Abstract
Normally ovulating women have been found to report greater sexual attraction to men other than their own partners when near ovulation relative to the luteal phase. One interpretation is that women possess adaptations to be attracted to men possessing (ancestral) markers of genetic fitness when near ovulation, which implies that women's interests should depend on qualities of her partner. In a sample of 54 couples, we found that women whose partners had high developmental instability (high fluctuating asymmetry) had greater attraction to men other than their partners, and less attraction to their own partners, when fertile.
Keywords: sexual selection, human, developmental instability, fluctuating asymmetry, ovulation
1. Introduction
Women's sexual preferences and interests change across their ovulatory cycles. Their preferences for a number of traits, including facial masculinity (Penton-Voak & Perrett 1999; Penton-Voak et al. 1999; Johnston et al. 2001), body scents associated with symmetry (Gangestad & Thornhill 1998; Rikowski & Grammer 1999; Thornhill & Gangestad 1999; Thornhill et al. 2003), masculine voice qualities (Putz in press) and behavioural displays of intrasexual competitiveness (Gangestad et al. 2004) peak near ovulation. These shifts appear when normally ovulating women rate men's short-term sexual attractiveness; studies have not detected changes in long-term mating preferences (Penton-Voak et al. 1999; Gangestad et al. 2004). In addition, women's extra-pair desires appear to change across the cycle. Gangestad et al. (2002) found that women reported greater attraction to extra-pair mates when fertile than when not; in-pair sexual attraction did not vary with cycle phase (cf. Pillsworth et al. 2004).
Female mate choice adaptations can evolve to favour males who confer material or genetic benefits on offspring. In humans and socially monogamous birds, for example, males may directly invest parental care or indirectly confer heritable genetic benefits (good genes) on offspring (Møller & Alatalo 1999; Gangestad & Simpson 2000; Jennions & Petrie 2000; Kokko et al. 2003). Females could benefit from both paternal care and good genes offered by long-term male partners. Though males displaying indicators of genetic quality are sexually attractive, they may provide less parental care. In the collared flycatcher (Ficedula albicollis), males who sport a large forehead patch—a sexually selected indicator of good genes (e.g. Sheldon et al. 1997)—invest less in offspring (Qvarnström 1999). In humans, symmetry may be an indicator of good genes (or may have been so ancestrally). Across species on average, symmetrical individuals experience greater mating success than less symmetrical individuals; research has effectively ruled out publication bias in this literature through both direct and indirect means (Møller et al. in press). In humans, it appears to be associated with cues of male sexual attractiveness (e.g. Gangestad & Thornhill 1998; Rikowski & Grammer 1999; Thornhill & Gangestad 1999; Thornhill et al. 2003). In addition, more symmetrical men tend toward a short-term mating strategy (e.g. Gangestad & Thornhill 1997).
In mating markets driven by supply and demand, some females fail to attract long-term mates offering good genes. These females may sometimes benefit from a strategy in which they secure investment from a long-term mate and obtain genetic benefits from extra-pair partners. In collared flycatchers, females whose social mates possess small forehead patches are more likely to engage in extra-pair copulation (Michl et al. 2002); their extra-pair partners are furthermore likely to possess large forehead patches (Sheldon & Ellegren 1999). In addition, females time their extra-pair copulations to occur in the middle of their fertile periods, increasing the chance that extra-pair partners will sire their offspring (Michl et al. 2002). Hence, females in this species appear to possess adaptations for obtaining good genes through extra-pair copulation.
Ancestrally, women may have benefited from a similar strategy (though, naturally, extra-pair copulation could play other roles as well; Greiling & Buss 2000). A woman could obtain genetic benefits of extra-pair mating only when fertile, but its costs (e.g. as a result of partner jealousy) extend throughout her cycle. Gangestad & Thornhill (1998) proposed selection for extra-pair mating to obtain genetic benefits should therefore have produced adaptation analogous to that seen in the collared flycatcher: increased extra-pair desires and preferences for indicators of good genes near ovulation, when conception is most likely. Although the notion that genetic benefits (in ancestral populations) account for the preference shifts remains speculative (e.g. Gangestad & Thornhill 2003; Fuller & Houle 2003), systematic shifts of preferences are well established.
Additional predictions about changes in women's sexual interests follow from this adaptationist hypothesis. The benefits of extra-pair mating for good genes outweigh its costs only for women with primary partners who offer relatively low genetic benefits to offspring. Hence, the ovulatory cycle shift in women's extra-pair desires and flirtation should be strongest for women with partners who lack traits preferred by women when fertile. Women whose partners lack these traits may furthermore be less sexually attracted to their partners when fertile. We tested this hypothesis by examining the moderating effect of male partners' fluctuating asymmetry (FA) on women's extra-pair and in-pair sexual interests across the cycle.
2. Material and Methods
(a) Participants
Participants were 54 romantically involved heterosexual couples recruited at the University of New Mexico. All women (ages 18–44) were normally ovulating (i.e. they were not using a contraceptive pill or other hormone-based contraceptive). Participants were paid for their participation. Some were given credit toward a psychology course research requirement in addition to $20. Others were paid either $50 (if male) or $70 (if female; women did luteinizing hormone (LH) tests). Women were, on average, 20.78 years old (s.d.=4.30) and men averaged 21.91 years old (s.d.=4.79). Relationship duration had a median of 14 months (range=1 month to 20 years; male and female reports of duration correlated 0.99 and were averaged). The majority of couples were exclusively dating one another (N=48, of which six cohabited); six were married. None had been divorced. Few had children (N=5 of each sex, of which four had children with their current partner).
(b) Procedures
Couples reported for three questionnaire sessions: an introductory session and two additional sessions, one scheduled during the fertile period of the cycle (as established by an LH surge) and one scheduled during the luteal phase. In each of the latter two sessions, women reported their sexual attraction and fantasy to their primary partner and to men other than their primary partner in the previous two days. For each male participant, asymmetries of 10 features were measured and composited into an FA index.
Women were asked to come to the lab for LH tests (using the over-the-counter Ovusign tester) for up to 5 days, plus self-test on weekends (verified by our inspection of the strip) if necessary. All surged within 4 days after or 2 days prior to their scheduled high fertility session, hence falling into the fertile window (Wilcox et al. 1995). Low fertility sessions were conducted at least 5 days after an LH surge. The last three days of the cycle were avoided for scheduling.
All questionnaires were administered to individuals in separate private rooms. During the sessions administered on low and high fertility days, we asked participants for reports of their events, thoughts and feelings in the past two days. Two questions were asked to assess women's sexual attraction to their own partners during this period: ‘I felt strong sexual attraction toward my primary current partner’ and ‘I fantasized about sex with a current partner’. Three questions assessed women's sexual attraction to men other their primary partner: ‘I felt strong sexual attraction toward someone other than a current partner’, ‘I fantasized about sex with a stranger or acquaintance’ and ‘I fantasized about sex with a past partner’. Women responded on 5-point scales, where 0= ‘not at all’ and 4= ‘a great deal’. Values were summed across items to create composite measures of attraction to partner and extra-pair men.
Relationship satisfaction was assessed during the initial questionnaire session using Hendrick et al.'s (1998) 7-item Relationship Assessment Scale, a reliable and validated instrument. (Sample item: ‘In general, how satisfied are you with your relationship?’) Reliability in the current sample was 0.73.
Men's FA was measured on 10 body traits: ear length, ear width, elbow width, wrist width, length of the fingers, ankle width and foot width. The right and left sides were measured twice using metal calipers. The measurer called out measurements to a recorder. Multiple measurements intervened the two measurements and, because measurers could at best remember their previous measurements only with great difficulty even if they tried (and were instructed not to try), the two measures of right and left side were blind to one another. Directional asymmetry on the traits is minor; in a separate sample of about 700 individuals, only asymmetry of the foot was found to possess a small but significant directional bias, correction of which makes virtually no difference to the overall measure (Furlow et al. 1997). Each trait's FA was standardized (divided) by average trait size across all men in the sample. All 10 traits' FA were then summed to create the FA composite index. Repeatabilities of both signed and unsigned asymmetries were all highly significant; intraclass correlations ranged from 0.79 to 0.95 for signed and 0.58 to 0.88 for unsigned asymmetries, all p<0.000 001. The composite measure had a repeatability of 0.77, p<0.000 001. We asked all men whether they had broken or sprained any feature measured. Any feature broken or sprained was given the mean asymmetry if it had greater asymmetry than the mean. In total, 5.7% of features were affected. The adjusted measure correlated 0.95 with a measure involving no correction.
3. Results
We analysed women's sexual interests through general linear modelling (SPSS-PC 12.0). Target (extra-pair men versus primary partner) and cycle phase (fertile versus non-fertile) were treated as repeated measures. Order (fertile versus non-fertile questionnaire session first), Partner FA and relationship duration were predictors. Total N for all analyses was 54 couples. Women reported greater attraction to their own partners than to other men overall, F1,50=65.31, p<0.001, and greater attraction overall during the fertile phase of the cycle than during the luteal phase, F1,50=4.65, p=0.036. Replicating Gangestad et al. (2002), target and phase interacted, F1,50=7.29, p=0.009; women's attraction to extra-pair men was greater during the fertile phase, F1,50=10.51, p=0.002 (marginal means=2.32 and 1.31 for fertile and luteal phase, respectively), but their attraction to their own partners was not, F1,50=0.02, n.s. (marginal means=5.13 and 5.10). In support of our primary prediction, this two-way interaction was strongly moderated by partner FA, F1,50=15.64, p<0.001. Separate analyses on women's attraction to extra-pair men and primary partners revealed significant interactions between phase and partner FA for both—though, as expected, in opposite directions (see figures 1 and 2). Relative to women with more symmetrical men, women with less symmetrical partners experienced greater attraction to extra-pair men during the fertile phase, t50=2.85, p=0.006, but not during the luteal phase, t50=0.67, n.s.; interaction F1,50=6.58, p=0.013. By contrast, women with less symmetrical partners experienced less attraction to their own partners during the fertile phase, t50=−2.10, p=0.041, but not during the luteal phase, t50=0.02, n.s.; interaction F1,50=4.58, p=0.037.
Figure 1.
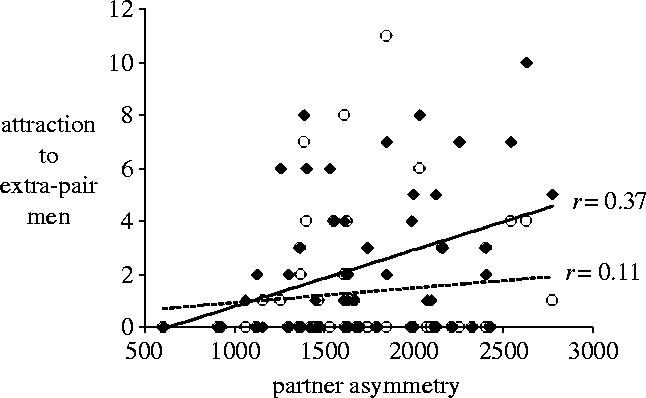
Scatterplots and regressions of women's sexual attraction to extra-pair men as a function of their primary partner's FA. Solid diamonds and solid regression line: fertile phase. Open circles and dashed regression line: luteal phase. r=0.37, p=0.006, and 0.11, n.s., respectively. Partner FA is the sum of the asymmetries across all 10 traits times 10 000 (to eliminate decimal places). A value of 2000 is equivalent to a mean of 2% asymmetry (relative to mean traits size in the sample) across the 10 traits.
Figure 2.
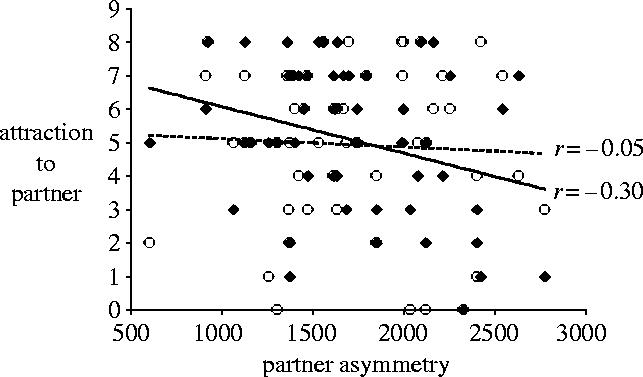
Scatterplots and regressions of women's sexual attraction to partners as a function of their primary partner's FA. Solid diamonds and solid regression line: fertile phase. Open circles and dashed regression line: luteal phase. r=−0.30, p=0.027, and −0.05, n.s., respectively.
Relationship satisfaction is known to strongly predict women's extra-pair sexual interests (e.g. Thompson 1983; Banfield & McCabe 2001). Hence, we added Hendrick et al.'s (1998) measure of women's relationship satisfaction as another predictor in additional analyses. As expected, relationship satisfaction interacted strongly with target, F1,49=23.58, p<0.001; women more satisfied in their relationships reported less extra-pair attraction, F1,49=12.37, p=0.001, and greater attraction to partners, F1,49=6.51, p=0.014. The predicted three-way interaction between target, phase and partner FA, however, was strengthened by inclusion of satisfaction as a predictor, F1,49=16.96, p<0.001. Within the fertile phase, partner FA was actually a stronger predictor of extra-pair attraction than satisfaction (within this sample), t49=2.70, p=0.010 and t49=−2.15, p=0.037, respectively. By contrast, satisfaction had a very strong effect on extra-pair attraction during the luteal phase, t49=−4.24, p<0.001, whereas partner FA had negligible effect, t49=0.32, n.s.
4. Discussion
In a sample of romantically involved couples, we replicated a previous finding that women experience greater attraction to and sexual fantasy about men other than primary partners when in the fertile phase of their cycles, but do not, on average, experience greater attraction toward their primary partners when fertile. More importantly, we found that the fluctuating asymmetry of women's partners moderates these effects. When fertile, women mated to relatively asymmetrical men do experience greater attraction to men other than their primary partners. Women mated to relatively symmetrical men, however, do not. By contrast, women mated to relatively symmetrical men report greater attraction to their partners when mid-cycle than do women mated to relatively asymmetrical men.
These findings importantly augment the empirical support for adaptation for seeking good genes in women. Not only do they provide additional support for increased attraction to men other than primary partners when women are fertile in the cycle; they show that this attraction is contingent on characteristics of the primary partner. We should note, however, that, although a number of findings are consistent with the claim that FA was an ancestral marker of good genes in humans (e.g. Gangestad & Thornhill 2003; see also Møller et al. in press), this claim remains speculative (e.g. Fuller & Houle 2003). We hence do not know for certain that female extra-pair interest mid-cycle is associated with a marker of partners' genetic fitness. One alternative is that women seek viable sperm at this time (see Thornhill & Gangestad (1999) for a discussion; see also Manning et al. (1998)).
Naturally, women's attraction to men other than their primary partners probably leads to extra-pair sex only occasionally. The costs associated with the potential loss of a relationship partner are often too high for women to act on their interests. Hence, relationship satisfaction importantly affects extra-pair attraction and actual infidelity.
The frequency of female extra-pair copulation (EPC) in humans appears to be, on average, modest and variable across populations. Of a large random sample of married women in the USA interviewed face-to-face, 15% admitted to extramarital sex (Laumann et al. 1994); anonymous questionnaire studies have yielded a mean rate of about 30% (see Thompson 1983). Studies using DNA or blood markers to ascertain paternity indicate that extra-pair paternity is rare in a Swiss population (1%; Sasse et al. 1994), moderate in Monterrey, Mexico (12%; Cerda-Flores et al. 1999), and high in that city's low-income subpopulation (20%; though only 5% in its high-income subsample).
Are these modest levels consistent with the existence of adaptation for seeking good genes in women? Imagine an experiment that will never be done, one that parallels Rice's (1996) seminal work on sexually antagonistic coevolution. Suppose that women were allowed to evolve in response to men but men not allowed to adapt to women. After many generations, women would likely gain an edge in the conflicts between the sexes—possibly evolving better means of circumventing male vigilance, reducing the costs of obtaining genetic benefits through extra-pair mating and, accordingly, doing so more often. Alternatively, if men but not women were allowed to evolve, men might evolve better means of detecting women's ovulation and avoiding cuckoldry, thereby reducing the frequency of women's extra-pair sex. Of course, neither scenario has occurred; the sexes have coevolved and, most likely, both sex's genetic interests are compromised by adaptations of the other sex. The mating strategies and tactics of both sexes have possibly undergone substantial revision through rounds of adaptation, counter-adaptation, counter–counter adaptation, etc.—without, ironically, the actual extra-pair paternity rate ever having been extraordinary. Whether 2 or 20% or, as we suspect, somewhere in between (and probably variable across ecological contexts), current estimates of extra-pair paternity may well be consistent with the idea that women have adaptation for seeking genetic benefits through EPC.
Acknowledgments
This work is based on a National Science Foundation grant awarded to the first two authors.
References
- Banfield S, McCabe M.P. Extra-relationship involvement among women: are they different from men? Arch. Sex. Behav. 2001;30:199–142. doi: 10.1023/a:1002773100507. 10.1023/A:1002773100507 [DOI] [PubMed] [Google Scholar]
- Cerda-Flores R.M, Barton S.A, Marty-Gonzalez L.F, Rivas F, Chakraborty R. Estimation of nonpaternity in the Mexican population of Nueveo Leon: a validation study with blood group markers. Am. J. Phys. Anthropol. 1999;109:281–293. doi: 10.1002/(SICI)1096-8644(199907)109:3<281::AID-AJPA1>3.0.CO;2-3. 10.1002/(SICI)1096-8644(199907)109%3A3%3C281%3A%3AAID-AJPA1%3E3.0.CO%3B2-3 [DOI] [PubMed] [Google Scholar]
- Fuller R.C, Houle D. Inheritance of developmental instability. In: Polak M, editor. Developmental instability: causes and consequences. Cambridge University Press; Cambridge, UK: 2003. pp. 157–186. [Google Scholar]
- Furlow B.F, Armijo-Prewitt T, Gangestad S.W, Thornhill R. Fluctuating asymmetry and psychometric intelligence. Proc. R. Soc. B. 1997;264:823–829. doi: 10.1098/rspb.1997.0115. 10.1098/rspb.1997.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S.W, Simpson J.A. The evolution of human mating: the role of trade-offs and strategic pluralism. Behav. Brain Sci. 2000;23:573–587. doi: 10.1017/s0140525x0000337x. 10.1017/S0140525X0000337X [DOI] [PubMed] [Google Scholar]
- Gangestad S.W, Thornhill R. The evolutionary psychology of extrapair sex: the role of fluctuating asymmetry. Evol. Hum. Behav. 1997;18:69–88. 10.1016/S1090-5138(97)00003-2 [Google Scholar]
- Gangestad S.W, Thornhill R. Menstrual cycle variation in women's preference for the scent of symmetrical men. Proc. R. Soc. B. 1998;265:927–933. doi: 10.1098/rspb.1998.0380. 10.1098/rspb.1998.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S.W, Thornhill R. Fluctuating asymmetry, developmental stability, and fitness: toward model-based interpretation. In: Polak M, editor. Developmental instability: causes and consequences. Cambridge University Press; Cambridge, UK: 2003. pp. 62–80. [Google Scholar]
- Gangestad S.W, Thornhill R, Garver C.E. Changes in women's sexual interests and their partners' mate retention tactics across the menstrual cycle: evidence for shifting conflicts of interest. Proc. R. Soc. B. 2002;269:975–982. doi: 10.1098/rspb.2001.1952. 10.1098/rspb.2001.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S.W, Simpson J.A, Cousins A.J, Garver-Apgar C.E, Christensen J.N. Women's preferences for male behavioral displays change across the menstrual cycle. Psychol. Sci. 2004;15:203–207. doi: 10.1111/j.0956-7976.2004.01503010.x. 10.1111/j.0956-7976.2004.01503010.x [DOI] [PubMed] [Google Scholar]
- Greiling H, Buss D.M. Women's sexual strategies: the hidden dimension of short-term extra-pair mating. Pers. Indiv. Differ. 2000;28:929–963. 10.1016/S0191-8869(99)00151-8 [Google Scholar]
- Hendrick S.S, Dicke A, Hendrick C. The relationship assessment scale. J. Soc. Pers. Relat. 1998;15:137–142. [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. 10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Johnston V.S, Hagel R, Franklin M, Fink B, Grammer K. Male facial attractiveness: evidence for hormone mediated adaptive design. Evol. Hum. Behav. 2001;23:251–267. 10.1016/S1090-5138(01)00066-6 [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. 10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann E.O, Gagnon J.H, Michael R.T, Michaels S. The social organization of sexuality. University of Chicago Press; Chicago, IL: 1994. [Google Scholar]
- Manning J.T, Scutt D, Lewis-Jones D.I. Developmental stability, ejaculate size and sperm quality in men. Evol. Hum. Behav. 1998;19:273–182. 10.1016/S1090-5138(98)00024-5 [Google Scholar]
- Michl G, Torok J, Griffith S.C, Sheldon B.C. Experimental analysis of sperm competition mechanisms in a wild bird population. Proc. Natl Acad. Sci. USA. 2002;99:5466–5470. doi: 10.1073/pnas.082036699. 10.1073/pnas.082036699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A.P, Alatalo R.V. Good-genes effects in sexual selection. Proc. R. Soc. B. 1999;266:85–91. 10.1098/rspb.1999.0607 [Google Scholar]
- Møller, A. P., Thornhill, R., Gangestad, S. W. In press. Direct and indirect tests for publication bias: asymmetry and sexual selection. Anim. Behav.
- Pillsworth E.G, Haselton M.G, Buss D.M. Ovulatory shifts in female sexual desire. J. Sex Res. 2004;41:55–65. doi: 10.1080/00224490409552213. [DOI] [PubMed] [Google Scholar]
- Penton-Voak I.S, Perrett D.I. Female preference for male faces changes cyclically: further evidence. Evol. Hum. Behav. 1999;21:39–48. 10.1016/S1090-5138(99)00033-1 [Google Scholar]
- Penton-Voak I.S, Perrett D.I, Castles D, Burt M, Koyabashi T, Murray L.K. Menstrual cycle alters face preference. Nature. 1999;399:741–742. doi: 10.1038/21557. 10.1038/21557 [DOI] [PubMed] [Google Scholar]
- Putz, D. A. In press. Menstrual cycle and mating context affect women's preference for male voice pitch. Evol. Hum. Behav.
- Qvarnström A. Different reproductive tactics in male collared flycatchers signalled by size of secondary sexual character. Proc. R. Soc. B. 1999;262:2089–2093. [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. 10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rikowski A, Grammer K. Human body odour, symmetry and attractiveness. Proc. R. Soc. B. 1999;266:869–874. doi: 10.1098/rspb.1999.0717. 10.1098/rspb.1999.0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse G, Muller H, Chakraborty R, Ott J. Estimating the frequency of nonpaternity in Switzerland. Hum. Hered. 1994;44:337–343. doi: 10.1159/000154241. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Ellegren H. Sexual selection resulting from extrapair paternity in collared flycatchers. Anim. Behav. 1999;57:285–298. doi: 10.1006/anbe.1998.0968. 10.1006/anbe.1998.0968 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Merila J, Qvarnstrom A, Gustafsson L, Ellegren H. Paternal genetic contribution to offspring condition predicted by size of male secondary sexual character. Proc. R. Soc. B. 1997;264:297–302. 10.1098/rspb.1997.0042 [Google Scholar]
- Thompson A.P. Extramarital sex: a review of the research literature. J. Sex Res. 1983;19:1–22. [Google Scholar]
- Thornhill R, Gangestad S.W. The scent of symmetry: a human sex pheromone that signals fitness? Evol. Hum. Behav. 1999;20:175–201. 10.1016/S1090-5138(99)00005-7 [Google Scholar]
- Thornhill R, Gangestad S.W, Miller R, Scheyd G, McCollough J, Franklin M. MHC, symmetry and body scent attractiveness in men and women (Homo sapiens) Behav. Ecol. 2003;14:668–678. 10.1093/beheco/arg043 [Google Scholar]
- Wilcox A.J, Weinberg C.R, Baird B.D. Timing of sexual intercourse in relation to ovulation. N. Engl. J. Med. 1995;333:1517–1521. doi: 10.1056/NEJM199512073332301. 10.1056/NEJM199512073332301 [DOI] [PubMed] [Google Scholar]


