Abstract
The human pathogenic fungus Candida albicans has traditionally been classified as a diploid, asexual organism. However, mating-competent forms of the organism were recently described that produced tetraploid mating products. In principle, the C.albicans life cycle could be completed via a sexual process, via a parasexual mechanism, or by both mechanisms. Here we describe conditions in which growth of a tetraploid strain of C.albicans on Saccharomyces cerevisiae ‘pre-sporulation’ medium induced efficient, random chromosome loss in the tetraploid. The products of chromosome loss were often strains that were diploid, or very close to diploid, in DNA content. If they inherited the appropiate MTL (mating-type like) loci, these diploid products were themselves mating competent. Thus, an efficient parasexual cycle can be performed in C.albicans, one that leads to the reassortment of genetic material in this organism. We show that this parasexual cycle—consisting of mating followed by chromosome loss—can be used in the laboratory for simple genetic manipulations in C.albicans.
Keywords: chromosome loss/mating/meiosis/parasexual/tetraploid
Introduction
Although found as a commensal in the digestive tract, Candida albicans is also the most common human fungal pathogen, causing both mucosal and systemic infections, particularly in immunocompromised people (Edmond et al., 1999). Candida albicans exists naturally as a diploid yeast and until recently was thought to be asexual, as no direct observations of mating or meiosis had been reported. The lack of evidence for sexual reproduction was surprising since DNA-based taxonomies indicated that C.albicans was closely related to sexually reproducing yeast species such as Saccharomyces cerevisiae. Moreover, a number of genes in C.albicans were identified whose homologs function in mating and meiosis in S.cerevisiae; some of these C.albicans genes (e.g. STE20 and DMC1) have even been shown to complement their S.cerevisiae counterparts (Diener and Fink, 1996; Leberer et al., 1996; Tzung et al., 2001). Population studies suggested that while C.albicans was largely clonal, implying that recombination in the wild occurred rarely, there was evidence for some form of genetic exchange in natural populations (Graser et al., 1996; Tibayrenc, 1997).
Evidence for a sexual cycle in C.albicans came from the description of mating-type like (MTL) loci in this organism (Hull and Johnson, 1999). The MTL loci of C.albicans are similar to the mating-type (MAT) loci of S.cerevisiae in two respects. First, they encode three closely related transcriptional regulators (called MATa1, MATα1 and MATα2 in S.cerevisiae), and secondly, the loci are heterozygous in standard diploid laboratory strains with the MATa1-like genes on one chromosome and MATα1- and MATα2-like genes on the other chromosome. It was subsequently premised that C.albicans might be made to mate by genetic modification of the wild-type a/α strain to create both a- and α-type strains. These derivatives were indeed found to mate, albeit at low efficiency, either in a mammalian host (Hull et al., 2000) or in vitro (Magee and Magee, 2000).
More recently, it has been shown that mating in C.albicans is directly linked to white–opaque switching (Miller and Johnson, 2002). Certain strains of C.albicans were known to undergo a white–opaque phase transition; white cells appeared round and formed dome-shaped colonies on agar, while opaque cells were more elongated and formed flatter colonies on agar (Soll, 1997). The ability to switch phases was found to be controlled by the transcriptional regulatory proteins encoded by the MTL loci. Furthermore, opaque cells were shown to mate ∼106 times more efficiently than white-phase cells, explaining the very low mating efficiency observed in the original mating experiments, which had utilized white-phase cells. Thus, mating-competent cells must first switch from white to opaque to mate efficiently; the fact that the MTL locus controls both processes ensures that white–opaque switching is only carried out by mating-competent cells. Consistent with this idea, switching-competent clinical isolates of C.albicans were found to be either ‘a’ or ‘α’ strains (Lockhart et al., 2002).
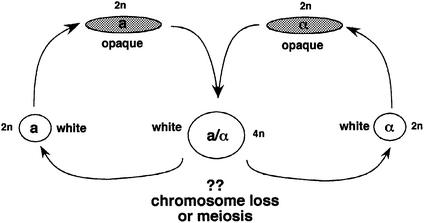
Although mating has now been amply documented for C.albicans, no study has reported whether the tetraploid products formed through mating can be induced to return to the diploid state. This process could, in principle, occur via a sexual cycle (meiotic divisions) or via a parasexual cycle (reductional mitotic divisions) in C.albicans (see Figure 1). In this study, we constructed a tetraploid strain of C.albicans containing several convenient genetic markers, and tested whether meiosis or chromosome loss could be induced under a number of different growth conditions. Although we did not observe meiosis, we did observe efficient and cooperative chromosome loss under certain conditions. Chromosome loss was particularly efficient in tetraploid strains that were grown at 37°C on a S.cerevisiae ‘pre-sporulation’ (pre-spo) medium, a medium that contains 1% yeast extract, 0.8% peptone and 10% glucose. The resultant progeny showed a range of ploidy, although approximately one-third of the cells had reduced their DNA content to (within experimental error) that of a control diploid strain. While chromosome reduction appears cooperative (i.e. the loss of one pair of chromosomes predisposes the strain to lose other pairs), it is not as concerted as typical fungal meioses. We show that induced chromosome loss in the tetraploid can be used to complete a parasexual genetic cycle, allowing a series of simple genetic manipulations to be performed with C.albicans. These include: (i) establishing linkage relationships between genes; (ii) carrying out backcrosses to establish that a phenotype arises from a single mutation; and (iii) constructing recombinant diploid strains that contain genetic information derived from two different diploid parents.
Fig. 1. Putative sexual cycle of C.albicans. Mating between a and α opaque cells produces an a/α tetraploid cell. A reduction in ploidy back to the diploid state, which could in principle occur by random chromosome loss or by meiosis, is the subject of this paper.
Results
Marking a tetraploid strain of C.albicans to follow chromosome loss
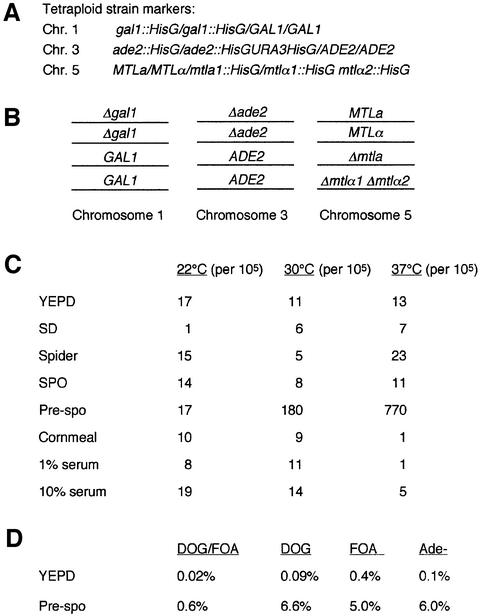
Previous studies have indicated that tetraploid strains of C.albicans might be unstable. For example, heat shock treatment reduced the chromosome number in tetraploid cells formed by protoplast fusion of two diploid strains (Hilton et al., 1985). Another study surmised that a tetraploid strain, constructed by mating two diploids, underwent chromosome loss to form a strain that was tri-allelic at the MTL locus (Hull et al., 2000). To test for growth conditions that could specifically induce chromosome loss or reduction in the tetraploid, a tetraploid strain was created (RBY18) in which chromosome loss could be easily monitored. The tetraploid strain was created by mating a and α cell types, and appeared to have undergone karyogamy based on DAPI staining (data not shown). The strain was constructed to be heterozygous for the URA3 and GAL1 genes. Both URA3 and GAL1 genes are counter-selectable: strains containing wild-type URA3 are unable to grow on medium containing 5-fluoroorotic acid (5-FOA) (Boeke et al., 1984), while strains containing the GAL1 gene are unable to grow on medium containing 2-deoxygalactose (2-DOG) as the carbon source (Platt, 1984). The tetraploid strain used in the present study contained one wild-type copy of URA3 and two copies of the wild-type GAL1 allele (see Figure 2A and B), and therefore is unable to grow on either 5-FOA- or 2-DOG-containing media. However, derivatives of this strain can grow on medium containing both 5-FOA and 2-DOG if they have lost both copies of the GAL1 gene and the single URA3 gene. The GAL1 and URA3 genes are located on chromosomes 1 and 3, respectively (Figure 2A and B). Note that URA3 is present at the ADE2 locus as a HisG-URA3-HisG allele, so that URA3 can be lost by intramolecular recombination in addition to chromosome loss. Experiments using a ura3/URA3; GAL1/GAL1 diploid strain confirmed that <1 in 100 000 colonies grew on medium containing 5-FOA and 2-DOG, demonstrating a very low background growth. Thus, efficient chromosome loss in a tetraploid would be expected to produce derivatives resistant to both 5-FOA and 2-DOG at frequencies well above the background level.
Fig. 2. Test of stability of a tetraploid strain grown on different media. (A and B) The tetraploid strain used in this study (RBY18) is hetero zygous at the GAL1, ADE2 and MTL loci on chromosomes 1, 3 and 5, respectively. (C) The tetraploid strain was grown for 8 days on different media at 22, 30 or 37°C, respectively. Cells were pooled from the different media and plated on FOA/DOG medium and YEPD. The number of cells that could grow on the FOA/DOG plates relative to YEPD was calculated (number of colonies per 105 cells). (D) The tetraploid strain was grown on YEPD or pre-spo medium for 8 days at 37°C. Following incubation, cells were recovered and tested for growth on several test media. The percentage of cells that could grow on the test plates (or gave red colonies indicating Ade– colonies) relative to YEPD was calculated.
The starting tetraploid strain was also heterozygous at two additional alleles. First, the ADE2 locus contained two wild-type and two mutant ADE2 alleles. Strains from which both wild-type copies of ADE2 were lost grew as characteristic red colonies, whereas all other configurations of the ADE2 locus gave rise to white colonies. In addition, the MTL locus on chromosome 5 was represented by four different MTL alleles: a, alpha, a1 deletion and alpha1/alpha2 deletion. All four of the MTL alleles were easily distinguishable using whole-cell PCR and primer pairs specific to each MTL allele.
Growth on pre-spo medium induces allele loss in a tetraploid strain
To test for chromosome loss, the marked tetraploid strain was plated on a variety of different media, including YEPD, SD, spider, spo, pre-spo and cornmeal medium, and incubated at 25, 30 or 37°C. Following incubation on solid media, colonies were pooled by scraping cells off the plate and the suspended cells were plated at different concentrations onto YEPD plates (control) or SD plates containing 5-FOA and 2-DOG. Preliminary experiments suggested that growth of the tetraploid at 37°C on a S.cerevisiae pre-spo medium resulted in at least 10 times as many colonies on the selective plates than any other condition tested (see Figure 2C).
Allele loss on pre-spo medium was then carefully compared with that on YEPD. Tetraploid cells were streaked on both media and allowed to grow for 14 days at 37°C. Subsequently, cells were pooled and tested for growth on several types of selective media; growth on each type of medium required the loss of a particular genetic marker. As shown in Figure 2D, the number of colonies observed on the selective plates was much greater for samples grown on pre-spo medium than on YEPD. For example, only 0.02% of cells picked from the YEPD plates were able to grow on medium containing DOG and FOA, whereas 0.6% of cells grown on pre-spo were DOGR and FOAR (a 30-fold difference). Similarly, whereas only 0.1% of cells grown on YEPD were Ade– (indicated by red colonies), 6% of cells grown on pre-spo were red (a 60-fold difference). These results suggested that allele loss (which may occur via chromosome loss, mitotic gene conversion or meiosis) was induced upon growth on pre-spo medium.
FACS analysis of progeny cells shows a reduction in ploidy
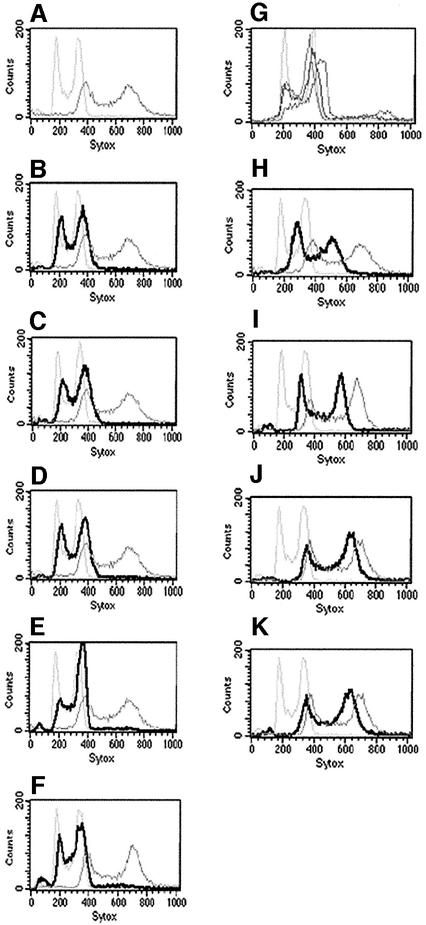
To test whether the loss of the URA3, GAL1, and ADE2 markers occurred by chromosome loss or mitotic gene conversion, we examined by FACS the ploidy of cells derived from tetraploids on pre-spo medium. Control diploid and tetraploid strains of C.albicans were readily distinguishable by FACS analysis, with tetraploids showing twice the DNA content of diploids (Figure 3A). Several colonies picked from pre-spo plates were also subjected to FACS analysis (Figure 3B–F). These strains, PS1–PS5, were all di-allelic at the MTL locus (see below). Strains PS1–PS5 all showed a greatly reduced ploidy relative to the parental tetraploid strain, with a DNA content very close to that of the diploid control (Figure 3B–F). We cannot be certain that these strains are true diploids as several appear to have a ploidy slightly higher than that of a parental diploid (Figure 3B–E). However, different laboratory diploid strains also show slight variations in DNA content as measured by FACS, suggesting that true diploid strains give a range of FACS signals (Figure 3G).
Fig. 3. FACS analysis of tetraploid strains that had been exposed to pre-spo medium. Single colonies were selected and grown up in liquid YEPD medium for FACS analysis, as described in Materials and methods. The x-axis of each graph (Sytox) represents a linear scale of fluorescence, and the y-axis (Counts) represents a linear scale of cell number. (A) Comparison of a control diploid strain (CAF2-1; left trace) with the starting tetraploid strain (RBY18; right trace). Control diploid and tetraploid traces are also included for reference in (B–F) and (H–K). (B–F) Five independent colonies (PS1–PS5) derived from the tetraploid strain RBY18 after incubation on pre-spo medium; all five colonies were di-allelic at MTL. (G) Comparison of the FACS profiles of four different laboratory diploid strains, CAF2-1, CHY257, CHY439 and CHY477 (Miller and Johnson, 2002). (H and I) Two colonies derived from the tetraploid strain after growth on pre-spo medium: these were chosen for FACS analysis because they were tri-allelic at the MTL locus. (J and K) Two colonies that were still tetra-allelic at MTL after incubation on pre-spo medium.
We also selected several additional pre-spo-derived colonies for FACS analysis that were tri-allelic or tetra-allelic at the MTL locus (see below). Significantly, the total DNA content of these strains appeared to mirror the number of MTL alleles they carried. For example, the FACS scans of three colonies that were tri-allelic at MTL showed DNA contents between that of a diploid and a tetraploid (examples shown in Figure 3H and I), suggesting they are trisomic for many of their chromosomes. Similarly, three colonies that were tetra-allelic at MTL after growth on pre-spo medium also appeared to be tetraploid or close to tetraploid in total DNA content (examples shown in Figure 3J and K). These results show that pre-spo conditions give rise to efficient chromosome loss and confirm that the allele loss observed in the previously described experiments is due to chromosome loss, and not to gene conversion or other types of allele loss.
Allele loss is concerted for all marked chromosomes
Based on the FACS analyses described above, chromosome loss in the tetraploids appeared to occur by an imprecise mechanism, i.e. it produced both diploid and triploid products. We addressed this point in more detail by directly measuring the rate at which genetically marked chromosomes were lost. We first used PCR to examine loss of alleles at the MTL locus. The tetraploid contains four unique alleles at the MTL locus (wt a1, wt alpha1/alpha2, Δa1 and Δalpha1/alpha2) and thus it is possible to follow the fate of each allele following exposure of the tetraploid to pre-spo medium. Figure 4 shows an analysis of tetraploid colonies plated on either pre-spo or YEPD plates. In this experiment, we again observed that a significantly higher fraction of cells grown on pre-spo were Ade– or DOGR compared with cells grown on YEPD (18-fold more colonies were DOGR and 46-fold more colonies were Ade–). In addition, analysis of the MTL locus in 32 colonies picked at random from YEPD revealed that 31 of the 32 colonies were still tetra-allelic at MTL and one was tri-allelic, consistent with relatively stable propagation of the tetraploid on this medium. In comparison, analysis of 32 colonies randomly picked from pre-spo plates indicated that 35% (14/40) of the colonies were di-allelic at MTL, 35% were tri-allelic (14/40) and 30% were tetra-allelic (12/40). The simplest interpretation of these results is that approximately one-third of the cells grown on pre-spo medium lost two copies of chromosome 5 (the chromosome containing MTL), one-third lost one copy of chromosome 5 and one-third retained the parental configuration of all four copies of chromosome 5. The fact that one-third of the products contained three copies of chromosome 5 suggested, like the FACS analysis, that chromosome loss was occurring by an imprecise mechanism and was therefore unlikely to be a result of a true meiosis.
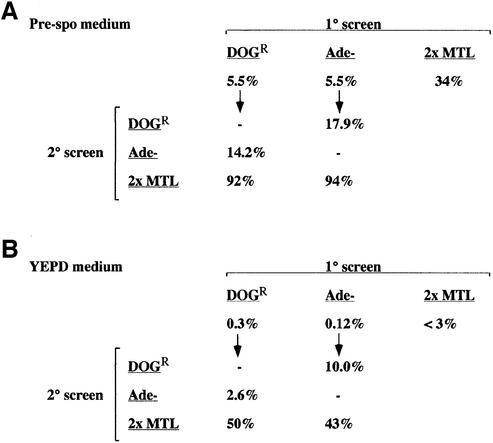
Fig. 4. Pre-spo medium induces allele loss in a tetraploid strain of C.albicans. (A) A detailed analysis of growth of the tetraploid strain on pre-spo medium was carried out. The tetraploid strain RBY18 was grown for 8 days at 37°C on pre-spo medium and pooled colonies were subsequently tested for growth on DOG or YEPD plates. The percentage of cells that were DOGR, Ade– or di-allelic at the MTL locus is indicated (1° screen). The DOGR and Ade– colonies were further tested to determine the percentage of these cells that had lost other chromosomal markers (2° screen). (B) The tetraploid strain was grown on YEPD for 8 days at 37°C, and subsequently analyzed as in (A). The results of (A) and (B) show that allele loss is concerted.
In order to compare the rates of loss of different chromosomes, we calculated the fraction of the population that had lost chromosome 1 (by monitoring GAL1), chromosome 3 (by monitoring ADE2) and chromosome 5 (by monitoring MTL). Note that if all of the cells underwent random chromosome loss to form diploids, we would expect that ∼17% of the cells would be Ade– and 17% of the cells would be DOGR. This is because one in six of the progeny (17%) would be ade2/ade2, whereas five out of six progeny would contain at least one wild-type copy of ADE2. A similar argument applies for GAL1. We observed that 5.5% of the colonies derived from pre-spo medium were Ade–, 5.5% were DOGR and 35% were di-allelic at MTL (Figure 4A). Thus, in this experiment, we can estimate that one-third of the tetraploid cells exposed to pre-spo medium lost two copies of chromo some 1, one-third lost two copies of chromosome 3 and one-third lost two copies of chromosome 5. These results indicate that chromosomes 1, 3 and 5 were lost at a similar rate upon exposure of the tetraploid to pre-spo medium.
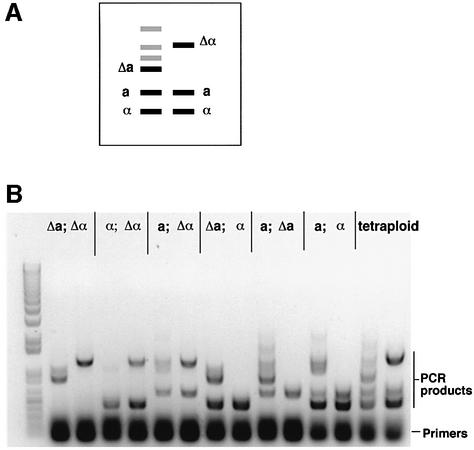
We also determined whether the alleles at the MTL locus segregated randomly, indicative of random loss of chromosome 5, by examining the different di-allelic combinations at MTL. All six possible combinations of MTL were obtained (see Figure 5), indicating that non-parental configurations of the chromosomes are generated by chromosome loss on pre-spo medium. In addition, the distribution of the six possible MTL alleles was analyzed in 259 di-allelic colonies. The number of colonies containing Δa/Δα, WTα/Δα, WTa/Δα, Δa/WTα, WTa/Δa and WTa/WTα alleles was 34 (13%), 29 (11%), 60 (23%), 34 (13%), 51 (20%) and 51 (20%), respectively. The relatively even distribution of MTL alleles strongly suggests that loss of chromosome 5 from tetraploids is random; in particular, the parental combinations are broken up and redistributed.
Fig. 5. Analysis of the MTL locus in cells grown on pre-spo medium. The configuration of the MTL was checked by PCR after tetraploid cells had been exposed to pre-spo medium at 37°C for 8 days. (A) Schematic representation of the PCR products used to identify the different MTL alleles. Black bands represent the position of the relevant PCR products, while gray bands represent non-specific products amplified in the PCR reaction. Each reaction contains three sets of primers and, in this ideal diagram, all four MTL alleles (a, α, Δa, Δα) are present in the strain. (B) PCR products from six different progeny colonies are shown, together with a tetraploid control. All six possible MTL configurations were observed, indicating the random nature of the chromosome loss.
To determine whether chromosome loss was concerted, we monitored strains that had lost one chromosome marker for the loss of other chromosomal markers. We picked the Ade– and DOGR colonies derived from pre-spo and YEPD, and examined the other markers in these colonies. After exposing tetraploids to pre-spo medium, we found that 14.2% of the DOGR colonies were Ade– and 92% were di-allelic at MTL (see Figure 4A). Similarly, of the Ade– colonies, we found that 17.9% were DOGR and 94% were di-allelic at MTL. These proportions are significantly higher than those for loss of a single marker (5.5% Ade–, 5.5% DOGR, 34% di-allelic at MTL), and are near the theoretical limits of fully concerted chromosome loss. Although the overall numbers were much lower, concerted loss was also observed with colonies from YEPD plates. Hence, while only 0.3% of the total colonies were DOGR and 0.12% were Ade–, 2.6% of DOGR colonies were Ade– and 10% of Ade– colonies were DOGR (see Figure 4B). In addition, 50% of DOGR colonies and 43% of Ade– colonies were di-allelic at MTL, whereas <3% (0/32) of the total colonies were di-allelic at MTL. Taken together, these results indicate that the efficient chromosome loss observed on pre-spo (and even the low frequency loss observed on YEPD) is concerted, i.e. cells that have lost two copies of one chromosome are much more likely to have lost copies of other chromosomes.
Time course of chromosome loss
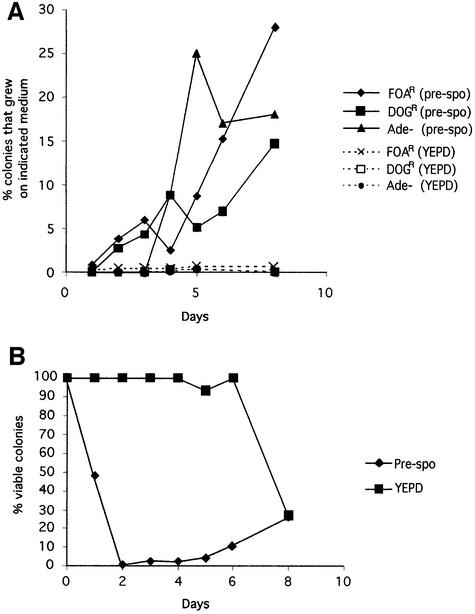
Chromosome loss on pre-spo and YEPD media at 37°C was followed over the course of 8 days. Cells were taken from pre-spo or YEPD plates and tested for the fraction of colonies able to grow on 5-FOA or 2-DOG plates and for Ade– colonies on YEPD plates. As shown in Figure 6A, significant numbers of resistant colonies began to appear on FOA and DOG plates after 2 days exposure to pre-spo medium (∼5% of viable colonies). The number of FOAR, DOGR and Ade– colonies from pre-spo plates continued to increase over time so that by 8 days 15% of the pre-spo colonies were DOGR and 18% were Ade–. Note that in this experiment, chromosome loss after 8 days is close to the theoretical maximum, with most of the colonies having lost two copies of chromosomes 1 and 3. The number of colonies from pre-spo plates that grew on FOA also increased daily and, by day 8, 28% of cells were FOAR. As there is only a single copy of the URA3 gene in the tetraploid, the theoretical limit is 50% FOAR colonies; thus, most of these cells have also undergone very efficient chromosome loss. In contrast to pre-spo plates, tetraploids exposed to YEPD gave rise to very few colonies that were FOA (0.7%) or DOG (0.04%) resistant, even after 8 days of growth (Figure 6A).
Fig. 6. Time course of chromosome loss and viability. (A) The tetraploid strain, RBY18, was grown on pre-spo (solid lines) or YEPD (dashed lines) medium at 37°C. Cells were collected at regular intervals and plated onto FOA, DOG or YEPD plates. The frequency of allele loss is proportional to the percentage of cells that grew on FOA or DOG plates relative to YEPD control plates, and by the percentage of Ade– (red) colonies on YEPD. (B) The percentage of viable cells was calculated by collecting cells, measuring the total OD600 and then plating a fixed number of cells onto YEPD plates.
Growth of tetraploid strains on pre-spo medium is accompanied by cell death
To quantitate the number of viable cells for the time-course experiment described above, cells were collected from pre-spo and YEPD plates at various time points, the OD600 was measured, and equal numbers of cells were plated on YEPD. The number of viable cells obtained from pre-spo plates fell precipitously so that after 2 days <1% of cells gave rise to viable colonies on YEPD (see Figure 6B). The number of viable colonies from pre-spo remained low until days 5–8, when the number of cells began to increase (viability increased to 26% by day 8). In contrast, the number of viable cells obtained from YEPD remained high and fell only at the end of the time course (Figure 6B). Examination of the cells from pre-spo plates by microsopy showed that a number of the cells appeared to have lysed (data not shown). Cells taken from YEPD plates did not exhibit cell lysis. There are two reasonable explanations for this phenomenon. (i) The chromosome loss induced by pre-spo medium could result in high lethality if recessive lethal mutations were present on some chromosomes, i.e. only certain combinations of chromosomes could be lost and still give rise to viable cells. However, the experiments described above indicate random chromosome loss of chromosomes 1, 3 and 5, suggesting that no recessive lethal mutations reside on these chromosomes. (ii) The pre-spo medium could induce stress that causes both chromosome loss and lethality, but the two are not directly linked.
Diploids do not undergo large-scale chromosome loss when grown on pre-spo
Given the efficiency with which pre-spo medium induced chromosome loss from tetraploid strains of C.albicans, we also tested whether these same conditions induced chromosome loss in diploid strains. Diploid strains that were heterozygous for the GAL1 gene or URA3 gene were streaked onto pre-spo medium and grown at 37°C for 9 days. After 9 days on pre-spo, only a very low fraction of the colonies were able to grow on DOG (0.08%) or FOA (0.03%), compared with the results with the tetraploid strain (5.5% were DOGR or FOAR; Figure 4A). In addition, when the MTL configuration of 16 of the DOGR and FOAR colonies was tested, all 16 were found to have the parental configuration at MTL (i.e. they were di-allelic). These results show that, in contrast to the tetraploid strain, the diploid strain does not readily undergo loss of chromosome 5. Since loss of one copy of chromosome 5 is not lethal in C.albicans (as shown by Janbon et al., 1998), this result suggests that pre-spo conditions do not induce chromosome loss in a diploid. The low fraction of DOGR and FOAR colonies may represent mutations or gene conversions of the wild-type GAL1 or URA3 genes when grown on pre-spo medium.
The analysis of chromosome loss from tetraploids, described earlier, also supports the idea that chromosomes are not readily lost from diploids. Examination of the MTL locus in a large number of tetraploids exposed to pre-spo media showed that while many had lost two alleles of MTL, only very few (2/261) had lost more than two alleles at MTL. These results suggest that the diploid state is much more stable than the tetraploid on pre-spo medium and may constitute the ‘ground’ state of ploidy on this medium.
Growth on sorbose medium also induces chromosome loss in the tetraploid
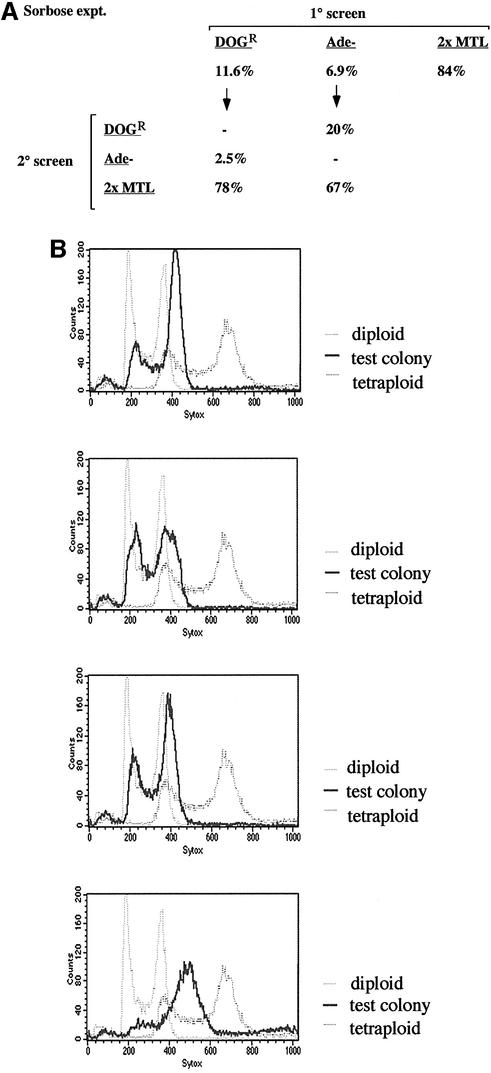
Diploid strains grown on sorbose medium have previously been shown to undergo loss of one copy of chromosome 5, the chromosome containing the MTL locus (Janbon et al., 1998; Magee and Magee, 2000). It is thought that an inhibitor of the sorbose utilization gene (SOU1) resides on chromosome 5, and that loss of one copy of the inhibitor gene allows C.albicans to metabolize sorbose. Based on these results, we expected that growth of a tetraploid strain on sorbose media might also lead to loss of copies of chromosome 5. To test the effect of sorbose on tetraploids, we grew the tetraploid strain RBY18 on sorbose medium for 5–7 days at 37°C. Similar to diploid strains, many of the tetraploid cells failed to grow on sorbose, and survivors began to appear by day 3. After 5–7 days, cells were collected from sorbose plates and tested for Ade– and DOGR phenotypes and MTL configuration. Eighty-four percent of cells were di-allelic at MTL, as expected if copies of chromosome 5 are being lost (Figure 7A). Unexpectedly, a high fraction of the cells had also lost copies of chromosomes 1 and 3, as evidenced by the significant number of DOGR (11.6%) and Ade– (6.9%) colonies, repectively (Figure 7A). The analysis of these data suggests that ∼70% of the cells had lost two copies of chromosome 1 and 41% of cells had lost two copies of chromosome 3. Testing of the DOGR colonies revealed that 2.5% of them were Ade– and 78% of them were di-allelic at MTL (Figure 7A). Similarly, of the Ade– colonies, 20% were DOGR and 67% of them were di-allelic at MTL. The tetraploid strain therefore appears to have undergone concerted chromosome loss of at least three different chromosomes on sorbose medium.
Fig. 7. Growth on sorbose medium induces chromosome loss from tetra ploids. The tetraploid strain RBY18 was grown on sorbose medium at 37°C for 7 days. (A) Chromosome loss was monitored by calculating the percentage of cells that were DOGR, Ade– or di-allelic at MTL (1° screen). DOGR colonies were also tested to see what percentage were Ade– or di-allelic at MTL, and Ade– colonies similarly tested to find the percentage that were DOGR or di-allelic at MTL (2° screen). (B) Four colonies picked from the sorbose medium and determined to be di-allelic at MTL were grown up in YEPD and analyzed by FACS. In each graph, a control diploid strain, the parent tetraploid strain and the test colony are included (see Figure 3 for details).
To confirm that the ploidy of the tetraploid is reduced upon growth on sorbose, we carried out FACS analysis on four of the sorbose-derived strains (Figure 7B). Three of the four showed a ploidy close to that of a diploid control, whereas one showed a ploidy intermediate between a diploid and tetraploid. In agreement with the genetic analysis, the FACS results suggest that the tetraploid has undergone extensive chromosome loss on sorbose medium, not only of chromosome 5, but of most of the other chromosomes as well. This is the same behavior as was observed for growth of tetraploids on pre-spo medium, with one significant difference. Whereas growth of the tetraploid on pre-spo medium very rarely gave rise to colonies with only one copy of chromosome 5 (<1%), a significant fraction of the colonies from sorbose contained only one copy of chromosome 5 (11.6%). This observation is consistent with previous results showing that specific loss of chromosome 5 gives cells a growth advantage on sorbose (Janbon et al., 1998).
Progeny from pre-spo media are mating competent
A major goal for pursuing chromosome loss in C.albicans is to be able to manipulate a genetic cycle in the organism. In order to show that mating followed by chromosome loss constitutes a true cycle, we set out to demonstrate that two diploid strains can be mated, the resulting tetraploid can be induced to produce diploid progeny, and the diploid progeny can in turn be mated to complete the cycle. For this experiment, we first mated an a and an α strain (see Materials and methods). The resulting tetraploid was grown on pre-spo medium to induce chromosome loss, and Ade– colonies were isolated, as these had probably undergone significant chromosome loss. These colonies were then analyzed by PCR to determine their MTL configuration. Potential mating strains, i.e. those that contained a copy of either the wild-type MTLa or MTLα locus, were then mated against control diploid strains (Ura–) of the opposite mating type. The tetraploid mating products were selected on Ura– Ade– plates. In every case, the pre-spo-derived a and α diploids proved to be mating competent and gave rise to new tetraploid colonies. Thus, mating of diploid strains, chromosome loss on pre-spo medium and re-mating constitutes a true parasexual cycle.
Use of a C.albicans parasexual cycle to link phenotype to genotype
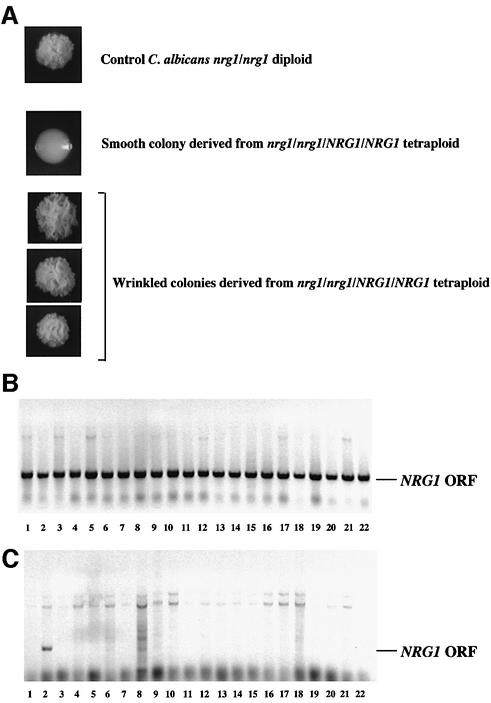
Two experiments were carried out to demonstrate that mating followed by chromosome loss could be used to carry out useful genetic manipulations. In the first experiment, we showed that a phenotype could be unambiguously linked to a gene deletion created in the laboratory. A Δnrg1/Δnrg1 strain of C.albicans is characterized by being highly filamentous and forming characteristic wrinkled colonies on YEPD (see Figure 8A; Braun et al., 2001; Murad et al., 2001). Although the nrg1 deletion was created in the laboratory, it is formally possible that the phenotype also involves additional unlinked mutations that were inadvertently introduced into the strain during construction. To show that the phenotype of this strain is caused by the nrg1 deletion, we crossed two nrg1/NRG1 heterozygous strains to generate a nrg1/nrg1/NRG1/NRG1 tetraploid strain. The tetraploid strain was then incubated on pre-spo medium for 10 days to induce chromosome loss, and cells were plated on to YEPD or DOG plates. Colonies that were wrinkled or smooth in appearance were chosen from both YEPD and DOG plates and re-streaked to confirm their phenotype (Figure 8A). PCR was then carried out on these colonies to determine whether the presence or absence of the intact NRG1 gene correlated with the smooth or wrinkled appearance of the colonies. Strikingly, 38/38 smooth colonies showed the presence of the NRG1 gene, while 29/30 wrinkled colonies lacked the NRG1 gene (see Figure 8B and C). The one wrinkled colony that tested positively for the NRG1 ORF by PCR (Figure 8C, lane 2) was re-streaked for single colonies and re-checked by PCR. Derivative colonies lacked the NRG1 ORF, suggesting that the original colony was a mixture of nrg1– and NRG1+ cells (data not shown). These results show that mating followed by chromosome loss on pre-spo can be used to link a phenotype with a specific mutation. This manipulation is especially important in C.albicans, as chromosome rearrangements are known to be common in this organism, and may occur during the routine construction of deletion strains.
Fig. 8. Chromosome loss in a tetraploid can be used to link a phenotype with a genotype. A tetraploid strain that is heterozygous for the NRG1 gene (nrg1/nrg1/NRG1/NRG1) was constructed by mating two Δnrg1/NRG1 strains, and then exposed to pre-spo medium to induce chromosome loss. (A) Both smooth and wrinkled colonies were obtained following chromosome loss on pre-spo. A control nrg1/nrg1 diploid strain is shown with its characteristic wrinkled appearance. Colony PCR was carried out on smooth colonies (B) or wrinkly colonies (C) generated from the tetraploid exposed to pre-spo medium in order to detect the presence or absence of the NRG1 ORF. Only smooth colonies showed the presence of the NRG1 gene. This analysis shows that the wrinkled appearance is caused by the NRG1 deletion (or a mutation closely linked to it). (Upper PCR bands are background bands from the PCR reaction.)
This experiment also shows that new diploid strains can be constructed that combine mutations from two diploid parents. We believe that, in many cases, this method of constructing strains with mutations in multiple genes will prove simpler than the current approach of creating the deletions sequentially in a single isolate. In addition, the ability to obtain homozygous mutants from heterozygotes via a parasexual cycle (as demonstrated above) could be particularly useful in determining whether a gene has an essential function in C.albicans. Finally, as shown above, this approach can also be used to circumvent the sometimes difficult task of deleting the second allele of a gene in generating a homozygous deletion strain.
Use of a C.albicans parasexual cycle for determining genetic linkage
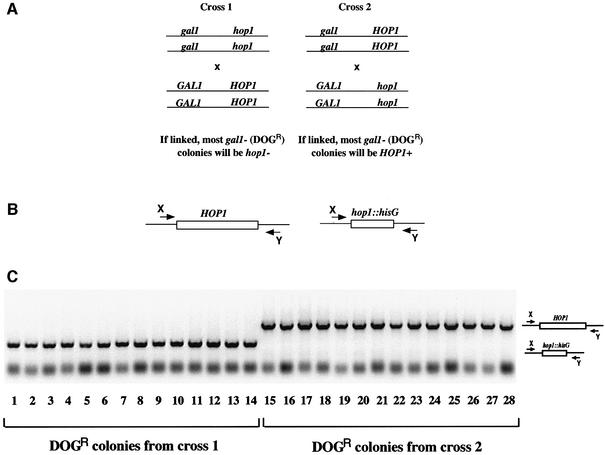
We also demonstrated that mating followed by chromosome loss could be used to determine genetic linkage. From unpublished studies on the HOP1 gene, we surmised that it resided on chromosome 1 together with the GAL1 gene (R.J.Bennett and A.D.Johnson, unpublished observations). To test this idea rigorously, we performed the genetic crosses shown in Figure 9A. A gal1/gal1/hop1/hop1 strain was crossed with a GAL1/GAL1/HOP1/HOP1 strain, and the reciprocal cross, a gal1/gal1/HOP1/HOP1 strain crossed with a GAL1/GAL1/hop1/hop1 strain, was also performed. The tetraploid strains were grown on pre-spo medium to induce chromosome loss. If the GAL1 and HOP1 genes were linked, then they should co-segregate. Thus, in the first cross, all gal1– strains would also be expected to be hop1–, while in the second cross all gal1– strains would be expected to be HOP1+ (barring recombination events). After incubation of the tetraploids on pre-spo plates, colonies lacking GAL1 were selected for on DOG media and tested for the presence of the wild-type HOP1 gene or the Δhop1 allele by PCR (Figure 9B and C). The results showed that 20/20 of the DOG-resistant colonies from the first cross contained only the Δhop1 allele, while 20/20 DOG-resistant colonies from the second cross contained only the wild-type HOP1 allele (Figure 9C). The GAL1 and HOP1 genes are therefore genetically linked; GAL1 has been shown to reside on chromosome 1 (Magee et al., 1988), and we conclude that HOP1 also lies on this chromosome.
Fig. 9. Chromosome loss induced by pre-spo medium can be used to demonstrate genetic linkage. (A) Two mating experiments were carried out in which a gal1 hop1 strain was crossed with a GAL1 HOP1 strain (cross 1), or a gal1 HOP1 strain was crossed with a GAL1 hop1 strain (cross 2). The resultant tetraploid strains were incubated on pre-spo medium at 37°C to induce chromosome loss. If the GAL1 and HOP1 genes are linked, all the DOGR (i.e. gal1–) progeny from cross 1 will lack the HOP1 gene, while all the DOGR progeny from cross 2 will contain the wild-type HOP1 gene. (B) Schematic of the PCR used to test whether a colony contains the wild-type HOP1 gene or the hop1 deletion. (C) Colony PCR was performed on 14 DOGR progeny from cross 1 (lanes 1–14) and 14 DOGR progeny from cross 2 (lanes 15–28). All PCR reactions contained primer sets to detect both the deleted and wild-type HOP1 alleles. The results show that HOP1 is genetically linked to GAL1.
Discussion
The lack of a sexual cycle in C.albicans has severely limited genetic analysis of this organism. In this study we provide evidence for a chromosome-loss pathway which, when combined with mating, completes a parasexual cycle for C.albicans that can be readily carried out in the laboratory. In particular, we show that the ploidy of a tetraploid strain of C.albicans (produced by the mating of two diploid strains) can be reduced by simple laboratory manipulations (growth on S.cerevisiae ‘pre-sporulation’ medium at 37°C) to that of a diploid. Although we do not understand its detailed mechanism, this ploidy decrease occurs through random chromosome loss that is concerted; that is, loss of one or more chromosomes seems to predispose cells to lose others, with the diploid state being the final product.
In this paper, we also show that concerted chromosome loss combined with mating allows for several important genetic manipulations to be performed in C.albicans. These include: (i) confirming that a phenotype is a consequence of a specific genotype; (ii) genetic linkage experiments; and (iii) construction of new strains. The ability to carry out these manipulations should greatly improve the ease with which C.albicans can be studied. For example, the ability to link a mutation with a certain phenotype (see Figure 8) is particularly important in this organism where chromosome rearrangments can occur at a high frequency (for a review, see Rustchenko and Sherman, 2003). Currently, gene ‘add-back’ experiments are typically carried out in which the gene of interest is re-introduced into the homozygous mutant. These experiments can be difficult to interpret, however, with multiple isolates containing the re-introduced gene often showing different degrees of complementation. The procedure described here eliminates this problem by relying on chromosome segregation to create a large collection of strains with defined sets of alleles. It is then possible to unambiguously link a phenotype with a mutation. Genetic linkage experiments (see Figure 9) should allow both confirmation of the physical map of C.albicans that is being generated from the sequencing of the genome (http://sequence-www.stanford.edu/group/candida) and the identification of regions of the genome that are ‘hot’ or ‘cold’ for mitotic recombination. Finally, the ability to construct new strains by a parasexual cycle will be of benefit as multiple gene knockouts in C.albicans are often laborious and time consuming. Establishing whether a gene is essential should be straightforward since mating of heterozygous strains followed by chromosome loss could reveal whether the homozygous mutant strain is viable. For these applications, ‘a’ and ‘α’ mating strains could be generated from existing mutant strains (which are predominantly ‘a/α’) by inducing the loss of chromosome 5 in diploid strains, as described by Magee and Magee (2000).
We have described new conditions for inducing particularly efficient chromosome loss from C.albicans tetraploids, and shown how chromosome loss can be combined with mating to form a robust parasexual cycle that can be carried out in the laboratory. However, chromosome loss itself has been observed previously. In several classical experiments, diploid cells of C.albicans were spheroplasted and fused to generate cells that were tetraploid following nuclear fusion. It was shown that the DNA content in these tetraploid cells could be reduced by chromosome loss induced by heat-shocking the cells at 50°C for 1–2 min (Hilton et al., 1985). This treatment created some cells with a diploid, or close to diploid, DNA content. In these pioneering studies, Hilton et al. were able to map ADE1, ARG1 and MET1 to a single chromosome through linkage analysis. In another study, tetraploid cells heterozygous for a partially dominant selectable marker were constructed by protoplast fusion. Following selection for drug resistance, 70% of the colonies picked were found to have lost chromosomes, with about half of these cells close to a diploid level (Poulter and Hanrahan, 1983). These results indicate that heat shock or drug selection can induce mitotic instability and chromosome loss, with diploid cells a significant product. Finally, growth of diploid strains of C.albicans on sorbose medium at 37°C has previously been shown to result in the loss of one copy of chromosome 5, the chromosome containing the MTL locus (Janbon et al., 1998, 1999). The majority of diploid cells die when grown on sorbose medium, but the survivors are often monosomic for chromosome 5. It has been proposed that the level of expression of the SOU1 gene, which is essential for l-sorbose assimilation in C.albicans, is regulated by the copy number of chromosome 5 (Janbon et al., 1998). We show in this paper that sorbose can, like pre-spo medium, induce efficient chromosome loss from a tetraploid strain. However, unlike the diploid, all chromosomes appear to be lost at random from the tetraploid, so that overall ploidy is reduced to close to that of a diploid strain. These results suggest the possibility that chromosomes other than chromosome 5 are also lost during growth of the diploid on sorbose media, but that this effect is masked either by reduplication of the missing chromosome or by cell death caused by exposure of recessive lethal alleles in the genome. In support of this idea, ∼10% of diploid strains grown on sorbose were found to have alterations in chromosomes other than chromosome 5, suggesting a general chromosomal instability on sorbose medium (Janbon et al., 1999).
All of the conditions found to induce chromosome loss in C.albicans also induce stress in the organism, as indicated by cell death of a significant fraction of the population. For example, in this paper we show that growth of the tetraploid strain on pre-spo medium resulted in a majority of the cells dying after 3 days at 37°C (Figure 6B). The number of viable cells then began to accumulate and most of these appeared to have lost at least one chromosome (after 8 days, 70% were either di- or trisomic for chromosome 5). Similarly, heat-shock treatment of tetraploids resulted in 10% viability, and growth of a diploid strain on sorbose medium for 3 days resulted in <1% viability of the original population (Hilton et al., 1985; Janbon et al., 1998). Unlike the case with sorbose medium and heat shock, it is not known why cell death and chromosome instability should occur on pre-spo medium, especially as its recipe is close to that of YEPD (see Materials and methods). Perhaps chromosome loss provides a tetraploid cell with some growth advantage on pre-spo medium, although, if so, the nature of this advantage is not known. Regardless of the mechanism, it appears that the cell may increase chromosome instability and missegregation in order to survive stress conditions (Janbon et al., 1999).
The present study leaves open the possibility that C.albicans is able to undergo meiosis. However, none of the conditions tested seemed to induce meiosis in our tetraploid strain, including conditions known to induce sporulation in S.cerevisiae. In addition, the reduction in ploidy on pre-spo medium was not blocked by deletion of the putative meiosis genes SPO11, DMC1(DLH1) or HOP1 (R.J.Bennett and A.D.Johnson, unpublished observations). If meiosis does occur in C.albicans, it may occur by a different signaling pathway, perhaps requiring a stimulus from its mammalian host. Curiously, while homologs of several S.cerevisiae genes required for meiosis are present in the C.albicans genome, including SPO11, DMC1 and NDT80 (Diener and Fink, 1996; Tzung et al., 2001), a number of conserved meiotic genes are conspicuous by their absence. These include IME1, ZIP2 and SPO13, which are strictly required for meiosis in S.cerevisiae (Tzung et al., 2001). In any case, the question of whether C.albicans can undergo meiosis has not been resolved.
Studies of C.albicans suggest that while populations are largely clonal, there is evidence for some mechanism of genetic exchange in natural populations (Graser et al., 1996; Tibayrenc, 1997). Although the present study does not rule out a meiotic phase in C.albicans, we demonstrate that mating and chromosome loss can constitute an alternative mechanism for genetic exchange. We speculate that chromosome loss could be induced in vivo when C.albicans is stressed by environmental conditions inside its mammalian host. Thus, chromosome loss may have evolved to provide C.albicans with an alternative pathway to meiosis and lead to the rearrangement of genetic material in this organism.
Materials and methods
Strains
All strains were derived from strain CAI4 (Δura3::imm434/Δura3::imm434; Fonzi and Irwin, 1993). Both copies of GAL1 were sequentially disrupted by means of a hisG-URA3-hisG cassette in the strain CHY444 (a derivative of CAI4 in which the MTL alpha1 and alpha2 genes have been deleted; Hull et al., 2000) to produce the strain RBY16. SD plates containing 5-FOA and uridine were used to counterselect against the URA3 marker (Fonzi and Irwin, 1993). RBY16 was then mated (mating conditions described below) to CHY477, in which the MTLa1 and ADE2 genes had been deleted. The resulting tetraploid, RBY18, was used to assay for chromosome loss. The diploid strains used to test for chromosome loss were CAF2-1 (Fonzi and Irwin, 1993) and RBY09 (ura3/ura3 gal1::HisG-URA3-HisG/GAL1 MTLa/mtlα1::HisG mtlα2::HisG).
In addition, strain RBY110 (ura3/ura3, hop1::HisG/hop1::HisG, gal1:HisG/gal1::HisG, MTLa/mtlα1::HisG mtlα2::HisG) was mated with CHY477 (Miller and Johnson, 2002) to give RBY595. RBY16 was mated with RBY113 (ura3/ura3 hop1::HisG-URA3-HisG/hop1::HisG, ade2::HisG/ade2::HisG, mtla1::HisG/MTLα) to give RBY593. RBY51 (ura3/ura3 nrg1::HisG/NRG1, MTLa/mtlα1::HisG mtlα2::HisG) was mated with RBY54 (ura3/ura3 nrg1::HisG-URA3-HisG/NRG1 ade2::HisG/ade2::HisG, mtla1::HisG/MTLα) to give RBY540.
Chromosome loss experiment
To test for chromosome loss, the tetraploid strain RBY18 or diploid strains CAF2-1/RBY09 were streaked onto different media and incubated at 25, 30 or 37°C for 5–10 days. Following incubation, cells were picked, resuspended in H2O and the OD600 measured. Cells were then plated on test conditions, i.e. YEPD containing uridine and adenine, 2-DOG plates, 5-FOA plates or 2-DOG/5-FOA plates. While the exact degree of chromosome loss varied significantly from experiment to experiment, large-scale random chromosome loss was observed in six independent experiments with cells grown on pre-spo medium.
The tetraploid strain was also grown on sorbose medium (Janbon et al., 1998) at 37°C for 5–7 days. Resulting colonies were analyzed as above. Chromosome loss on sorbose medium was observed in three independent experiments.
FACS analyses
FACS analyses of C.albicans strains were carried out by growing logarithmic cultures of cells to OD600 ∼2.0. Cells were collected by centrifugation from 0.5 ml cultures and prepared for FACS analysis as described previously (Hull et al., 2000).
Media, mating conditions and MTL PCR assay
For details, see Supplementary data available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to Eric Summers and Gerald Fink for communicating the media conditions for using 2-deoxygalactose in C.albicans. We thank Richard Morreale for assistance with FACS analyses, and Annie Tsong and Hiten Madhani for comments on the manuscript. We are grateful to the Stanford Genome Technology Center (http://www-sequence.stanford. edu/group/candida) for providing sequence data for C.albicans. Sequencing of the C.albicans genome was accomplished with the support of the NIDR and the Burroughs Welcome Fund.
References
- Boeke J.D., LaCroute,F. and Fink,G.R. (1984) A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet., 197, 345–346. [DOI] [PubMed] [Google Scholar]
- Braun B.R., Kadosh,D. and Johnson,A.D. (2001) NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. EMBO J., 20, 4753–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener A.C. and Fink,G.R. (1996) DLH1 is a functional Candida albicans homologue of the meiosis-specific gene DMC1. Genetics, 143, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond M.B., Wallace,S.E., McClish,D.K., Pfaller,M.A., Jones,R.N. and Wenzel,R.P. (1999) Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis., 29, 239–244. [DOI] [PubMed] [Google Scholar]
- Fonzi W.A. and Irwin,M.Y. (1993) Isogenic strain construction and gene mapping in Candida albicans. Genetics, 134, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser Y., Volovsek,M., Arrington,J., Schonian,G., Presber,W., Mitchell,T.G. and Vilgalys,R. (1996) Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proc. Natl Acad. Sci. USA, 93, 12473–12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C. and Fink,G.R. (1991) Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego, CA.
- Hilton C., Markie,D., Corner,B., Rikkerink,E. and Poulter,R. (1985) Heat shock induces chromosome loss in the yeast Candida albicans. Mol. Gen. Genet., 200, 162–168. [DOI] [PubMed] [Google Scholar]
- Hull C.M. and Johnson,A.D. (1999) Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science, 285, 1271–1275. [DOI] [PubMed] [Google Scholar]
- Hull C.M., Raisner,R.M. and Johnson,A.D. (2000) Evidence for mating of the ‘asexual’ yeast Candida albicans in a mammalian host. Science, 289, 307–310. [DOI] [PubMed] [Google Scholar]
- Janbon G., Sherman,F. and Rustchenko,E. (1998) Monosomy of a specific chromosome determines l-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc. Natl Acad. Sci. USA, 95, 5150–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G., Sherman,F. and Rustchenko,E. (1999) Appearance and properties of l-sorbose-utilizing mutants of Candida albicans obtained on a selective plate. Genetics, 153, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E. et al. (1996) Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc. Natl Acad. Sci. USA, 93, 13217–13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kohler,J. and Fink,G.R. (1994) Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science, 266, 1723–1726. [DOI] [PubMed] [Google Scholar]
- Lockhart S.R., Pujol,C., Daniels,K.J., Miller,M.G., Johnson,A.D., Pfaller,M.A. and Soll,D.R. (2002) In Candida albicans, white–opaque switchers are homozygous for mating type. Genetics, 162, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B.B. and Magee,P.T. (2000) Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science, 289, 310–313. [DOI] [PubMed] [Google Scholar]
- Magee B.B., Koltin,Y., Gorman,J.A. and Magee,P.T. (1988) Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol. Cell. Biol., 8, 4721–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.G. and Johnson,A.D. (2002) White–opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell, 110, 293–302. [DOI] [PubMed] [Google Scholar]
- Murad A.M. et al. (2001) NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J., 20, 4742–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. (1984) Toxicity of 2-deoxygalactose to Saccharomyces cerevisiae cells constitutively synthesizing galactose-metabolizing enzymes. Mol. Cell. Biol., 4, 994–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R. and Hanrahan,V. (1983) Conservation of genetic linkage in nonisogenic isolates of Candida albicans. J. Bacteriol., 156, 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko E. and Sherman,F. (2003) Genetic instability of C.albicans. In Howard,D. (ed.), Pathogenic Fungi in Humans and Animals. Marcel Dekker, New York, NY.
- Soll D.R. (1997) Gene regulation during high-frequency switching in Candida albicans. Microbiology, 143, 279–288. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M. (1997) Are Candida albicans natural populations subdivided? Trends Microbiol., 5, 253–254. [DOI] [PubMed] [Google Scholar]
- Tzung K.W. et al. (2001) Genomic evidence for a complete sexual cycle in Candida albicans. Proc. Natl Acad. Sci. USA, 98, 3249–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]