Abstract
Oestrogens organize and activate circuits within the vertebrate central nervous system. Oestrogen synthesis occurs via the expression of aromatase, a P450 enzyme detected in microsomes and more recently in pre-synaptic boutons. Synaptic aromatase has only been described in brain regions that express aromatase in many subcellular compartments, so its function remains poorly understood. To more thoroughly study the role of oestrogen synthesis at synaptic terminals, we examined the ultrastructural compartmentalization of aromatase in the zebra finch; a species in which high aromatase activity can be measured in brain areas that do not contain somal aromatase. Here, we report the presence of aromatase in pre-synaptic boutons in the hippocampus and the high vocal centre brain areas with low and undetectable somal aromatase, respectively, in addition to areas with abundant somal aromatase such as the preoptic area and caudomedial nidopallium. At these brain areas, males had more total synapses, more aromatase pre-synaptic boutons and importantly, the proportion of total synaptic profiles that expressed aromatase was significantly higher in males relative to females. Aromatase-positive pre-synaptic boutons were always observed innervating aromatase-negative post-synaptic elements. We conclude that oestrogen may be provided to discrete oestrogen-sensitive targets by synaptic aromatization. Further, some targets may be exposed to more oestrogen in males. The expression of aromatase in individual synapses of projection neurons represents a unique mechanism of neuroendocrine action. Neurons with steroidogenic capability may modulate distant targets with the specificity of axonal innervation.
Keywords: oestrogen, testosterone, synapse, learning, electron microscopy
1. Introduction
Oestrogens have potent effects on the developing and adult brain. Oestrogen can be provided to steroid-sensitive circuits by the neural expression of aromatase, the enzyme that catalyses the conversion of circulating androgens to oestrogens. Aromatase in the vertebrate brain is well described (Vockel et al. 1990; Schlinger & Arnold 1991; Shen et al. 1995; Naftolin et al. 1996; Saldanha et al. 2000; Roselli & Resko 2001; Soma et al. 2003). Aromatase is particularly abundant in the songbird telencephalon (Schlinger & Arnold 1991) but is conspicuously absent in several areas that contain oestrogen receptor (ER) (Shen et al. 1995; Saldanha et al. 2000; Soma et al. 2003). Vockel et al. (1990) reported high levels of aromatase activity in the zebra finch high vocal centre (HVC) and robust nucleus of the archistriatum (RA), but subsequent studies using in situ hybridization or immunocytochemistry (ICC) show few or no aromatase-positive somata (Shen et al. 1995; Saldanha et al. 2000). The well documented action of oestrogen upon some song nuclei (see Schlinger 1997) in the absence of the predominant source of oestrogen creates a paradox. How are these oestrogen-sensitive song-control nuclei provided with oestrogen?
Since 1989, evidence has supported the concept that oestrogen is present in synaptic terminals. Biochemical analyses of subcellular fractions of quail (Coturnix japonica) hypothalami (Schlinger & Callard 1989) and whole telencephalons of a songbird (Schlinger & Arnold 1992) revealed high aromatase activity in synaptosomal preparations. Using immuno-electron microscopy (EM; Naftolin et al. 1996), the pre-synaptic localization of aromatase was confirmed in the hypothalamus of rat (Rattus norvegicus), monkey (Cercopithecus aethiops), human (Homo sapiens) and quail. These brain regions were shown also to contain aromatase in many somata, so the function of aromatase in synapses per se remained unclear.
Very little is known about the role of aromatase expression at the synapse. Aromatase may be trafficked via axonal processes to pre-synaptic terminals that innervate steroid-sensitive targets in regions devoid of somal aromatase. In order to better understand synaptic aromatase, it is important to evaluate a system where synaptic aromatase expression is found separate from aromatase-positive somata. Using ICC, we previously showed that aromatase-positive neurons in the zebra finch project into HVC; a region devoid of somal aromatase (Saldanha et al. 2000). These cells are located in the shelf region outside HVC and send beaded, axon-like, processes dorsally into its neuropil. Taken together with the biochemical reports of aromatase activity in songbird synaptosomal preparations (Schlinger & Arnold 1992) these data make the zebra finch an excellent model in which to examine the role of synaptic aromatization. We, therefore, utilized immuno-electron microscopy to determine if synaptic aromatase is found in areas of low somal aromatase expression. In addition, the brain of male zebra finches may be exposed to more oestrogen than that of females (for review see Schlinger 1997) but the mechanism for male-biased oestrogen provision has not been identified (Vockel et al. 1990; Schlinger & Arnold 1992; Wade et al. 1995; Freking et al. 2000). Therefore, we also looked for sex differences in synaptic aromatase.
2. Material and methods
All experiments were conducted in accordance with the Lehigh University and UCLA IACUC rules and regulations. Subjects were adult (>120 days) zebra finches (Taeniopygia guttata, N=4 per sex). Birds were housed in same-sex groups in a room maintained at 20±2 °C and a 14 : 10 LD cycle. Food, water, grit and a cuttle-bone were available ad libitum. Birds were deeply anaesthetized (ketamine/xylazine) and transcardially perfused with 0.1 M phosphate buffer (PB; 5 ml) followed by 40 ml 4% paraformaldehyde (pH=7.35) containing 15% picric acid (PARAPIC). The gonads were removed and the size of the testes (males) and two largest follicles (females) was recorded. The brain was removed and immersed in 4% paraformaldehyde for 48 h at 4 °C, gel-embedded in 8% gelatin (following a coat of 4% gelatin) and re-immersed in 4% paraformaldehyde for 72–96 h at 4 °C. Coronal, 50 μm-sections were cut on a vibratome into 0.1 M PB.
The specificity of the antibody used in the present experiments has been previously validated. Briefly, in Western blots, this polyclonal antibody is known to recognize a single band in whole cell lysates from gonads, telencephalons and diencephalons but not pectoral muscle in adult zebra finches of both sexes (Saldanha et al. 2000). Further, in ICC experiments, absorption of the antibody with the antigen (absorption control) and removal of the antibody from the protocol (no primary control) results in total absence of immunoproduct (Saldanha et al. 2000). In order to control for variations across multiple ICC runs in the present experiment, each reaction included a complete set of sections from one male and one female (4 ICC runs in total). Thus, for each of four pairs of subjects all solutions in the protocol were identical.
Aromatase ICC was conducted using previously published protocols (Saldanha et al. 2000) except for the following. Saponin (0.05%) was used instead of TritonX100 and sections were exposed to the primary antibody (AZAC) for 96 h at 4 °C at 1 : 2500. All washes were done in 0.1 M PB and for the colour reaction, peroxide was generated in situ via the action of glucose oxidase on β-d-glucose in the presence of ammonium chloride (see Saldanha et al. 2001).
Areas that contained immunoproduct within the hypothalamic preoptic area (HPOA), caudomedial nidopallium (NCM) and hippocampus (HP) were viewed under low power and sections (approximately 4 mm2) were dissected into 0.9% saline. In the HPOA and NCM, to constrain dissections within the appropriate brain area, care was taken to excise only the centre of the area while leaving the periphery untouched. Since, aromatase expression in NCM can be relatively diffuse, only the centre of the field was excised and corresponded to the area immediately medial to Field L on the coronal section that first demonstrated a clear HVC. For HP, in each coronal section rostral to the anterior commissure, the entire left and right HP were embedded separately. Only sections from the medial portions of these blocks were analysed for EM. For HVC, the condenser was moved slightly out of alignment rendering the nucleus visible despite lack of observable immunoproduct. Dissections were made in the centre of HVC and care was taken to avoid its dorsal ependymal wall and ventral shelf. The remainder of the coronal section was mounted, dehydrated, coverslipped and examined under the light microscope to reconfirm that microdissections were indeed within the appropriate nucleus and restricted to the centre of the nucleus.
Microdissected sections were washed in 0.9% saline, exposed to 2% osmium tetroxide in 1.5% potassium ferricyanide (2 h), serially dehydrated through alcohols, embedded in Epon (Electron Microscopy Sciences) and polymerized at 65 °C overnight. Thick sections (1 μm) were cut on an ultratome (Sorvall MT2) and examined for presence of immunoproduct on a light microscope. Once encountered, sections were thin sectioned (50–70 nm) and collected onto Formvar coated copper grids. For HVC, thin sectioning began as soon as tissue was encountered. Grids were air dried in a dust-free compartment in preparation for EM.
(a) Data collection and analysis
Thick sections (1 μm) were examined under a light microscope and all visible somata were counted in every section that was subsequently examined under EM (5–18 sections per area per bird). For EM, sections were viewed using a Phillips 420 microscope equipped with a 10 μm aperture and a CCD camera. Photomicrographs of labelled soma (HPOA, NCM and HP) were collected at low power (1000–3000×). Subsequently, high power images (5500×) of the adjacent neuropil and HVC were sampled. For each brain area we initially scanned the tissue under high magnification to ensure the presence of immunoproduct, whereupon 15–20 photomicrographs of non-overlapping fields were taken from each area per bird. In all, data were collected from 555 photomicrographs.
Images were optimized for brightness and contrast only (Photoshop) and a grid (7×7″, intersections at every square inch) was placed over the picture. An experimenter then recorded the type of ultrastructural element under each of the 36 points within the grid (margins were ignored) and whether the element was stained or unstained. Dendrites (identified by the presence of rough endoplasmic reticula (RER) and lack of microtubule bundles), axons (identified as fine processes with bundled microtubules and no RER), terminals (identified by the presence of aggregations of clear vesicles) and pre-synaptic boutons (identified as terminals but with clusters of vesicles around putative active zones and distinct pre- and post-synaptic membranes and a post-synaptic density) were counted and classified as containing immunoproduct (stained) or devoid of detectable immunoproduct (unstained).
Photomicrographs were re-scored by an independent investigator and the resultant datasets did not differ significantly. The original data were averaged within brain area and then within sex. Data were analysed using ANOVA with sex and brain area as the main variables. Fisher least significant difference (LSD) (main effects) and least square means (LSM) comparisons (interactions) were used to determine the source of variation.
3. Results
All data in the present report reflect means±s.e.m. All male subjects had well developed testes (5.4±0.8 mm, major diameter; left testis). Females had follicles in various states of development (1.24–3.72 mm diameter; largest follicle).
As described previously (Saldanha et al. 2000; Peterson et al. 2001), aromatase is detected in somata and fibres in several areas of the songbird brain. The overall distribution of immunoproduct was replicated in the current studies using ICC techniques optimized for EM (see §2). Due to the large disparity in size of cell bodies and fine processes, somal aromatase was quantified in thick sections (1 μm), whereas extrasomal aromatase was examined and quantified using stereological counts from high magnification ultraphotomicrographs.
(a) Somal aromatase—light microscopy
Immunoproduct was abundantly seen at many loci in the songbird brain including areas rich in immunoreactive somata and fibres such as the HPOA and NCM (figure 1a,b). The HP was found to contain fewer aromatase-expressing somata. Importantly, somal aromatase was undetectable in HVC. In keeping with the above, statistical analyses revealed that somal aromatase varied across brain area (F(3,24)=36.7; p<0.001 (ANOVA); NCM=HPOA>HP=HVC (Fisher LSD); figure 3a). Notably, there was no difference between sexes in somal aromatase expression (F(1,24)=0.30; p=0.86) and the interaction between brain area and sex was not significant (F(3,24)=2.32; p=0.09).
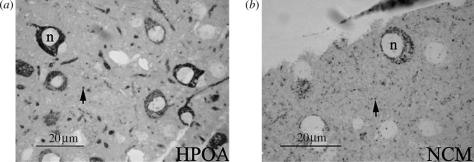
Figure 1.
Photomicrographs (light microscopy) of aromatase expression in sections (1 μm) of the zebra finch brain. While immunoreactivity is seen in the cytoplasm but not the nucleus (n) of several somata in the (a) HPOA and (b) NCM, it is also observed in multiple puncta (arrows) distributed throughout the parenchyma. Magnification bars=20 μm.
Figure 3.
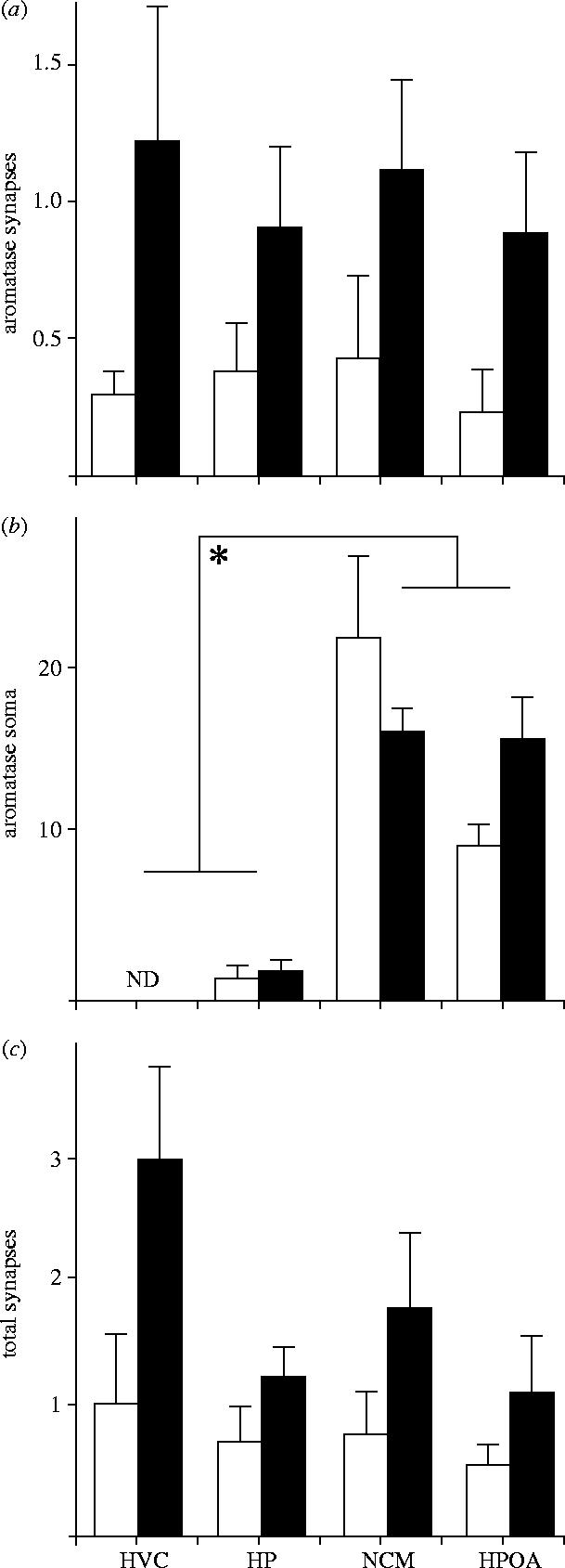
Histograms showing the frequency of (a) aromatase-positive pre-synaptic boutons, (b) aromatase-positive somata and (c) total synapses in adult zebra finches (males are shown as black bars). Aromatase expressing synapses are observable in HVC, where aromatase-positive soma are non-detectable (ND). Further, the total number of synaptic profiles is higher in males compared to females overall in (c) as is the frequency of stained profiles in (a). ND=not detected. F values (and the degrees of freedom used in their computation) for the main effects and interaction are depicted.
(b) Punctal aromatase—light microscopy
At the light microscopic level, all brain regions were found to contain intensely immunoreactive beaded processes and puncta. Importantly, all examined areas of the zebra finch brain contain aromatase in punctate fibres regardless of whether these areas contained aromatase in somata. In order to accurately define and describe the ultrastructural components reflected in these puncta, we quantified aromatase expression at the ultrastructural level using immuno-electron microscopy.
(c) Ultrastructural aromatase
Use of PARAPIC permitted excellent preservation of ultrastructure and antigenicity, allowing for unequivocal determination of the subcellular compartmentalization of aromatase. At the EM level, we documented several somata in the HPOA and NCM expressing aromatase. In these regions, somal aromatase was restricted to the cytoplasm with no evidence of expression in the nucleus, nucleolus, outer cell membrane or nuclear envelope. Stained cell bodies in the HPOA were often fusiform and large (12–20 μm—major cell diameter), while those in the NCM were pyriform and small (8–12 μm—major cell diameter). Golgi apparati were only occasionally noted in stained soma, but when observed contained complex cisternae and multiple invaginations. In the cytoplasm, aromatase immunoproduct is almost invariably revealed as flocculent diaminobenzidene (DAB) deposits along RER. Dendrites (identified by the presence of RER and lack of microtubule bundles), axons (identified as fine processes with bundled microtubules and no RER), terminals (identified by the presence of aggregations of clear vesicles) and pre-synaptic boutons (identified as terminals but with clusters of vesicles around putative active zones and distinct pre- and post-synaptic membranes and a post-synaptic density) were found to contain immunoproduct.
RER-associated aromatase is detectable in proximal and distal dendritic processes in these brain areas, but also at neural loci with low to undetectable somal aromatase. Specifically, in HP and HVC, immunoreactive dendrites were observable despite the paucity (HP) and absence (HVC) of somal immunoproduct. Correspondingly, at all brain areas examined, pre-synaptic boutons, terminals and axons contained aromatase immunoproduct regardless of the presence of abundant (HPOA, NCM), sparse (HP) or undetectable (HVC) aromatase-expressing somata. The fine structure of axonal processes combined with the flocculent nature of DAB immunoproduct made it difficult to precisely identify the organelles containing aromatase. Nevertheless, the presence of aromatase immunoproduct in these components was unequivocally observable. Immunostain for aromatase expression in somata, terminals and pre-synaptic boutons is shown in figure 2a–d.
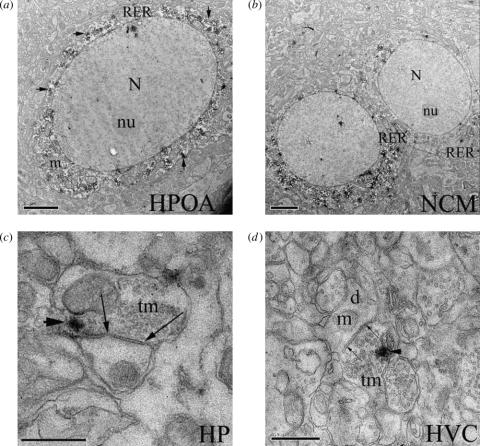
Figure 2.
Ultraphotomicrographs showing aromatase expression in several brain areas of the zebra finch. Immunoproduct (arrowheads) is visible along tracks of rough endoplasmic reticulum (RER) within the cytoplasm but not the nucleus (N) or nucleolus (Nu) of soma in (a) HPOA and (b) NCM (magnification bars=2 μm). Aromatase expression is also detectable in pre-synaptic boutons in all brain areas studied, including (c) those with low (HP) or (d) undetectable (HVC) somal aromatase expression, where immunoproduct is visible within pre-synaptic terminals (tm; arrows) that innervate immunonegative dendrites (d). m, mitochondrion. Magnification bars for HP=1 μm and HVC=0.5 μm.
Stereological counts of ultraphotomicrographs revealed that aromatase expression in pre-synaptic boutons was strongly affected by sex, but not brain area (table 1). Specifically, at all brain areas examined males had about twice as many pre-synaptic boutons containing aromatase than females (figure 3c). Two-way ANOVA showed a significant effect of sex (F(1,24)=11.15; p<0.003) but no effect of brain area or the interaction. The sex effect reflects a male-biased frequency of immunoreactive pre-synaptic boutons (0.978±0.177 versus 0.343±0.09, p<0.05 Fisher LSD). No sex, brain area or interaction effects were detected in the frequency of immunoreactive dendrites, axons or terminals (table 1). Interestingly, the frequency of total pre-synaptic boutons was also found to be male biased (F(1,24)=9.51; p<0.006; 0.978±0.177 versus 0.343±0.09, p<0.05 Fisher PLSD). Given this finding, we also analysed the potential effect of sex and brain area on the frequency of aromatase-positive synapses as a proportion of total synapses. The number of aromatase expressing synapses per total synapses was significantly affected by sex (F(1,24)=4.757; p<0.05) and was higher in males relative to females (0.612±0.05 versus 0.405±0.07; p<0.05 Fisher LSD). No effects of sex, brain area or their interaction were apparent for any other ultrastructural components including dendritic profiles, axons or terminals (table 1).
Table 1.
Sex and brain area effects of aromatase ultrastructure.
| HPOA | NCM | HP | HVC | F(1,24) (sex) | F(3,24) (area) | F(3,24) (sex×area) | ||
|---|---|---|---|---|---|---|---|---|
| total dendrites | M | 9.28±0.70 | 10.24±0.88 | 11.59±0.08 | 10.65±0.00 | 0.004 | 0.208 | 0.129 |
| F | 10.46±0.37 | 10.53±0.09 | 11.26±0.14 | 9.08±2.19 | ||||
| stained | M | 1.66±0.24 | 1.58±0.56 | 2.65±0.47 | 2.51±0.98 | 0.942 | 0.34 | 0.647 |
| F | 2.70±0.68 | 3.14±0.93 | 3.19±1.76 | 1.76±0.34 | ||||
| unstained | M | 7.61±0.78 | 8.66±2.35 | 8.94±1.08 | 8.14±3.08 | 0.325 | 0.094 | 0.059 |
| F | 7.76±0.95 | 7.39±1.36 | 8.08±1.16 | 7.31±1.89 | ||||
| total axons | M | 1.05±0.49 | 0.79±0.46 | 0.61±0.45 | 1.86±0.80 | 0.873 | 0.91 | 0.6 |
| F | 0.95±0.21 | 0.75±0.24 | 0.59±0.24 | 0.79±0.54 | ||||
| stained | M | 0.04±0.04 | 0.34±0.26 | 0.20±0.14 | 0.34±0.14 | 0.031 | 1.644 | 0.516 |
| F | 0.08±0.03 | 0.36±0.05 | 0.29±0.18 | 0.12±0.06 | ||||
| unstained | M | 1.01±0.45 | 0.45±0.22 | 0.41±0.31 | 1.53±0.81 | 0.943 | 1.537 | 0.396 |
| F | 0.88±0.23 | 0.39±0.25 | 0.30±0.11 | 0.67±0.56 | ||||
| total terminals | M | 23.43±0.72 | 22.15±0.88 | 21.85±0.57 | 19.55±0.91 | 0.024 | 0.011 | 0.554 |
| F | 20.59±0.68 | 22.01±0.94 | 21.86±0.78 | 23.70±0.13 | ||||
| stained | M | 5.70±0.26 | 6.28±1.84 | 7.39±1.40 | 5.49±1.60 | 0.057 | 0.239 | 0.265 |
| F | 6.13±0.67 | 5.15±1.60 | 6.25±1.29 | 6.35±1.93 | ||||
| unstained | M | 17.73±0.98 | 5.88±2.27 | 14.46±0.91 | 14.06±0.36 | 0.199 | 0.227 | 1.284 |
| F | 14.46±0.78 | 16.86±0.20 | 15.61±0.98 | 17.35±0.28 | ||||
| total synapses | M | 1.18±0.47 | 1.86±0.64 | 1.31±0.22 | 3.10±0.75 | 9.571 | 2.61 | 1.014 |
| F | 0.60±0.12 | 0.85±0.35 | 0.75±0.30 | 1.10±0.56 | ||||
| stained | M | 0.89±0.31 | 1.13±0.33 | 0.91±0.30 | 1.24±0.50 | 11.15 | 0.252 | 0.164 |
| F | 0.24±0.16 | 0.44±0.30 | 0.39±0.18 | 0.31±0.09 | ||||
| unstained | M | 0.29±0.19 | 0.74±0.36 | 0.40±0.10 | 0.86±0.31 | 0.222 | 1.473 | 0.206 |
| F | 0.37±0.12 | 0.41±0.15 | 0.36±0.12 | 0.79±0.49 |
Effects across sex were detected in the total number of synapses and the number of aromatase-expressing synapses (bold).
Aromatase-expressing dendrites, axons and terminals were often found in apposition with other stained elements. However, when aromatase-expressing pre-synaptic boutons were encountered, the post-synaptic element was always unstained (aromatase-negative) and was found most frequently to be a dendrite. Thus, the majority of aromatase-pre-synapses are axo-dendritic and always innervate elements lacking the ability to synthesize oestrogen.
4. Discussion
Here, we report that aromatase is expressed in terminals and synapses within many areas of the zebra finch brain. Our results point to two important findings. First we find pre-synaptic aromatase in areas devoid of somal aromatase expression suggesting that oestrogen synthesis can occur in projection neurons that impinge upon distant targets. Second in the brain areas studied, we find more aromatase-positive pre-synapses in males than females indicating that synaptic aromatization may function in a sex-specific manner. These findings suggest that synaptic aromatase is a key feature of the songbird brain. Its distribution or abundance in males and females may resolve questions about oestrogen delivery to steroid-sensitive circuits.
The goal of the current experiment was to test the idea that aromatase-positive synaptic terminals were observable in areas of the zebra finch brain that: (i) are sensitive to oestrogen and (ii) lack appreciable amounts of somal aromatase. This hypothesis was confirmed in that aromatase expressing pre-synaptic boutons were observable in the HP and HVC, areas with low to no somal aromatase, respectively, (see figure 3). Additionally, in confirmation of previous work (Naftolin et al. 1996) aromatase was also detected in pre-synaptic boutons within areas of abundant somal aromatase such as the HPOA and NCM (figures 1 and 2). These data suggest that the songbird brain is capable of expressing aromatase far from its traditional microsomal location. Thus, pre-synaptic packaging of aromatase appears to be a significant source of oestrogen to steroid-sensitive loci lacking somal aromatase. Males exhibit a significantly higher proportion of synaptic profiles that express aromatase, suggesting that synaptic aromatization may regulate sexually dimorphic function and point to the possibility that the male brain may be exposed to higher oestrogen from synaptic aromatization relative to females. Stereological counts on serially reconstructed sections, where aromatase had been stained using post-embedding EM protocols would be very helpful in replicating these findings.
Specificity of the aromatase antibody was assured by procedures previously validated (Saldanha et al. 2000). Aromatase was localized primarily on RER in neuronal somata and dendrites. Aromatase was also expressed in terminals, some of which formed synapses onto unstained dendrites. These synapses were observed some distance from immunopositive somata and dendrites. The compartmentalization of aromatase in synaptic organelles could not be defined unequivocally. The packaging of aromatase in synaptic vesicles has been suggested in other species (Naftolin et al. 1996). Synaptic aromatase was observed in all regions containing varying amounts of somal aromatase including HPOA, NCM and HP. More importantly, synaptic aromatase was also reported in HVC, a region devoid of somal aromatase expression. Previous reports of high aromatase activity (Vockel et al. 1990) but little to no aromatase expression have been a source of debate (Shen et al. 1995; Saldanha et al. 2000). The expression of aromatase in synapses within HVC probably accounts for this discrepancy. Apparently, aromatase is trafficked via axonal processes into steroid-sensitive song-control regions. Some of these cells are located in the shelf region beneath HVC (Saldanha et al. 2000), but others may originate elsewhere in the brain.
Pre-synaptic aromatase was expressed at a greater frequency in males compared to female zebra finches. While trends suggested the possibility of sexually dimorphic pre-synaptic aromatase expression in HVC no significant differences were observed. At the light-level, male zebra finches have approximately twice the number of aromatase-positive fibres in the HPOA and 60% more aromatase-positive fibres in the NCM than do females (Saldanha et al. 2000). Despite these differences in aromatase-positive fibres and terminals adult male and female zebra finches have similar numbers of aromatase-positive somata, suggesting aromatase-positive neurons in males are more complex than in females. Alternatively, aromatase may be preferentially trafficked to pre-synapses in males relative to females. Discrepancies in sex differences in aromatase have been evaluated using biochemistry (Vockel et al. 1990), in situ hybridization (Shen et al. 1995) and ICC (Saldanha et al. 2000). Only the resolution of ultrastructural analysis provides direct evidence for differences in aromatase that may account for sex differences in behaviour.
The EM analysis performed herein replicated sexual dimorphisms in other vertebrates. Specifically, the frequency of total pre-synapses was found to be higher in males relative to females. These findings are in good agreement with previous reports showing similar patterns of results in canary (Serinus canarius) song nuclei (DeVoogd & Nottebohm 1981) and the rodent hypothalamus (Raisman & Field 1971). It is necessary, however, to point out that the observed sex differences may not be generalized to the entire zebra finch brain. The present study specifically examined areas with abundant aromatase (Shen et al. 1995; Saldanha et al. 2000). Further studies that explicitly differentiate between areas of high and low aromatase expression will be necessary to understand the true extent of this sexual dimorphism.
Presumably, synaptic aromatase expression creates locally high levels of oestrogen in close proximity to specific cells of the male HPOA, HP, NCM and HVC. These brain areas are critical for the expression of reproductive behaviour (for review Balthazart 1990), learning and memory (Patel et al. 1997; Saldanha et al. 1999), song-recognition (Mello et al. 1992; Chew et al. 1996) and singing (Nottebohm et al. 1976). The synthesis of oestrogen at specific synapses in these regions may participate in the activation and expression of these behaviours in males. A test of this hypothesis would involve biochemical assays of aromatase activity within the HVC at physiological concentrations of androgenic substrate and/or direct measurements of oestrogen titres in micropunches of HVC. To the best of our knowledge neither experiment has been successfully conducted perhaps due to the technical difficulty involved. The mechanism creating this sex difference is unknown, but the amount of aromatase in synapses of males and females could be established when the brain undergoes sexually dimorphic brain development (Balthazart et al. 1996) or could be subject to transient regulation in adults by photoperiod induced changes in circulating hormones (Balthazart 1997).
Interestingly, while we found the numbers of pre-synaptic profiles containing aromatase to be male biased, no sex difference was detectable in the number of terminals (axonal specializations without synaptic clefts). We believe this pattern of results cannot be accounted for by procedural biases such as plane of section. Perhaps the transformation of aromatase-positive terminals into functional synapses is regulated in a male biased manner, developmentally or in adulthood. Indeed, circulating testosterone may itself be part of this process as treatment of female canaries with exogenous testosterone induces singing and increases synaptic density in RA (Devoogd et al. 1985). This idea may be tested structurally by recording the relative frequencies of terminals and synapses in serial sections through HVC in males and females. Additionally, this idea may be tested functionally by determining if electrical activity and/or synaptic transmission in the song circuit are affected by aromatase inhibition in males, but not (or to a lesser extent) in females.
Synapses containing aromatase could influence target cells expressing either androgen or oestrogen receptors (ARs or ERs). Milner et al. (2001) revealed the post-synaptic localization of ER on dendritic spines of rodent hippocampal neurons suggesting that post-synaptic membranes are indeed potential oestrogen targets. Oestrogens synthesized at the synapse might also mediate neurotransmitter release (Gazzaley et al. 1996) and/or modulate membrane electrical properties (Mermelstein et al. 1996; Gu et al. 1999). Aromatase could also reduce androgen concentrations lessening post-synaptic androgen action on specific AR-expressing cells. In songbirds, the presence of aromatase in synapses may have especially important actions on the development or activation of song circuits. ER and/or AR (in varying relative abundance) are present in several song nuclei, including HVC, in all species of songbirds examined so far (Nordeen et al. 1987; Brenowitz & Arnold 1989; Metzdorf et al. 1999; Soma et al. 2003). Irrespective of the action of aromatase, its presence in individual synapses provides a mechanism to alter the steroidal milieu in and around the synaptic cleft.
The presence of aromatase in synapses also creates a unique opportunity to couple specific electrical and hormonal signalling systems. Aromatase activity can be rapidly modulated by fluxes in Ca2+ and K+ (Balthazart et al. 2001); K+-induced depolarizations increase intracellular Ca2+ concentrations to downregulate aromatase activity (Balthazart et al. 2001). The functional status of synaptic aromatase might depend, therefore, upon the excitation state of its parent neuron. Aromatase-containing synapses of non-stimulated neurons would remain unaffected. Thus, in the presence of a uniform supply of a substrate like testosterone (presumably from the gonads), steroids could act differently on different neural circuits depending on whether those circuits are activated during behavioural or sensory stimulation. Coordinate electrical and hormonal signalling like this may be a key feature in the actions of sex steroids on steroid-sensitive neural networks in songbirds and in other vertebrate species as well.
Steroids are provided to the brain by peripheral synthesis and release into the circulation via endocrine pathways. Additionally, active steroids are synthesized in the brain by the somal expression of steroidogenic enzymes and provided to nearby targets by paracrine and autocrine pathways (Schlinger 1997). Synaptic aromatase may be part of a larger steroidogenic capability of the synapse that includes the androgen synthetic enzyme CYP17 (Hojo et al. 2004). We believe synaptic aromatase is a mechanism of oestrogen provision that represents a fundamentally unique neuroendocrine mechanism. Synaptic provision involves the transport of steroidogenic enzyme to terminals far from their source. This action combines the relatively long-range characteristic of an endocrine mechanism with the targeted specificity of axonal innervation. Synaptic aromatase enables distinct neural circuits to influence and act on other circuits by altering local steroid concentrations.
Acknowledgements
We thank Paul Micevych and Guido Zamphegi for assistance with the preliminary EM studies. This work was supported by NIH NS 042767 (CJS), MH 61994 (BAS) and HD 07228-18.
References
- Balthazart J. Brain aromatization of testosterone regulates male reproductive behavior in birds. Prog. Clin. Biol. Res. 1990;342:92–98. [PubMed] [Google Scholar]
- Balthazart J. Steroid control and sexual differentiation of brain aromatase. J. Steroid Biochem. Mol. Biol. 1997;61:323–339. 10.1016/S0960-0760(96)00235-X [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Ball G.F. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm. Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. 10.1006/hbeh.1996.0066 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball G.F. Phosphorylation processes mediate rapid changes of brain aromatase activity. J. Steroid Biochem. Mol. Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. 10.1016/S0960-0760(01)00143-1 [DOI] [PubMed] [Google Scholar]
- Brenowitz E.A, Arnold A.P. Accumulation of estrogen in a vocal control brain region of a duetting songbird. Brain Res. 1989;480:119–125. doi: 10.1016/0006-8993(89)91574-6. [DOI] [PubMed] [Google Scholar]
- Chew S.J, Vicario D.S, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc. Natl Acad. Sci. USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. 10.1073/pnas.93.5.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Devoogd T.J, Nixdorf B, Nottebohm F. Synaptogenesis and changes in synaptic morphology related to acquisition of a new behavior. Brain Res. 1985;329:304–308. doi: 10.1016/0006-8993(85)90539-6. 10.1016/0006-8993(85)90539-6 [DOI] [PubMed] [Google Scholar]
- Freking F, Nazairians T, Schlinger B.A. The expression of the sex steroid-sythesizing enzymes CYP11A1, 3-beta-HSD, CYP17, and CYP 19 in gonads and adrenals of adult and developing zebra finches. Gen. Comp. Endocrinol. 2000;119:140–151. doi: 10.1006/gcen.2000.7503. 10.1006/gcen.2000.7503 [DOI] [PubMed] [Google Scholar]
- Gazzaley A.H, Weiland N.G, McEwen B.S, Morrison J.H. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach K.S, Moss R.L. Rapid action of 17 beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. 10.1210/en.140.2.660 [DOI] [PubMed] [Google Scholar]
- Hojo Y, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl Acad. Sci. USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C.V, Vicario D.S, Clayton D.F. Song presentation induces gene expression in the songbird forebrain. Proc. Natl Acad. Sci. USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein P.G, Becker J.B, Surmeier D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 1999;407:115–129. 10.1002/(SICI)1096-9861(19990428)407:1%3C115::AID-CNE9%3E3.0.CO;2-W [PubMed] [Google Scholar]
- Milner T.A, McEwen B.S, Hayashi S, Li C.J, Reagan L.P, Alves S.E. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429:355–371. 10.1002/1096-9861(20010115)429:3%3C355::AID-CNE1%3E3.0.CO;2-# [PubMed] [Google Scholar]
- Naftolin F, Horvath T.L, Jakab R.L, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Nordeen K.W, Nordeen E.J, Arnold A.P. Estrogen accumulation in zebra finch song control nuclei: implications for sexual differentiation and adult activation of song behavior. J. Neurobiol. 1987;18:569–582. doi: 10.1002/neu.480180607. 10.1002/neu.480180607 [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes T.M, Leonard C.M. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. 10.1002/cne.901650405 [DOI] [PubMed] [Google Scholar]
- Patel S.N, Clayton N.S, Krebs J.R. Hippocampal tissue transplants reverse lesion-induced spatial memory deficits in zebra finches (Taeniopygia guttata) J. Neurosci. 1997;17:3861–3869. doi: 10.1523/JNEUROSCI.17-10-03861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R.S, Saldanha C.J, Schlinger B.A. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J. Neuroendocrinol. 2001;13:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. 10.1046/j.1365-2826.2001.00647.x [DOI] [PubMed] [Google Scholar]
- Raisman G, Field P.M. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- Roselli C.E, Resko J.A. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J. Steroid Biochem. Mol. Biol. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Saldanha C.J, Clayton N.S, Schlinger B.A. Androgen metabolism in the juvenile oscine forebrain: a cross-species analysis at neural sites implicated in memory function. J. Neurobiol. 1999;40:397–406. doi: 10.1002/(sici)1097-4695(19990905)40:3<397::aid-neu11>3.0.co;2-6. 10.1002/(SICI)1097-4695(19990905)40:3%3C397::AID-NEU11%3E3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- Saldanha C.J, Tuerek M.J, Kim Y.-H, Fernandes A.O, Arnold A.P, Schlinger B.A. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific anitbody. J. Comp. Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. 10.1002/1096-9861(20000807)423:4%3C619::AID-CNE7%3E3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- Saldanha C.J, Silverman A.J, Silver R. Direct innervation of GnRH neurons by encephalic photoreceptors in birds. J. Biol. Rhythms. 2001;16:39–49. doi: 10.1177/074873040101600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B.A. Sex steroids and their actions on the birdsong system. J. Neurobiol. 1997;33:619–631. 10.1002/(SICI)1097-4695(19971105)33:5%3C619::AID-NEU9%3E3.0.CO;2-7 [PubMed] [Google Scholar]
- Schlinger B.A, Arnold A.P. Brain is the major site of estrogen synthesis in a male songbird. Proc. Natl Acad. Sci. USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B.A, Arnold A.P. Plasma sex steroids and tissue aromatization in hatchling zebra finches: implications for the sexual differentiation of singing behavior. Endocrinology. 1992;130:289–299. doi: 10.1210/endo.130.1.1727704. 10.1210/en.130.1.289 [DOI] [PubMed] [Google Scholar]
- Schlinger B.A, Callard G.V. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger B.A, Campagnoni A.T, Arnold A.P. An atlas of aromatase mRNA expression in the zebra finch brain. J. Comp. Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. 10.1002/cne.903600113 [DOI] [PubMed] [Google Scholar]
- Soma K.K, Schlinger B.A, Wingfield J.C, Saldanha C.J. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J. Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. 10.1002/neu.10225 [DOI] [PubMed] [Google Scholar]
- Vockel A, Pröve E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990;511:291–302. doi: 10.1016/0006-8993(90)90174-a. 10.1016/0006-8993(90)90174-A [DOI] [PubMed] [Google Scholar]
- Wade J, Schlinger B.A, Arnold A.P. Aromatase and 5 beta-reductase activity in cultures of developing zebra finch brain: an investigation of sex and regional differences. J. Neurobiol. 1995;27:240–251. doi: 10.1002/neu.480270210. 10.1002/neu.480270210 [DOI] [PubMed] [Google Scholar]