map them onto long-term data of environmental variation for these populations. The models could be used to compare predicted and observed body size distributions and analyse evolutionary stable body size strategies for each population. Integral projection models would provide the adequate mechanistic tool (Childs et al. 2003, 2004).
(f) Evolution of body size beyond marine iguanas
Organisms can evolve extreme body sizes in a very short time (Losos et al. 1997, 1998). For example, Australian tiger snakes (genus Notechis) evolved island dwarfs and giants independently five times during the past 5000–10 000 years (Keogh et al. 2005). While many factors influence body size evolution, it appears that the most prevalent mechanism causing evolutionary changes in body size is the size and availability of an animal's food. For example, most body size shifts in island populations of snakes are caused by different sizes of prey items available for adult island snakes (Boback 2003). The size of food items available to Darwin's finches (Geospiza) influences their evolutionary trajectory towards smaller or larger beak sizes, which are highly correlated with general body size (Grant 1986). I hypothesize that the most important reasons why animals grow large in the first place are related to sexual selection. Mate choice is now recognized as an ubiquitous selective agent and could be the prime mechanism driving sexual selection (Bradbury et al. 1985).
Assuming that the above generalizations will hold true when scrutinized by formal comparative explorations, I suggest that a promising and important avenue for future work on body size could be twofold: first, field researchers should use integral projection models to gain insights into the properties of evolutionary stable body size strategies in a life history context (Easterling et al. 2000; Rees & Rose 2002; Childs et al. 2003). Second, a physiology of life history approach could help expand our mechanistic understanding of the physiological and genetic factors that enable and permit rapid body size changes (Ricklefs & Wikelski 2002). Field ecologists could borrow approaches and physiological and genetic techniques from laboratory studies, for example from work on insects (Stern 2003), where insulin signalling appears to control final body size. The ultimate goal will be to understand evolutionarily stable body size strategies on a mechanistic level down to changes in gene regulation. Such studies are doable in the wild and have already been pioneered in Darwin's finches (Abzhanov et al. 2004).
Acknowledgments
I am indebted to Andrew Laurie who had the foresight to start these studies in the best way possible. I thank Ela Hau, Victor Carrillo, Ebo Gwinner, Michael Romero, Stan Rand, Lynn Erckman, John Wingfield, Fritz Trillmich, Wolfgang Wickler, Pat Whealan, Fin Walsh, Franz Kümmeth, Dustin Rubenstein and a large superb contingent of field assistants, local fisherman and dinghy drivers for invaluable help throughout. Thomas Dellinger, Fritz Trillmich and Andrew Laurie kindly permitted the use of their data throughout the project. Laura Spinney, Dustin Rubenstein, Ela Hau, Silke Berger and Kelly Lee-Pooh provided expert comments on this manuscript. The Galapagos National Park Service permitted our work and together with the Charles-Darwin Station helped in many essential, sometimes survival-relevant ways. TAME provided transportation. This study was supported by grants from the Alexander-von-Humboldt Society, the Smithsonian Tropical Research Institute, the Max-Planck-Gesellschaft, the Universities of Illinois and Princeton and the National Science Foundation. This contribution is thankfully dedicated to George Bartholomew and Ray Huey, pioneers of physiological ecology.
References
- Abzhanov A, Protas M, Grant B.R, Grant P.R, Tabin C.J. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. doi:10.1126/science.1098095 [DOI] [PubMed] [Google Scholar]
- Arnold S.J. Morphology, performance and fitness. Am. Zool. 1983;23:347–361. [Google Scholar]
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection: applications. Evolution. 1984;38:720–734. doi: 10.1111/j.1558-5646.1984.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Bartholomew G.A. A field study of temperature relations in the Galapagos marine iguana. Copeia. 1966;2:241–250. [Google Scholar]
- Boback S.M. Body size evolution in snakes: evidence from island populations. Copeia. 2003;39:81–94. [Google Scholar]
- Boersma P.D. An ecological study of the Galapagos marine iguana. In: Bowman R.I, Berson M, Leviton A.E, editors. Patterns of evolution in Galapagos organisms. AAAS, Pacific Division; San Francisco: 1984. pp. 157–176. [Google Scholar]
- Bonner J.T. Lines of investigation. Nature. 1984;312:475. doi:10.1038/312475a0 [Google Scholar]
- Bradbury J.W, Vehrencamp S.L, Gibson R. Leks and the unanimity of female choice. In: Greenwood P.J, Harvey P.H, Slatkin M, editors. Essays in the honour of John Maynard-Smith. Cambridge University Press; Cambridge, UK: 1985. pp. 301–314. [Google Scholar]
- Brown J.H, Maurer B.A. Macroecology: the division of food and space among species on continents. Science. 1989;243:1145–1150. doi: 10.1126/science.243.4895.1145. [DOI] [PubMed] [Google Scholar]
- Burness G.P, Diamond J, Flannery T. Dinosaurs, dragons, and dwarfs: the evolution of maximal body size. Proc. Natl Acad. Sci. USA. 2001;98:14518–14523. doi: 10.1073/pnas.251548698. doi:10.1073/pnas.251548698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P.J, Frappell P.B, Wang T, Wikelski M. The relationship between heart rate and rate of oxygen consumption in Galagagos marine iguanas (Amblyrhynchus cristatus) at two different temperatures. J. Exp. Biol. 2002;205:1917–1924. doi: 10.1242/jeb.205.13.1917. [DOI] [PubMed] [Google Scholar]
- Buttemer W.A, Dawson W.R. Temporal pattern of foraging and microhabitat use by Galapagos marine iguanas, Amblyrhynchus cristatus. Oecologia. 1993;96:56–64. doi: 10.1007/BF00318031. doi:10.1007/BF00318031 [DOI] [PubMed] [Google Scholar]
- Carpenter C. The marine iguana of the Galapagos Islands, its behavior and ecology. Proc. Calif. Acad. Sci. 4th Ser. 1966;34:329–376. [Google Scholar]
- Case T.J. Body size differences between populations of the chuckwalla, Sauromalus obesus. Ecology. 1976;57:313–323. [Google Scholar]
- Case T.J, Schwaner T.D. Island mainland body size differences in Australian varanid lizards. Oecologia. 1993;94:102–109. doi: 10.1007/BF00317309. doi:10.1007/BF00317309 [DOI] [PubMed] [Google Scholar]
- Charnov E. Some explorations of symmetry in evolutionary biology. Oxford University Press; New York: 1993. Life history invariants. [Google Scholar]
- Childs D.Z, Rees M, Rose K.E, Grubb P.J, Ellner S.P. Evolution of complex flowering strategies: an age- and size-structured integral projection model. Proc. R. Soc. B. 2003;270:1829–1838. doi: 10.1098/rspb.2003.2399. doi:10.1098/rspb.2003.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs D.Z, Rees M, Rose K.E, Grubb P.J, Ellner S.P. Evolution of size-dependent flowering in a variable environment: construction and analysis of a stochastic integral projection model. Proc. R. Soc. B. 2004;271:425–434. doi: 10.1098/rspb.2003.2597. doi:10.1098/rspb.2003.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D.M, Duncan R.A, McBirney A.R, Richards M.A.M.W.W, Harpp K.S, Fox C.G. Drowned islands downstream from the Galapagos hotspot imply extended speciation times. Nature. 1992;355:246–258. doi:10.1038/355246a0 [Google Scholar]
- Curio E. Zur geographischen Variation des Feinderkennens einiger Darwinfinken (Geospizinae) Z. Tierpsychol. 1965;26:394–487. [Google Scholar]
- Darwin C. new edition. Appleton; New York: 1883. Journal of researches. [Google Scholar]
- Drent J, Lichtenbelt W.D.V, Wikelski M. Effects of foraging mode and season on the energetics of the marine iguana, Amblyrhynchus cristatus. Funct. Ecol. 1999;13:493–499. doi:10.1046/j.1365-2435.1999.00337.x [Google Scholar]
- Easterling M.R, Ellner S.P, Dixon P.M. Size-specific sensitivity: applying a new structured population model. Ecology. 2000;81:694–708. [Google Scholar]
- Grant P.R. Princeton University Press; Princeton, NJ: 1986. Ecology and evolution of Darwin's finches. [Google Scholar]
- Harvey P.H, Purvis A. Understanding the ecological and evolutionary reasons for life history variation: mammals as a case study. In: McGlade J, editor. Advanced ecological theory: principles and applications. Blackwell Science; Oxford: 1999. pp. 232–248. [Google Scholar]
- Hobson E.S. Observations on diving in the Galapagos marine iguana, Amblyrhynchus cristatus (Bell) Copeia. 1965;1965:249–250. [Google Scholar]
- Hofmann H, Benson M.E, Fernald R.D. Social status regulates growth rate: consequences for life-history strategies. Proc. Natl Acad. Sci. USA. 1999;96:14 171–14 176. doi: 10.1073/pnas.96.24.14171. doi:10.1073/pnas.96.24.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illius A.W, Gordon I.J. Modeling the nutritional ecology of ungulate herbivores: evolution of body size and competitive interactions. Oecologia. 1992;89:428–434. doi: 10.1007/BF00317422. [DOI] [PubMed] [Google Scholar]
- Keogh J.S, Scott I.A.W, Hayes C. Rapid and repeated origin of insular gigantism and dwarfism in Australian tiger snakes. Evolution. 2005;59:226–233. [PubMed] [Google Scholar]
- Laurie W.A. Effects of the 1982–1983 El Niño-Southern oscillation event on marine iguanas (Amblyrhynchus cristatus, Bell, 1825) populations in the Galápagos islands. In: Glynn P, editor. Global Ecological Consequences of the 1982–1983 El Niño-Southern Oscillation. Elsevier; New York: 1989. pp. 121–141. [Google Scholar]
- Laurie W.A, Brown D. Population biology of marine iguanas (Amblyrhynchus cristatus). 2. Changes in annual survival rates and the effects of size, sex, age and fecundity in a population crash. J. Anim. Ecol. 1990a;59:529–544. [Google Scholar]
- Laurie W.A, Brown D. Population biology of marine iguanas (Amblyrhynchus cristatus). 3. Factors affecting survival. J. Anim. Ecol. 1990b;59:545–568. [Google Scholar]
- Losos J.B, Warheit K.I, Schoener T.W. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature. 1997;387:70–73. doi:10.1038/387070a0 [Google Scholar]
- Losos J.B, Jackman T.R, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- Luiselli L. Snakes don't shrink, but ‘shrinkage’ is an almost inevitable outcome of measurement error by the experimenters. Oikos. 2005;110:199–202. doi:10.1111/j.0030-1299.2005.14034.x [Google Scholar]
- Mackie R.I, Rycyk M, Ruemmler R.L, Aminov R.I, Wikelski M. Biochemical and microbiological evidence for fermentative digestion in free-living land iguanas (Conolophus pallidus) and marine iguanas (Amblyrhynchus cristatus) on the Galapagos archipelago. Phys. Biochem. Zool. 2003;77:127–138. doi: 10.1086/383498. doi:10.1086/383498 [DOI] [PubMed] [Google Scholar]
- Madsen T, Shine R. Phenotypic plasticity in body sizes and sexual size dimorphism in European grass snakes. Evolution. 1993;47:321–325. doi: 10.1111/j.1558-5646.1993.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Madsen T, Shine R. Do snakes shrink? Oikos. 2001;92:187–188. doi:10.1034/j.1600-0706.2001.920122.x [Google Scholar]
- Meiri S, Dayan T. On the validity of Bergmann's rule. J. Biogeogr. 2003;30:331–351. [Google Scholar]
- Nagy K.A, Shoemaker V.H. Field energetics and food consumption of the Galápagos marine iguana, Amblyrhynchus cristatus. Physiol. Zool. 1984;57:281–290. [Google Scholar]
- Niewiarowski P.H, Roosenburg W. Reciprocal transplant reveals sources of variation in growth-rates of the lizard Sceloporus undulatus. Ecology. 1993;74:1992–2002. [Google Scholar]
- Partecke J, von Haenseler A, Wikelski M. Territory establishment in lekking marine iguanas, Amblyrhynchus cristatus: support for the hotshot mechanism. Behav. Ecol. Sociobiol. 2002;6:579–587. doi:10.1007/s00265-002-0469-z [Google Scholar]
- Perrin N. On body size, energy and fitness. Funct. Ecol. 1998;12:500–502. doi:10.1046/j.1365-2435.1998.00217.x [Google Scholar]
- Peters R.H. Cambridge University Press; Cambridge, UK: 1983. The ecological implications of body size. [Google Scholar]
- Rassmann K. Evolutionary age of the galapagos iguanas predates the age of the present Galapagos islands. Mol. Phylognet. Evol. 1997;7:158–172. doi: 10.1006/mpev.1996.0386. doi:10.1006/mpev.1996.0386 [DOI] [PubMed] [Google Scholar]
- Rassmann K, Tautz D, Trillmich F, Gliddon C. The microevolution of the Galapagos marine iguana Amblyrhynchus cristatus assessed by nuclear and mitochondrial genetic analyses. Mol. Ecol. 1997;6:437–452. doi:10.1046/j.1365-294X.1997.00209.x [Google Scholar]
- Rees M, Rose K.E. Evolution of flowering strategies in Oenothera glazioviana: an integral projection model approach. Proc. R. Soc. B. 2002;269:1509–1515. doi: 10.1098/rspb.2002.2037. doi:10.1098/rspb.2002.2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss M.J. Cambridge University Press; Cambridge, UK: 1989. The allometry of growth and reproduction. [Google Scholar]
- Ricklefs R, Wikelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Romero L.M, Wikelski M. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proc. Natl Acad. Sci. USA. 2001;98:7366–7370. doi: 10.1073/pnas.131091498. doi:10.1073/pnas.131091498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein D.R, Wikelski M. Seasonal changes in food quality: a proximate cue for reproductive timing in marine iguanas. Ecology. 2003;84:3013–3023. [Google Scholar]
- Rubenstein, D. R. & Wikelski, M. In press. Steroid hormones and aggression in female Galapagos marine iguanas. Hormon. Behav (doi:10.1016/j.yhbeh.2005.04.006) [DOI] [PubMed]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, UK: 1984. Scaling. Why is animals size so important? [Google Scholar]
- Snell H.L, Jennings R.D, Snell H.M, Harcourt S. Intrapopulation variation in predator-avoidance performance of Galapagos lava lizards: the interaction of sexual and natural selection. Evol. Ecol. 1988;2:353–369. [Google Scholar]
- Stamps J.A, Andrews R.M. Estimating asymptotic size using the largest individuals per sample. Oecologia. 1992;62:33–40. doi: 10.1007/BF00317842. [DOI] [PubMed] [Google Scholar]
- Stern D.L. Body-size control: how an insect knows it has grown enough. Curr. Biol. 2003;13:R267–R269. doi: 10.1016/s0960-9822(03)00197-0. doi:10.1016/S0960-9822(03)00197-0 [DOI] [PubMed] [Google Scholar]
- Tracy C.R. Differences in body size among chuckwalla (Sauromalus obesus) populations. Ecology. 1999;80:259–271. [Google Scholar]
- Trillmich K.G.K. The mating system of the marine iguana (Amblyrhynchus cristatus) Z. Tierpsychol. 1983;63:141–172. [Google Scholar]
- Trillmich K.G.K, Trillmich F. Foraging strategies of the marine iguana, Amblyrhynchus cristatus. Behav. Ecol. Sociobiol. 1986;18:259–266. doi:10.1007/BF00300002 [Google Scholar]
- VanDenburgh J, Slevin J.R. Expedition of the California academy of sciences to the Galapagos islands, 1905–1906. IX. The Galapagoan lizards of the genus Tropidurus; with notes on the iguanas of the genera Conolophus and Amblyrhynchus. Proc. Calif. Acad. Sci. 1913;4:133–202. [Google Scholar]
- West-Eberhard M.J. Oxford University Press; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- Wikelski M, Bäurle S. Pre-copulatory ejaculation solves time constraints during copulations in marine iguanas. Proc. R. Soc. B. 1996;263:439–444. [Google Scholar]
- Wikelski M, Carbone C. Environmental scaling of body size in island populations of Galápagos marine iguanas. In: Alberts A.C, Carter R.L, Hayes W.K, Martins E.P, editors. Biology and conservation of Iguanas. University of California Press; Berkeley: 2004. pp. 148–157. [Google Scholar]
- Wikelski M, Hau M. Is there an endogenous tidal foraging rhythm in marine iguanas? J. Biol. Rhythms. 1995;10:345–360. doi: 10.1177/074873049501000407. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Romero L.M. Body size, performance and fitness in Galapagos marine iguanas. Integr. Comp. Biol. 2003;43:376–386. doi: 10.1093/icb/43.3.376. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Thom C. Marine iguanas shrink to survive El Niño. Nature. 2000;403:37–38. doi: 10.1038/47396. doi:10.1038/47396 [DOI] [PubMed] [Google Scholar]
- Wikelski M, Trillmich F. Foraging strategies of the Galápagos marine iguana (Amblyrhynchus cristatus): adapting behavioral rules to ontogenetic size change. Behaviour. 1994;128:255–279. [Google Scholar]
- Wikelski M, Trillmich F. Body size and sexual size dimorphism in marine iguanas fluctuate as a result of opposing natural and sexual selection: an island comparison. Evolution. 1997;51:922–936. doi: 10.1111/j.1558-5646.1997.tb03673.x. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Wrege P.H. Niche expansion, body size and survival in Galapagos marine iguanas. Oecologia. 2000;124:107–115. doi: 10.1007/s004420050030. doi:10.1007/s004420050030 [DOI] [PubMed] [Google Scholar]
- Wikelski M, Gall B, Trillmich F. Ontogenetic changes in food-intake and digestion rate of the herbivorous marine iguana (Amblyrhynchus cristatus, Bell) Oecologia. 1993;94:373–379. doi: 10.1007/BF00317112. doi:10.1007/BF00317112 [DOI] [PubMed] [Google Scholar]
- Wikelski M, Carbone C, Trillmich F. Lekking in marine iguanas: female grouping and male reproductive strategies. Anim. Behav. 1996;52:581–596. doi:10.1006/anbe.1996.0199 [Google Scholar]
- Wikelski M, Carrillo V, Trillmich F. Energetic limits to body size in a grazing reptile, the Galapagos marine iguana. Ecology. 1997;78:2204–2217. [Google Scholar]
- Wikelski M, Carbone C, Bednekoff P.A, Choudhury C, Tebbich S. Female choice in marine iguana leks: a wider selection of males obtained at a cost. Ethology. 2001;107:623–638. doi:10.1046/j.1439-0310.2001.00701.x [Google Scholar]
- Wikelski M, Steiger S, Gall B, Nelson K.N. Sex, drugs and mating role: testosterone-induced phenotype-switching in Galapagos marine iguanas. Behav. Ecol. 2005;16:260–268. doi:10.1093/beheco/arh160 [Google Scholar]
- Woodley S.K, Painter D.L, Moore M.C, Wikelski M, Romero L.M. Effect of tidal cycle and food intake on the baseline plasma corticosterone rhythm in intertidally foraging marine iguanas. Gen. Comp. Endocrinol. 2003;132:216–222. doi: 10.1016/s0016-6480(03)00085-6. doi:10.1016/S0016-6480(03)00085-6 [DOI] [PubMed] [Google Scholar]


