Abstract
There is now considerable evidence that female choice drives the evolution of song complexity in many songbird species. However, the underlying basis for such choice remains controversial. The developmental stress hypothesis suggests that early developmental conditions can mediate adult song complexity by perturbing investment in the underlying brain nuclei during their initial growth. Here, we show that adult male canaries (Serinus canaria), infected with malaria (Plasmodium relictum) as juveniles, develop simpler songs as adults compared to uninfected individuals, and exhibit reduced development of the high vocal centre (HVC) song nucleus in the brain. Our results show how developmental stress not only affects the expression of a sexually selected male trait, but also the structure of the underlying song control pathway in the brain, providing a direct link between brain and behaviour. This novel experimental evidence tests both proximate and ultimate reasons for the evolution of complex songs and supports the Hamilton–Zuk hypothesis of parasite-mediated sexual selection. Together, these results propose how developmental costs may help to explain the evolution of honest advertising in the complex songs of birds.
Keywords: sexual selection, signal evolution, developmental stress, brain, behaviour, avian parasites
1. Introduction
Many bird species produce songs, which are complex in structure and are used for both intra- and inter-sexual communication (Catchpole & Slater 1995; Searcy & Yasukawa 1996). Females of a wide range of oscine species show preferences for males that sing more complex songs (Catchpole & Slater 1995), and large song repertoires are thought to have evolved under selection through female choice (Searcy & Yasukawa 1996). A functional understanding of the factors affecting repertoire size in songbirds thus gives an insight into the evolution of such epigamic traits. Song is a learned behaviour, occurring in most songbirds in the first few months of life when the discrete nuclei of the song control pathway in the forebrain are still developing (Nowicki et al. 1998). Song complexity has been positively related both within and across species to the volume of these nuclei, in particular the high vocal centre (HVC) (DeVoogd et al. 1993; Airey & DeVoogd 2000; Garamszegi & Eens 2004), although this is not universal (Leitner & Catchpole 2004). Further, in the canary (Serinus canaria), lesions that reduced the volume of the HVC were followed by a reduction in repertoire size in adulthood, suggesting a direct link between brain and behaviour (Halle et al. 2003b). The underlying neural pathways of song learning and production are well studied (Brainard & Doupe 2002) and it is thought that the costs of development or maintenance of these brain nuclei might mediate the cost of the behavioural trait (Catchpole 1996). The developmental stress hypothesis suggests that stressful conditions during the nestling period could disrupt the initial growth of these nuclei and significantly affect the quality of a male's song in adulthood through impaired song learning, thereby maintaining song complexity as an honest indicator of an individual's developmental history and potentially genetic quality (Nowicki et al. 1998, 2002; Spencer et al. 2003). Recent experimental studies have provided support for this hypothesis, showing that both elevated stress hormones and nutritional restriction during post-natal development can reduce the quality of the song signal (Nowicki et al. 1998, 2002; Buchanan et al. 2003; Spencer et al. 2003, 2004).
Parasites have detrimental effects on their hosts, drawing energy away from important physiological functions (Clayton & More 1997), and during development young birds may be exposed to a number of different parasites. We suggest that parasitic stress during development could affect song nuclei growth and mediate the expression of adult song signals, providing more evidence in support of the developmental stress hypothesis. Comparative studies have linked song complexity to parasite loads (Hamilton & Zuk 1982, but see Read & Weary 1990), However, to date, there are only two single-species studies that address this issue directly, and both are correlational. Field studies on the barn swallow (Hirundo rustica; Moller 1991) and the sedge warbler (Acrocephalus schoenobaenus; Buchanan et al. 1999) have respectively shown detrimental effects of ectoparasites on song output and of haematozoan parasites on song complexity.
In this study, we experimentally infected 12 juvenile canaries (S. canaria) with avian malaria (Plasmodium relictum) a common avian blood parasite (Huff & Coulston 1946) during the early post-fledging period (20–45 days). The experimental infections were timed to correspond with the growth period of the main nuclei in the song control pathway (Alvarezbuylla et al. 1988). The adult song of the malarial infected birds was then compared to a control group (n=8). Female canaries have been shown to prefer males with larger repertoires, investing more in reproduction when paired with their chosen male (Kroodsma 1976). Females have also shown preferences for particular types of rapidly repeated two-note song sub-units or syllables called ‘A’ syllables (Vallet & Kreutzer 1995; Vallet et al. 1998). Therefore in order to determine the consequences of parasitic infection on song quality we quantified the total syllable repertoire size and the number of ‘A’ syllables. In addition, we quantified the number of syllables containing two or more notes, here termed ‘complex’ syllables to give an indication of syllable complexity in our birds. Whilst it is not known if such syllables are sexually selected, the inclusion of this measure allowed us to more fully investigate the effects of parasites on a range of variables pertaining to song complexity.
2. Material and methods
(a) Nestling rearing and parasitic infections
Twenty Fife and Gloster canary chicks were hand reared on a diet of hand rearing mix (Quiko, UK) until independence, when they were fed on ad libitum plain and mixed canary seed (Haiths, UK). To supplement this small sample, extra birds were obtained from a local supplier (35–40 days post-hatch, n=26) and kept under the same diet and conditions as the hand reared birds. Birds were randomly allocated to either a parasitic treatment group or control, however, treatment allocation was counterbalanced for canary strain type and origin (hand reared or supplier bought). Birds in the parasitic group received two intra-peritoneal injections of 150 μl of pigeon (Columba livia) blood (50% dilution in phosphate buffered saline, PBS) from a pigeon previously infected with a commercially available culture of P. relictum (ATCC, USA). The experimental infections were transferred when parasitaemia was gauged to be 10–20% in the pigeon blood, and were transferred twice between the ages of 20–45 days post-hatch for all canary chicks, with at least a 10 day separation period. Hand reared birds received injections at day 20 and 35 post-hatching, whilst the supplier bought animals were injected at both 35–40 and 45–50 days of age. Transmission of the parasite was confirmed by a single blood smear 3 days post-infection. Birds in the control group received 150 μl injections of PBS at similar time intervals. Twenty of the 46 birds originally obtained were found to be males (n=8 control (n=4 hand reared), n=12 parasitized (n=4 hand reared)) and all data presented here refer to this sub-sample of animals.
(b) Physiological measurements
Haematocrit levels were used here to determine health indices in both parastized and control birds. Fifty microlitres of blood was taken by venepuncture 10 days after initial parasite infection in capillary tubes and spun for 5 min at 13 000 r.p.m. in a centrifuge (Jouan A13, VA, USA). The resultant packed cell length was measured to the nearest mm and the percentage of red blood cells in each sample was calculated. Corticosterone and testosterone levels were measured in blood samples taken immediately before the second experimental infection. This was done to determine if parasitic infection elevated stress hormone levels. Blood samples (100 μl) were collected within 3 min of capture in heparinized capillary tubes after puncture of the brachial vein with a 25 gauge needle, centrifuged and the plasma stored at −20 °C for later hormone assay. Corticosterone concentrations were measured after extraction of 20 μl aliquots of plasma in diethyl ether, by radioimmunoassay (Wingfield 1994) using anti-corticosterone antiserum (code B21–42, Endocrine Sciences, Tarzana, CA) and [1,2,6,7-3H]-corticosterone label (Amersham, UK). The extraction efficiency was 75–90%. The assay was run with 50% binding at 123 pg tube−1, and the detection limit was 0.45 ng ml−1 (for 7.3 μl aliquots of extracted plasma). Testosterone concentrations were measured in plasma samples by direct radioimmunoassay using anti-testosterone antiserum (code 8680–6004, Biogenesis, UK) and [125I]-testosterone label (code 07-189126, ICN, UK) (Parkinson & Follett 1995). The assay was run with 50% binding at 5 pg tube−1 and a detection limit of 0.03 ng ml−1. Experimental samples was measured in duplicate 10 μl volumes. Immune function was tested at 120–150 days post-hatch using a cell-mediated immune challenge, via injection of phytohaemoglutinin (PHA) into the wing web (Lochmiller et al. 1993). The response (swelling) to the challenge was tested 5 and 24 h after a 50 μl suspension of PHA was injected into the wing using standard techniques (Lochmiller et al. 1993).
(c) Song analysis
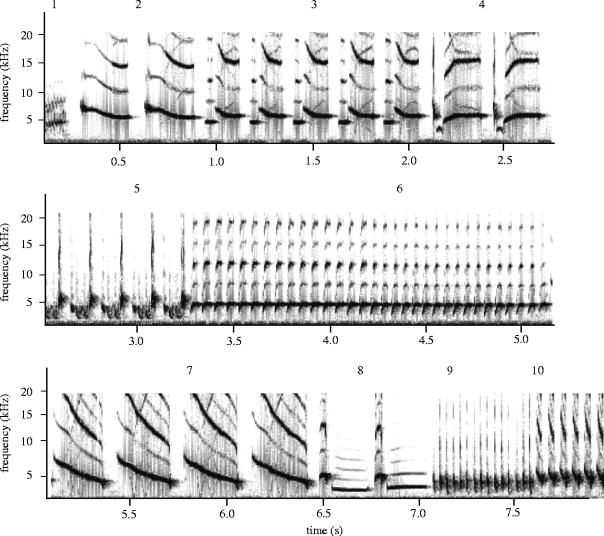
Male canaries learn their songs over a period of several months; therefore, we waited until full adult song had crystallized (age 240 days post-hatch (Alvarezbuylla et al. 1988)) to record their songs. During the period between hand rearing and song recording all males were housed in sex- and treatment-specific groups in visual and acoustic contact with the same adult male conspecific tutors (n=8). Between February and April 2003 the song of each male was recorded (total n=20; 12 parasitized and 8 control). Males were placed in an aviary (1.8×0.9×1.8 m3) in a sound attenuated room in small treatment-specific groups (n=5 each) along with a single female. Recordings were made for 1 h periods until at least 300 s of song had been recorded for each male, using Avisoft SAS-Lab Pro (R. Specht, Germany: figure 1) via a Sennheisser K6 microphone and a laptop computer (Dell Latitude LS, UK). We estimated the repertoire size of each male by determining the number of different syllable types within 300 s of song. In two cases (one control, one experimental) less than 300 s of song could be scrutinized (203 and 160 s available), thus the repertoire of these birds may be underestimated in this study. However, current evidence suggests that 200 s of song is sufficient to satisfactorily determine repertoire size in this species (Halle et al. 2003a). Removal of these birds did not significantly alter any statistical values. Within each repertoire the number of different syllables with more than two notes was noted, as was the number of ‘A’ syllables. Syllables were categorized as ‘A’ syllables if they had a two-note structure and a repetition rate of more than 17 elements per second (Vallet & Kreutzer 1995).
Figure 1.
Example sonogram of Canary song produced using Avisoft SASLab Pro (R. Specht, Germany), containing ten different syllables.
(d) Analysis of brain nuclei
At 298±18 (s.d.) days old male canaries were weighed and then killed with an overdose of chloroform. Birds were perfused transcardially with 0.9% saline followed by 4% phosphate-buffered formaldehyde solution. Brains were post-fixed and their mass recorded. One half of each brain was immersed in RNAse free 10%, followed by 30% phosphate-buffered sucrose. Brains were then sectioned parasagittally on a freezing microtome at 40 μm, collected in PBS and alternate sections were mounted onto Superfrost Plus microscope slides for Nissl staining. Slides were analysed under brightfield with a Leitz Aristoplan microscope (Leitz Wetzlar, Germany). The areas of the brain regions HVC and RA were video-digitized using a PC equipped with an image analysis system (MetaMorph 4.6, Visitron Systems, Germany). Volumes were calculated from every third section and the sum of the area sizes multiplied by 120 μm (section interval×section thickness).
(e) Statistical analysis
To investigate sources of variation in song parameters (repertoire size, number of A syllables and number of two-note syllables), brain parameters (HVC and RA volume), stress hormone (corticosterone) levels and immune response a series of General Linear Models (GLM, Minitab, PA, USA) was undertaken. As well as our experimental treatments, immune response; basal corticosterone; mass at adulthood and haematocrit counts were all entered into the model as independent covariates, where appropriate. Stepwise deletion of non-significant terms minimized the chances of spurious variables being included in the final minimal adequate model. In all statistical analyses the origin of the birds (i.e. hand reared or supplier bought) was controlled for. A single interaction term was also entered into the models (bird origin×treatment) to determine if our treatments had differential effects on birds from our two sources. Residuals were checked for normality and homogeneity of variance.
3. Results
The physiological costs of our experimental infections were confirmed via significantly depressed haematocrit levels (percentage of red blood cells) in parasitized birds soon after infection (14±3 days) (figure 2a; table 1). Parasitic infection caused a 17% reduction in red blood cell levels compared to controls. As predicted by the developmental stress hypothesis, infection with malaria resulted in significantly reduced repertoire sizes (figure 2b; table 1). This decrease represented a 12.5% decline in song complexity. Parasitic infection had significant specific effects on neural development. The volume of the HVC nucleus was significantly smaller in parasitized birds when compared to controls (figures 2c and 3; table 1). The observed reduction represents a decrease of 36% in nucleic volume. The number of complex syllables in an individual's repertoire was also significantly correlated with HVC volume (table 1). Overall brain size and RA volume were unaffected by experimental infection (F1,17=0.02; p=0.898; F1,17=0.37, p=0.551).
Figure 2.
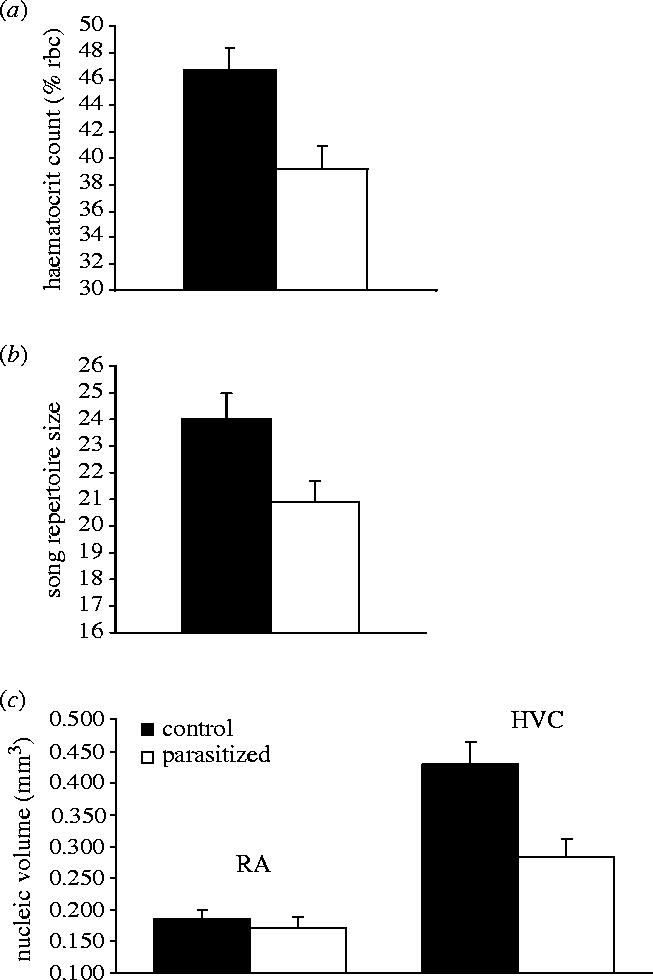
The effects of experimental malarial infection during development on (a) haematocrit levels, (b) song repertoire size (number of different syllables) and (c) neural development of the HVC and Robust nucleus of the Arcopallium (RA) in adult canaries. Graphs show fitted means +s.e.
Table 1.
Sources of variation in (a) repertoire size and (b) HVC volume in adult male canaries (Serinus canaria).
| F-ratio | coefficient | p | |
|---|---|---|---|
| (a) independent variables in the final model | |||
| treatment | 25.67 | NA | 0.0001 |
| haematocrit reading (%rbc) | 18.63 | −0.4334 | 0.001 |
| bird origin (hand reared or supplier bought) | 0.56 | NA | 0.467 |
| origin×treatment | 3.51 | NA | 0.081 |
| excluded independent variables | |||
| basal corticosterone (ng ml−1) | 0.05 | 0.0209 | 0.821 |
| cell-mediated immune response | 0.16 | 0.7350 | 0.693 |
| adult mass (g) | 0.19 | −1.0250 | 0.673 |
| testosterone (ng ml−1) | 1.25 | 0.9427 | 0.282 |
| number complex syllables | 1.53 | 0.4871 | 0.237 |
| final model R2=0.67, residual d.f.=15 | |||
| (b) independent variables in the final model | |||
| treatment | 8.14 | NA | 0.013 |
| number complex syllables | 5.29 | −0.0317 | 0.037 |
| haematocrit reading (%rbc) | 4.08 | −0.0084 | 0.063 |
| bird origin (hand reared or supplier bought) | 4.01 | NA | 0.065 |
| origin×treatment | 4.28 | NA | 0.058 |
| excluded independent variables | |||
| adult mass (g) | 0.01 | 0.0012 | 0.910 |
| testosterone (ng ml−1) | 0.41 | 0.0221 | 0.530 |
| basal corticosterone (ng ml−1) | 0.76 | −0.0021 | 0.399 |
| cell-mediated immune response | 1.86 | 0.0686 | 0.195 |
| final model R2=0.51, residual d.f.=14 |
Table shows the results of a General Linear Model (Minitab, PA, USA). Terms excluded due to non-significance are shown at the bottom of each table. Significant terms are shown in bold. The model also controlled for the origin of the birds. NA signifies a coefficient is not applicable as this term is a factor.
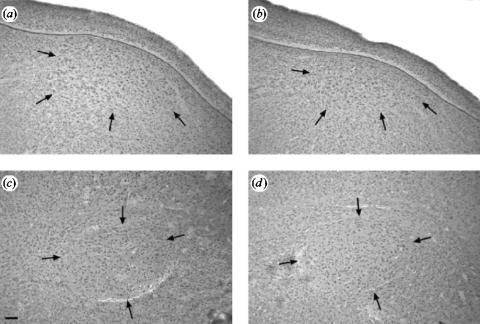
Figure 3.
Photomicrographs showing HVC of control (a) and parasitized (b) male canaries and Robust nucleus of the Archopallium (RA) of control (c) and parasitized (d) birds. HVC was smaller in the parasitized birds (b) compared to the control (a) group, whereas RA showed no reduction in size (c) and (d). Scale bar is 100 μm.
We found very low incidences of ‘A’ syllables within our canary population, permitting only qualitative analysis of any treatment effects. In total four birds exhibited ‘A’ syllables, two from the parasitized group and two controls. The low incidence in the attractive high-repetition ‘A’ syllables seen in this study is possibly due to the age of the birds used. All birds sang some syllables with a two-note structure, however, we found no effects of parasitic infection on the total number of these complex syllables within a repertoire (F1,17=0.13, p=0.722). We found no evidence of any change in cell-mediated immune response, or basal corticosterone level (samples taken within 3 min of capture) due to our experimental infections (F1,17=0.44, p=0.515; F1,17=0.13, 0.725, respectively). There was also no significant relationship between immune response and repertoire size within the parasitized group of birds (F1,10=0.80, p=0.393).
4. Discussion
These data provide novel experimental evidence for a causal link between parasitic infection during postnatal development and complex song repertoires, confirming that parasites can influence a sexually selected trait. Our results suggest that individual differences in song quality and their underlying neural circuitry may be subject to sexual selection. Malarial infection clearly had an impact on the development of a sexually selected acoustic trait and its controlling mechanism, specifically the brain HVC nuclei. This provides clear support for the developmental stress hypothesis, and demonstrates its relevance for a wide range of potential physiological stressors (Nowicki et al. 2002; Spencer et al. 2003; Buchanan et al. 2004). As such, this leads to the suggestion that complex song repertoires could have evolved in a number of songbird species as honest indicators of parasitized status. This also offers support for a major assumption underpinning the several models of parasite-mediated sexual selection, including the controversial Hamilton–Zuk hypothesis (Hamilton & Zuk 1982). As we have shown that parasites can cause a reduction in song complexity, any male that is more resistant, and by definition has zero or few parasites, is more likely to have a larger repertoire.
The underlying physiological basis for these effects is still not clear, however. A recent study has shown that another potential mechanism, namely corticosterone, can play a significant mediating role in the expression of the song signal (Spencer et al. 2003), however, we did not find elevated basal corticosterone levels in the parasitized birds. This does not preclude the potential role of stress hormones in the expression of such characters, as prolonged exposure to elevated stress hormones can have serious negative effects on neuronal growth and survival (McEwen & Sapolsky 1995; Sapolsky 1996; Buchanan 2000). Further work perhaps including stress responses to standardized stressors in such cases would be necessary to clarify the role of stress hormones. At the level of the individual, it is possible there is a trade-off between trait expression and investment in immune maintenance, although we found no effects of our treatments on cell-mediated immune response. Estimates of the energetic costs of immune responses have revealed significant increases in basal metabolism (Martin et al. 2003). Such a trade-off could be causally linked to investment in growth of the brain nuclei. A possible alternative or additional way in which parasitic infection might have affected singing behaviour is by reducing the amount of sub-song produced and thereby limiting auditory feedback. More detailed experiments examining the timing and behavioural effects of such an infection would be required to further elucidate the mechanisms underlying the results described here. The observation that parasites significantly affected the HVC nucleus but not the RA also suggests differential sensitivity of the HVC nucleus to our experimental infections. A comparable result has recently been shown in another songbird species (Buchanan et al. 2004).
One potential confounding factor in our experimental design is the use of pigeon blood as an intermediary host. The injection of pigeon blood would have caused an immune response in our experimental canaries, which was not mirrored in the control group which received injections of PBS only. We therefore cannot completely discount the possibility that the effects seen here on both song complexity and neural morphology are partly due to an immune reaction against pigeon antigens. We would argue that this is unlikely. Foreign red blood cells are non-pathogenic and therefore only stimulate a short-term immune response. Any effects seen as a result of the immune response against pigeon blood cell antigens would therefore be due to the short-term energetic costs of an elevated immune response. The majority of studies using such manipulations and demonstrating costs of such a response, report limited short-term effects in avian species (Svensson et al. 1998; Horak et al. 2003; Verhulst et al. 2005). In addition, there was a significant reduction in haematocrit levels in our parasitized birds, characteristic of malarial infection (Williams 2005). Furthermore, active malarial infection was confirmed in the experimental birds by observing presence of the parasites in stained blood smears.
Previous experimental studies have shown links between parasitism and ornament expression and intensity (Moller 1990; Zuk et al. 1990; Moller 1991). However, few intra-specific studies have concentrated on the aspect of song complexity. The best evidence to date to link parasitism to song complexity comes from a correlative study on the sedge warbler (A. schoenobaenus), where parasitized males exhibited lower repertoire sizes than non-parasitized birds (Buchanan et al. 1999). Our work exposes the potential role of parasites in mediating the process of sexual selection and shaping the evolution of the song control pathway in the brain. We suggest that parasitic infection could mediate many sexual signals. Developmental stress provided by environmental factors such as parasite infection could provide an explanation for the evolution of complex song repertoires as well as other ‘honest advertisements’, which have to date, eluded satisfactory explanation.
Acknowledgments
All work conducted under Home office license PPL 30/1777. K.A.S. and S.L. were funded by BBSRC research grant code 7/S14062. K.L.B. was funded by research fellowship from the Royal Commission for the Exhibition of 1851. We thank Sadie Iles-Ryan and Diane Flower for animal husbandry, many undergraduate research assistants for help with hand rearing and Roger Francis for assistance with licensing. Two anonymous referees provided helpful comments which improved an earlier draft of this paper.
References
- Airey D.C, DeVoogd T.J. Greater song complexity is associated with augmented song system anatomy in zebra finches. Neuroreport. 2000;11:2339–2344. doi: 10.1097/00001756-200007140-00054. [DOI] [PubMed] [Google Scholar]
- Alvarezbuylla A, Theelen M, Nottebohm F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc. Natl Acad. Sci. USA. 1988;85:8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard M.S, Doupe A.J. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. 10.1038/417351a [DOI] [PubMed] [Google Scholar]
- Buchanan K.L. Stress and the evolution of condition-dependent signals. Trends Ecol. Evol. 2000;15:156–160. doi: 10.1016/s0169-5347(99)01812-1. 10.1016/S0169-5347(99)01812-1 [DOI] [PubMed] [Google Scholar]
- Buchanan K.L, Catchpole C.K, Lewis J.W, Lodge A. Song as an indicator of parasitism in the sedge warbler. Anim. Behav. 1999;57:307–314. doi: 10.1006/anbe.1998.0969. 10.1006/anbe.1998.0969 [DOI] [PubMed] [Google Scholar]
- Buchanan K.L, Spencer K.A, Goldsmith A.R, Catchpole C.K. Song is an honest signal of past developmental stress in the European starling (Sturnus vulgaris) Proc. R. Soc. B. 2003;270:1149–1156. doi: 10.1098/rspb.2003.2330. 10.1098/rspb.2003.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K.L, Leitner S, Spencer K.A, Goldsmith A.R, Catchpole C. Developmental stress selectively affects development of the brain nuclei HVC in a songbird. Proc. R. Soc. B. 2004;271:2381–2386. doi: 10.1098/rspb.2004.2874. 10.1098/rspb.2004.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole C.K. Song and female choice: good genes and big brains? Trends Ecol. Evol. 1996;11:358–360. doi: 10.1016/0169-5347(96)30042-6. 10.1016/0169-5347(96)30042-6 [DOI] [PubMed] [Google Scholar]
- Catchpole C.K, Slater P.J.B. Cambridge University Press; 1995. Bird Song: biological themes and variations. [Google Scholar]
- Clayton D.H, More J. Oxford University Press; 1997. Host parasite evolution: general principles and avian models. [Google Scholar]
- DeVoogd T.J, Krebs J.R, Healy S.D, Purvis A. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc. R. Soc. B. 1993;254:75–82. doi: 10.1098/rspb.1993.0129. [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z, Eens M. Brain space for a learned task: strong intraspecific evidence for neural correlates of singing behaviour in songbirds. Brain Res. Rev. 2004;44:187–193. doi: 10.1016/j.brainresrev.2003.12.001. 10.1016/j.brainresrev.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Halle F, Gahr M, Kreutzer M. Effects of unilateral lesions of HVC on song patterns of male domesticated canaries. J. Neurobiol. 2003a;56:303–314. doi: 10.1002/neu.10230. 10.1002/neu.10230 [DOI] [PubMed] [Google Scholar]
- Halle F, Gahr M, Kreutzer M. Impaired recovery of syllable repertoires after unilateral lesions of the HVC of male domesticated canaries. Anim. Biol. 2003b;53:113–128. 10.1163/157075603769700322 [Google Scholar]
- Hamilton A, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Horak P, Saks L, Ots I, Kullissaar T, Kollist H, Zilmer M. Physiological effects of immune challenge in captive greenfinches (Carduelis chloris) Can. J. Zool. 2003;81:371–379. 10.1139/z03-020 [Google Scholar]
- Huff C.G, Coulston F. The relation of natural and acquired immunity of various avian hosts to the Cryptozoites and Metacryptozoites of Plasmodium gallinaceum and Plasmodium relictum. J. Infect. Dis. 1946;78:99–117. doi: 10.1093/infdis/78.2.99. [DOI] [PubMed] [Google Scholar]
- Kroodsma D.E. Reproductive development in a female songbird: differential stimulation by quality of male song. Science. 1976;192:574–575. doi: 10.1126/science.192.4239.574. [DOI] [PubMed] [Google Scholar]
- Leitner S, Catchpole C.K. Syllable repertoire size and the size of the song control system in captive canaries (Serinus canaria) J. Neurobiol. 2004;60:21–27. doi: 10.1002/neu.10331. 10.1002/neu.10331 [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Vestey M.R, Boren J.C. Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. Auk. 1993;110:503–510. [Google Scholar]
- Martin L.B, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. 10.1098/rspb.2002.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S, Sapolsky R.M. Stress and congnitive function. Curr. Opin. Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. 10.1016/0959-4388(95)80028-X [DOI] [PubMed] [Google Scholar]
- Moller A.P. Effects of a hematophagous mite on the barn swallow (Hirundo rustica)—a test of the Hamilton and Zuk Hypothesis. Evolution. 1990;44:771–784. doi: 10.1111/j.1558-5646.1990.tb03804.x. [DOI] [PubMed] [Google Scholar]
- Moller A.P. Parasite load reduces song output in a passerine bird. Anim. Behav. 1991;41:723–730. [Google Scholar]
- Nowicki S, Peters S, Podos J. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 1998;38:179–190. [Google Scholar]
- Nowicki S, Searcy W.A, Peters A. Brain development, song learning and mate choice in birds: a review and experimental test of the nutritional stress hypothesis. J. Comp. Physiol. Sens. Neural Behav. Physiol. 2002;188:1003–1014. doi: 10.1007/s00359-002-0361-3. 10.1007/s00359-002-0361-3 [DOI] [PubMed] [Google Scholar]
- Parkinson T.J, Follett B.K. Thyroidectomy abolishes seasonal testicular cycles of Soay rams. Proc. R. Soc. B. 1995;259:1–6. doi: 10.1098/rspb.1995.0001. [DOI] [PubMed] [Google Scholar]
- Read A.F, Weary D.M. Sexual selection and the evolution of bird song-a test of the Hamilton–Zuk Hypothesis. Behav. Ecol. Sociobiol. 1990;26:47–56. 10.1007/BF00174024 [Google Scholar]
- Sapolsky R.M. Why stress is bad for your brain. Science. 1996;272:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Searcy W.A, Yasukawa K. Song and female choice. In: Kroodsma D.E, Miller E.H, editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; New York: 1996. pp. 454–473. [Google Scholar]
- Spencer K.A, Buchanan K.L, Goldsmith A.R, Catchpole C.K. Song as an honest indicator of developmental history in the zebra finch (Taeniopygia guttata) Horm. Behav. 2003;44:132–139. doi: 10.1016/s0018-506x(03)00124-7. 10.1016/S0018-506X(03)00124-7 [DOI] [PubMed] [Google Scholar]
- Spencer K.A, Buchanan K.L, Goldsmith A.R, Catchpole C.K. Developmental stress, social rank and song complexity in the European starling (Sturnus vulgaris) Proc. R. Soc. B. 2004;271(Suppl. 3):S121–S123. doi: 10.1098/rsbl.2003.0122. 10.1098/rsbl.2003.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Raberg L, Coch C, Hasselquist D. Energetic stress, immunosupression and the costs of an antibody response. Funct. Ecol. 1998;12:132–140. 10.1046/j.1365-2435.1998.00271.x [Google Scholar]
- Vallet E, Kreutzer M. Female canaries are sexually responsive to special song phrases. Anim. Behav. 1995;49:1603–1610. 10.1016/0003-3472(95)90082-9 [Google Scholar]
- Vallet E, Beme I, Kreutzer M. Two-note syllables in canary songs elicit high levels of sexual display. Anim. Behav. 1998;55:291–297. doi: 10.1006/anbe.1997.0631. 10.1006/anbe.1997.0631 [DOI] [PubMed] [Google Scholar]
- Verhulst S, Riedstra B, Wiersma P. Brood size and immunity costs in zebra finches Taeniopygia guttata. J. Avian Biol. 2005;36:22–30. 10.1111/j.0908-8857.2005.03342.x [Google Scholar]
- Williams R.B. Avian malaria: clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian Pathol. 2005;34:29–47. doi: 10.1080/03079450400025430. 10.1080/03079450400025430 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C. Modulation of the adrenocortical response to stress in birds. In: Davey K, Peter R, Tobey S, editors. Perspectives in Endocrinology. National Research Council of Canada; Ottawa: 1994. pp. 520–528. [Google Scholar]
- Zuk M, Johnson K, Thornhill R, Ligon J.D. Parasites and male ornaments in free-ranging and captive Red Jungle Fowl. Behaviour. 1990;114:232–248. [Google Scholar]