Abstract
Regardless of their origins, mate preferences should, in theory, be shaped by their benefits in a mating context. Here we show that the female preference for carotenoid colouration in guppies (Poecilia reticulata) exhibits a phenotypically plastic response to carotenoid availability, confirming a key prediction of sexual selection theory. Earlier work indicated that this mate preference is genetically linked to, and may be derived from, a sensory bias that occurs in both sexes: attraction to orange objects. The original function of this sensory bias is unknown, but it may help guppies find orange-coloured fruits in the rainforest streams of Trinidad. We show that the sensory bias also exhibits a phenotypically plastic response to carotenoid availability, but only in females. The sex-specificity of this reaction norm argues against the hypothesis that it evolved in a foraging context. We infer instead that the sensory bias has been modified as a correlated effect of selection on the mate preference. These results provide a new type of support for the hypothesis that mate preferences for sexual characters evolve in response to the benefits of mate choice—the alternatives being that such preferences evolve entirely in a non-mating context or in response to the costs of mating.
Keywords: sexual selection, phenotypic plasticity, mate preference, carotenoids, Poeciliidae
1. Introduction
Darwin's (1871) famous hypothesis that female mate preferences are responsible for the evolution of elaborate male ornaments has been confirmed beyond reasonable doubt (Andersson 1994). Recent research in sexual selection has largely focused on the question of how mate preferences themselves evolve. Mate preference evolution can be initiated by a pre-existing sensory bias, i.e. an incipient preference that inadvertently enables males with certain trait values to mate more frequently (or with higher quality females) than others (reviewed in Andersson 1994; Kokko et al. 2003; Mead & Arnold 2004). As the male trait evolves in response to selection caused by the incipient preference, the preference itself should evolve in a manner determined, in part, by the net effect of the male trait, and any genetically correlated traits, on the fitness of females and their offspring (Payne & Pagel 2001; Kokko et al. 2003; Hunt et al. 2004; Mead & Arnold 2004). Much empirical effort has been devoted to measuring the costs and benefits of female mate preferences and the relevant genetic correlations, but there have been few attempts to determine whether mate preferences actually have been shaped by selection in a mating context (Jennions & Petrie 1997; Widemo & Saether 1999; Grether 2000; Qvarnstrom et al. 2000). Recent comparative and experimental evolution studies have shown that female resistance to male sexual traits can evolve in response to the costs of mating (e.g. Holland & Rice 1999; Arnqvist & Rowe 2002), but we lack comparable data for systems in which females are thought to receive direct or indirect fitness benefits from choosing males with more elaborate sexual traits. This is an especially salient issue for mate preferences that may be derived from sensory biases that have an important function in another context (e.g. food detection) (West-Eberhard 1984; Kirkpatrick 1987; Basolo 1990; Ryan et al. 1990; Endler 1992; Proctor 1992; Basolo 1998; Endler & Basolo 1998; Rodd et al. 2002; Rodriguez & Snedden 2004; Schaefer et al. 2004; Smith et al. 2004). Are such mate preferences evolutionarily constrained by selection in a non-mating context (Rodriguez & Snedden 2004)? Do they eventually become genetically decoupled from their original sensory functions? Or do the underlying sensory biases evolve as a correlated effect of selection in a mate choice context? These possibilities are not mutually exclusive but instead represent different outcomes along a theoretical continuum.
Using guppies (Poecilia reticulata), a well established model system for sexual selection studies, we investigated whether a female mate preference and the sensory bias to which it is genetically linked have evolved in response to variation in an environmental factor that is thought to affect the benefits of the mate preference. Female guppies prefer males with high concentrations of carotenoid pigments in the orange spots on their bodies (Kodric-Brown 1989; Grether 2000), and guppies of both sexes are attracted to inanimate orange objects outside a mating context (Rodd et al. 2002). In the rainforests to which guppies are native, being attracted to orange objects may help these fish exploit nutritious orange fruits that occasionally fall into the streams from the forest canopy (Rodd et al. 2002). In laboratory-born fish, the degree of attraction to orange objects and the strength of the female preference for carotenoid colouration were highly, positively correlated across populations, which indicates that they share a common genetic basis (Rodd et al. 2002). Other poeciliid fishes besides guppies are also attracted to orange objects (F. H. Rodd et al. unpublished data), but whether this sensory bias predates the evolution of orange colouration is not yet known. While it seems likely that the mate preference for carotenoid colouration was originally a by-product of the sensory bias, the reverse scenario cannot be ruled out (Rodd et al. 2002). Either way, given the genetic correlation between these two traits, evolutionary changes in one should be mirrored by changes in the other. We now describe a specific prediction about how the mate preference would be expected to be shaped by selection in a mating context.
Carotenoid-based traits in general, and the sex-limited orange spots of male guppies in particular, have long been viewed as indicators of mate quality (Endler 1980; Kodric-Brown 1989; Hill 1991; Olson & Owens 1998). Animals are unable to synthesize these pigments (Goodwin 1984), and in some species, including guppies, a trade-off exists between the use of ingested carotenoids in colouration and in the immune system (Olson & Owens 1998; Blount et al. 2003; Faivre et al. 2003; McGraw & Ardia 2003; Grether et al. 2004). The primary source of carotenoids for guppies in nature is unicellular algae, the availability of which is largely a function of the openness of the forest canopy (i.e. light availability) (Grether et al. 1999, 2001a). Carotenoid deposition in the orange spots is a diminishing returns function of carotenoid intake, which means that as carotenoid availability increases, it should become harder for females to use carotenoid colouration to distinguish between males that are fit (e.g. good foragers with a strong immune system and few diseases) and those that are not (Olson & Owens 1998; Grether 2000). Mate quality in guppies could include the heritable components of variation in foraging ability, parasite resistance and male attractiveness, as well as any direct benefits associated with choosing healthy mates (Endler 1980; Kennedy et al. 1987; Houde & Torio 1992; Houde 1997; Lopez 1998; Brooks 2000; Evans et al. 2004). The availability of carotenoids in the environment could influence the indicator value of the orange spots with respect to any of these components of mate quality (Grether 2000). For example, if carotenoid assimilation efficiency was heritable at a site where dietary carotenoids were scarce, females preferentially mating with high-carotenoid males would tend to produce high-carotenoid (i.e. attractive) sons.
Given that mate choice entails costs (Jennions & Petrie 1997), an optimally choosy female would place more weight on traits that are strongly correlated with mate quality than on those that are not. This is a key prediction of the indicator models of sexual selection (Schluter & Price 1993; Andersson 1994; Iwasa & Pomiankowski 1999; Kokko et al. 2003; Mead & Arnold 2004). Thus, in guppies, the strength of the mate preference for carotenoid colouration is predicted to covary negatively with carotenoid availability in the wild (Grether 2000). That is, females in low-carotenoid availability environments should place more weight on carotenoid colouration versus other male traits when choosing mates, in comparison to females in high-carotenoid availability environments. Negative covariation between carotenoid availability and the strength of the female preference for carotenoid colouration could arise along two different evolutionary paths, corresponding to two alternative predictions: populations exposed to different levels of carotenoid availability could diverge genetically in preference strength; or a reaction norm could evolve such that carotenoid intake (or a correlated environmental factor) affects the development of the preference. Grether (2000) tested the first prediction and showed that while the mate preference for carotenoid colouration varies genetically among populations, the pattern of divergence appears to be random with respect to carotenoid availability in the wild. Here we test the second prediction.
The reaction norm prediction could be satisfied if the strength of the female mate preference for carotenoid colouration was reduced either by carotenoid intake or overall food intake, because carotenoid availability is positively linked to food availability in the wild (algae is the main source of food and carotenoids; Grether et al. 1999, 2001b). We, therefore, manipulated carotenoid intake and food intake in a factorial experiment in which female guppies were raised from birth on one of four diets: low food/trace carotenoid, low food/high carotenoid, high food/trace carotenoid or high food/high carotenoid. The purpose of manipulating life-long food intake, as opposed to short-term access to food, was to simulate food availability differences between streams in the wild. In a separate experiment, we examined the influence of carotenoid intake on the degree to which guppies of both sexes were attracted to orange objects in a non-mating context.
2. Material and Methods
(a) Study populations and experimental diets
We used first generation (G1) laboratory descendants of fish collected from four low predation (Endler 1980) sites in the Northern Range of Trinidad: two in the Madamas drainage (Universal Transverse Mercator Grid coordinates, Zone 20: PS 939.2 886.6, PS 950.1 880.0) and two in the Upper Quare (Crayfish) drainage (PS 965 835.2, PS 965 832.2). Twenty-five to 35 wild females per population contributed offspring to the G1 generation. Fish were housed at UCLA in a temperature-controlled (24.0±1.5°C water temperature) windowless room on a 12 : 12 photoperiod (mixed daylight spectrum fluorescent and incandescent light). The tanks were treated with 2-chloro-4,6-bis(ethylamino)-s-triazine (Algae Destroyer, Aquarium Pharmaceuticals) to retard algae growth and visible algae were removed regularly.
Newborn G1 fish were randomly assigned to one of two food levels (low or high) and one of three carotenoid concentrations (trace, low or high) and housed in 8 l plastic tanks at densities of 1–6 fish per tank. Because of tank space limitations, females in the intermediate (low) carotenoid diet group were removed from the experiment. The high food level was about as much as guppies of a given age were willing to eat on a twice-daily feeding schedule (as determined in pilot feeding trials based on the presence of uneaten food); the low food level was one third of the high food level. Food amounts were adjusted as the fish aged, on a predetermined schedule and delivered with a specialized feeding device. The low- and high-carotenoid diets were designed to contain carotenoid pigments found in the natural diets of guppies. Nine different types of carotenoids have been found in the periphyton in Trinidad streams (Grether et al. 1999; 2001b). Of these, only lutein, zeaxanthin and β-carotene are likely to be converted into skin pigments by guppies (J. Hudon, personal communication). The basal (trace-carotenoid) diet contained white fish meal (46.0%), wheat flour (45.4%), fish gelatine (1.5%), sucrose (2.1%), cornstarch (1.9%), vegetable oil (2.0%) and a vitamin premix (1.0%) containing vitamin A palminate but no carotenoids. The low- and high-carotenoid diets also contained synthetic lutein and β-carotene obtained from Roche Vitamins (Parsippany, NJ). The basal recipe was adjusted to ensure that each of the three diets contained equal proportions of the non-carotenoid constituents of the Roche products (fish gelatine, sucrose and cornstarch). The diets were manufactured by Ocean Star International, Inc. (Snowville, Utah) in multiple batches and stored before use at −80°C. Based on high performance liquid chromatography (HPLC) analyses of diet samples, the carotenoid content of the trace-carotenoid diet was negligible (≤0.2 μg g−1), the low-carotenoid diet contained 2.85 μg g−1 lutein, 0.21 μg g−1 zeaxanthin, 1.99 μg g−1 β-carotene (5.05 μg g−1 total carotenoids), and the high-carotenoid diet contained 745.57 μg g−1 lutein, 71.43 μg g−1 zeaxanthin, 522.30 μg g−1 β-carotene (1339.30 μg g−1 total carotenoids; D. F. Millie, personal communication). The estimated protein (40%) and fat content (10%) of these diet are similar to high-quality commercial feeds for tropical fish.
The sexes were separated well before sexual maturity (Houde 1997). Males were housed in 8 l tanks at densities of 1–4 males per tank, and females were housed in 38 l tanks at densities of ≤20 fish per tank. To give males courtship experience, we housed one mature stock female with each male group for at least 7 days prior to the mate choice tests. Females remained virgins until they were used in the mate choice tests.
(b) Mate choice tests
Following Grether (2000), we measured the strength of the mate preference for carotenoid colouration from the responses of females to the courtship displays of males raised on three different carotenoid levels. Carotenoid consumption increases the carotenoid content and thus the chroma of the orange spots (Kodric-Brown 1989; Grether 2000). We used an open-aquarium design (Houde 1997) in which groups of three males and three females were allowed to interact freely in an 180 l observation tank. Males within a group were from the same population but from different carotenoid diet treatments; females within a group were from the same population and diet treatment. Each male group was tested twice, on consecutive days, with females from a single diet treatment: once with females from their own population and once with females from the other population in the same drainage. Each male was observed for a minimum of six 5 min focal samples (15 min per male per female group) during which all sigmoid displays and female responses were recorded (see Grether (2000) for further details). Male attractiveness was measured as the proportion of a male's courtship (sigmoid) displays that elicited a sexual (glide) response from a female during focal-male observations (Grether 2000). This measure of male attractiveness has been used by other authors (Houde 1988; Houde & Endler 1990; Houde 1994; Endler & Houde 1995) and has been shown to correlate with mating success as determined from paternity analyses (Houde 1988). To maintain a balanced design, we excluded from analysis all male groups in which a male was not observed courting females of both groups. In total, 285 males (66–75 per population) and twice as many females contributed to the final data set. The fish were fed to satiation (with trace-carotenoid flakes) approximately 15 min before the mate choice observations were begun, to prevent possible treatment group differences in hunger levels. All other details of the experimental set-up and behaviour observation protocol follow Grether (2000).
Data were analysed using ANOVA with female population, female food level, and male and female carotenoid diets as fixed factors and male group as a random factor nested within female carotenoid diet and female food level (male group refers to the trios of males who were tested together with the same two groups of females; see above). The influence of the independent variables on male attractiveness was measured from the model's main effects. Population and treatment group differences in preference strength were measured from the interactions between male carotenoid diet and the other factors. The four-way interaction and the interaction between male group and male carotenoid diet were removed from the model after being tested for significance (p>0.6).
Preference strength was measured as the slope of male attractiveness residuals on the log carotenoid concentration of the male diet, following Grether (2000). Attractiveness residuals were obtained from single drainage ANOVA models with the following terms: male population, female population, male population×female population, male group nested within male population and female population×male group. This procedure removed variation in male attractiveness associated with male group and population, thereby making the preference slopes comparable across populations.
To generate figure 2, preference slopes for the six populations previously studied (Grether 2000; Rodd et al. 2002) were recalculated using improved estimates of the total carotenoid content (μg g−1) of the diets used in that study (trace-carotenoid diet, 0.2; low-carotenoid diet, 32.5; high-carotenoid diet, 861.2; D. F. Millie, personal communication). The revised preference slopes were shallower than the original slopes (mean difference, 0.013) because the improved HPLC estimates for the low- and high-carotenoid diets were higher than the original estimates. Nevertheless, the correlation between the revised and original preference slopes was quite high (r=0.9999, n=6 populations), and neither the original results (Rodd et al. 2002) nor the results presented here were sensitive to whether the revised or original preference slopes were used in the analysis.
Figure 2.
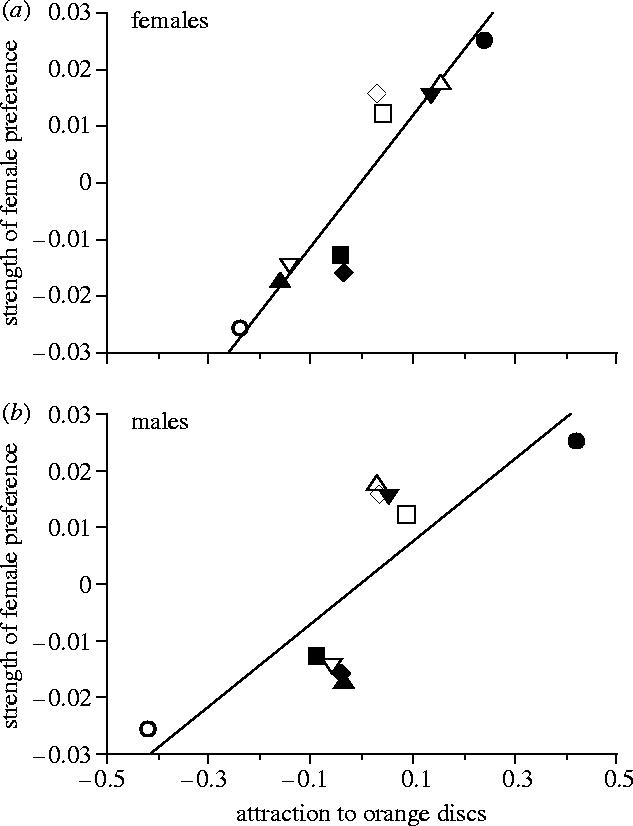
Relationship between the strength of the female mate preference for carotenoid coloration and the orange-attraction sensory bias in both sexes (n=10 populations). (a) Females (r2=0.86, p<0.0001). (b) Males (r2=0.63, p=0.004). Attraction to orange was measured as the rate at which guppies pecked orange discs (see § 2). The plotted values are residuals from river drainage means, to control for genetic differentiation between drainages. River drainage is indicated by the shape of the symbol (circle, Paria; square, Marianne; upward pointing triangle, Quare; downward pointing triangle, Crayfish; diamond, Madamas). Sites with relatively open (closed) forest canopies in their respective drainages are indicated by unfilled (filled) symbols.
(c) Coloured disc attraction tests
The colour attraction tests followed the protocol described in Rodd et al. (2002), except that a larger test aquarium was used (92×40×40 cm3). Fish were tested between the regular morning and afternoon feeding times, and thus should not have been unusually hungry during the tests. As described in Rodd et al. (2002), a 12 cm wide×6 cm high cylindrical zone was visually established around each of the eight 1.3 cm diameter coloured discs at the bottom of the test aquarium. Fish were tested individually and their responses to the discs (entering zones and pecking) was recorded in 7-min focal samples. Fish that did not enter any zones during the 7-min tests were excluded from the analysis. In total, 127 virgin females (27–36 per population) and 128 males (22–37 per population) contributed to the final data set. Data were analysed with an ANOVA with population, sex, food level and carotenoid diet as fixed factors. For reflectance spectra of the coloured discs and other details, see Rodd et al. (2002).
(d) Female body size and condition
The mass (±0.1 mg) and standard length (0.01 mm readout) of each female was measured after the mate choice tests. An allometric condition index, Ka=(weight)(length)−b, was constructed using females on the low-food, trace-carotenoid diet as the reference group (Bolger & Connolly 1989). Exponent b was estimated from the slope of least squares regressions of log(weight) on log(length) for the reference group (b±s.e.: 3.12±0.03). The results were essentially the same when the standard isometric condition index, K=(weight)(length)−3, was used instead of Ka. The influence of the experimental diets on body size (log of standard length) and condition (Ka) was assessed in ANOVAs with food level and carotenoid level as factors. Three bivariate outliers (most likely measurement errors) were excluded from the analysis (N=687).
3. Results
Females raised from birth on the trace-carotenoid diets showed a stronger preference for high-carotenoid males, compared with females raised on the high-carotenoid diets (figure 1). Statistically, this result was confirmed by the interaction between male carotenoid diet and female carotenoid diet (table 1). While the mean strength of the female preference for carotenoid colouration varied significantly among populations (interaction between female population and male carotenoid diet; table 1), the sensitivity of the preference to female carotenoid intake did not (interaction between female population, male carotenoid diet and female carotenoid diet; F6,464=0.36, p=0.90). Female food intake had a significant negative effect on the sensitivity of the female preference to carotenoid intake (interaction between male carotenoid diet, female carotenoid diet and female food level; table 1), to the extent that the effect of female carotenoid diet on the strength of the preference was only apparent in low-food females (figure 1). However, food level, per se, had no overall effect on the strength of the female preference (interaction between female food level and male carotenoid diet; F2,464=0.40, p=0.67).
Figure 1.
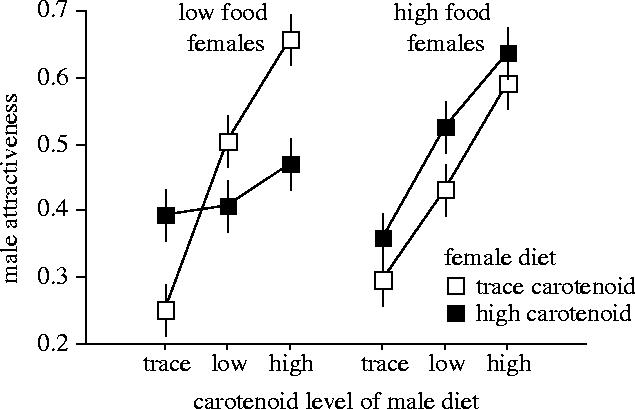
Effects of diet on the strength of the female mate preference for carotenoid coloration. Points represent least squares means (+s.e.m.) from the ANOVA in table 1.
Table 1.
Sources of variation in mate attractiveness in the mate choice tests. (Male attractiveness was measured from the responses of females to male courtship displays. Nonsignificant two- and three-way interactions were included in the model but are not shown here.)
| source | F | p | d.f.a |
|---|---|---|---|
| female population | 2.62 | 0.05 | 3, 464 |
| male carotenoid diet | 46.46 | <0.0001 | 2, 464 |
| female carotenoid diet | 0.07 | 0.80 | 1, 68.2 |
| female food level | 0.48 | 0.49 | 1, 68.2 |
| female pop.×male carot. diet | 3.05 | 0.006 | 6, 464 |
| male carot. diet×female carot. diet | 5.03 | 0.007 | 2, 464 |
| female pop.×male carot.×female food | 4.14 | 0.0005 | 6, 464 |
| female pop.×female carot.×female food | 4.18 | 0.006 | 3, 464 |
| male carot.×female carot.×female food | 4.94 | 0.008 | 2, 464 |
| male group (female carot., female food) | 2.57 | <0.0001 | 64, 464 |
Satterthwaitte method.
Females raised on the high food level were larger (standard length, F1,683=698.36, p<0.0001) and in better condition (Ka, F1,683=68.94, p<0.0001) than their low-food counterparts. In contrast, the level of carotenoids in the diet did not affect female body size (F1,683=2.76, p=0.10) or condition (F1,683=2.40, p=0.12).
The basic results of the colour attraction tests replicate and strengthen the findings of Rodd et al. (2002). Both sexes were more strongly attracted to orange discs than to discs of other colours, as measured by number of pecks (Wilcoxon matched pairs tests; orange versus all other colours individually; females: all p≤0.001; n=127; males: all p≤0.015; n=128). This held even for the subset of females raised on the trace-carotenoid diet (p≤0.004; n=49), which demonstrates that attraction to orange objects is indeed an innate sensory bias. In an earlier study, fish used in the colour attraction tests had been exposed to multicoloured flake food and orange Artemia nauplii, as well as to mature male guppies, which left open the possibility that attraction to orange was a learned response. In the present study, females raised on the trace-carotenoid diet had no opportunity to be rewarded for approaching orange objects (the trace-carotenoid diet is pale brown in colour). The four guppy populations tested in this study also followed the same geographic pattern as the six previously tested populations (Rodd et al. 2002) with respect to the relationship between preference strength and orange attraction (figure 2).
In parallel to the mate preference results, females raised on the trace-carotenoid diets showed stronger attraction (as measured by the number of pecks) to orange discs, compared with females raised on the high-carotenoid diets (figure 3). In males, however, carotenoid intake had no significant effect on the sensory bias (figure 3). There was a significant effect of carotenoid diet (F1,226=4.31, p=0.04) and a significant sex-by-carotenoid diet interaction (F1,226=4.55, p=0.03). The difference between the trace- and high-carotenoid diet groups was significant for females (planned contrast t=2.96, p=0.003) but not for males (t=−0.06, p=0.9). Females were more strongly attracted to the orange discs than males were within the trace-carotenoid diet group (t=2.58, p=0.01) but not within the high-carotenoid diet group (t=−0.39, p=0.7). Neither sex nor food level had significant main effects (sex F1,226=2.44, p=0.11; food F1,226=0.92, p=0.34), nor were the interactions involving food level significant (p≥0.3). Population had a marginally significant main effect (F3,226=2.63, p=0.05) and did not interact significantly with the other factors (p≥0.2). No three-way interactions were significant (p≥0.3); the four-way interaction was removed from the model after being tested for significance (p=0.86).
Figure 3.
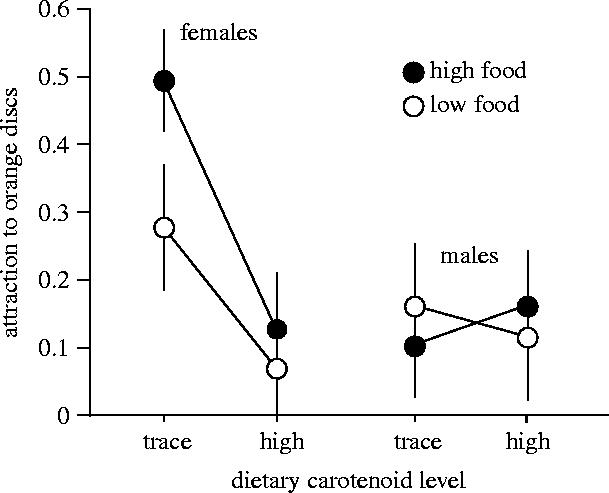
Effects of diet on the rate at which guppies pecked orange discs. Points represent least squares means (+s.e.m.) from the ANOVA described in § 2(c).
Male guppies spend a smaller fraction of their time foraging than females do (Magurran & Seghers 1994), and thus a sex difference in attraction to coloured objects might reflect a general sex difference in foraging motivation. Overall, however, the sexes did not differ significantly in the rate at which they pecked orange discs (p=0.11) or coloured discs in general (Mann–Whitney test, z=−1.29, p=0.20, nmales=128, nfemales=127). Thus, males and females differ specifically in regard to their sensitivity to carotenoids.
4. Discussion
We found that the strength of the female mate preference for carotenoid colouration in guppies is influenced by carotenoid availability in the direction predicted by sexual selection theory. As carotenoid intake declines, the responsiveness of females to the carotenoid content of the orange spots on potential mates increases. In the wild, the sensitivity of females to carotenoid intake may enable them to choose higher quality mates, because carotenoid availability influences the value of the orange spots as indicators of male mate quality. We are aware of only one other example of carotenoid ingestion altering an animal's behaviour. Parsnip webworms (Depressaria pastinacella) show reduced avoidance of ultraviolet light when carotenoids are present in their diet, a response that may enable them to forage diurnally because carotenoids offer protection against photooxidative stress (Carroll et al. 1997).
The modulating influence of food availability on the mate preference strength–carotenoid intake reaction norm (figure 1) can be explained in terms of the costs of mate choice. Under conditions of chronic food limitation in nature, choosy females could suffer a net reduction in fitness unless the indicator value of the preferred male trait is relatively high because, for example, evaluating males takes time and energy (Jennions & Petrie 1997; Widemo & Saether 1999). Although food availability is closely linked to carotenoid availability at the population level (Grether et al. 1999, 2001b), females vary in condition within populations and carotenoid availability can vary substantially among pools within low-carotenoid-availability streams (e.g. because fallen trees create temporary light gaps). Thus, females with a history of low food intake, or who are in poor condition for other reasons, may find themselves in a high-carotenoid-availability environment regardless of the environment experienced by their parents. This may be why the mate preference shows a plastic response to carotenoid intake, as opposed to genetically matching the average level of carotenoid availability in each stream. In any case, the plasticity of the mate preference explains why it has not diverged genetically between populations in response to geographic variation in carotenoid availability (Grether 2000). This is an example of how phenotypic plasticity can preclude or eliminate divergent selection on mate preferences.
Adaptive plasticity in mate preferences has been reported previously (Milinski & Bakker 1993; Rosenqvist & Houde 1997; Bakker et al. 1999; Lesna & Sabelis 1999; Lopez 1999; Qvarnstrom et al. 2000), but we are aware of no other examples in which a mate preference has been shown to covary with the indicator value of the trait it favours. Our results provide a new type of support for the hypothesis that mate preferences for secondary sexual characters evolve in response to the benefits of mate choice—the alternatives being that such preferences evolve entirely in a non-mating context or in response to the costs of mating (Schluter & Price 1993; Iwasa & Pomiankowski 1999; Kokko et al. 2003; Mead & Arnold 2004). Our results also present a new modelling opportunity for sexual selection theorists because no existing model allows mate preferences to vary phenotypically with the indicator value of the traits they favour.
Paradoxically, the intensity of sexual selection on carotenoid colouration in guppies may not be affected by carotenoid availability at the population level, because most females in the wild are likely to experience conditions conducive to expressing a strong preference for carotenoid colouration (i.e. high food availability or low carotenoid availability). However, if sexual selection on other male traits (e.g. size, courtship vigour) is more intense in high-food-availability streams (e.g. because the overall cost of choosiness is lower when females are in better condition; Jennions & Petrie 1997), then the relative intensity of selection on carotenoid colouration may indeed be higher in low-carotenoid streams.
The responsiveness of the sensory bias to the level of carotenoids in the diet in females, and the lack thereof in males, suggests that carotenoid-dependence of the sensory bias evolved as a correlated effect of selection acting directly on the mate preference, not vice versa. If female guppies benefit from enhanced attraction to orange fruit when other sources of carotenoids are scarce, then males should too. Males benefit in at least two ways from consuming carotenoids. First, carotenoids increase a male's attractiveness to females (Kodric-Brown 1989; Grether 2000). Second, carotenoid consumption has a positive effect on the ability of males to reject foreign tissue (a measure of immune system strength). The latter effect was not found in females, perhaps because females do not face a trade-off between allocating carotenoids to immune function versus sexual attractiveness (Lozano 1994; Møller et al. 2000; Grether et al. 2004). Although female guppies transfer carotenoids to their offspring via the egg yolk (G. F. Grether et al. unpublished data), we have no evidence that females are carotenoid-limited in the wild (Grether et al. 1999). Thus, while attraction to orange objects may originally have evolved in response to the nutritional benefits of consuming carotenoid-rich orange fruits (Rodd et al. 2002), it is difficult to explain the carotenoid-dependence of the sensory bias as a foraging adaptation.
The simplest explanation for our results is that the mate preference for carotenoid colouration was modified by the indicator process and that the sensory bias changed, in females, as a correlated response. Further research is needed, however, to distinguish between the alternative hypotheses. We are currently testing the colour attraction responses of other species of poeciliid fishes. If species basal to guppies in the phylogeny show attraction to orange objects but lack orange colouration, this would imply that the sensory bias predates the evolution of orange colouration (see Basolo 1990; Ryan 1990; Proctor 1992). The evolutionary order of events could be further resolved by examining the effects of carotenoid intake on orange attraction in other species. Only species with orange colouration would be expected to show a plastic response to carotenoid intake, if this reaction norm evolved in a mating context.
Acknowledgments
We thank R. Brooks, R. Calsbeek, J. A. Endler, M. den Hollander, A. E. Houde, T. Pitcher, D. Punzalan, L. Rowe, D. M. Shier and three anonymous referees for discussion and comments and many dedicated University of California undergraduates for assistance with the laboratory experiments. Ocean Star International, Inc. produced and donated the experimental diets, Roche Vitamins, Inc. donated carotenoids, and D. F. Millie analysed the carotenoid content of the diets. In Trinidad, we thank the Sinanan family for accommodation, the University of West Indies for sponsorship and the government of Trinidad for permits to collect guppies and to work in the Quare watershed. This research was supported by National Science Foundation grants IBN-0001309 to GFG and IBN-0130893 to GFG and GRK.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Bakker T.C.M, Kunzler R, Mazzi D. Sexual selection: condition-related mate choice in sticklebacks. Nature. 1999;401:234. 10.1038/45727 [Google Scholar]
- Basolo A.L. Female preference predates the evolution of the sword in swordtail fish. Science. 1990;250:808–810. doi: 10.1126/science.250.4982.808. [DOI] [PubMed] [Google Scholar]
- Basolo A.L. Evolutionary change in a receiver bias: a comparison of female preference functions. Proc. R. Soc. B. 1998;265:2223–2228. doi: 10.1098/rspb.1998.0563. 10.1098/rspb.1998.0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount J.D, Metcalfe N.B, Birkhead T.R, Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. 10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- Bolger T, Connolly P.L. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 1989;34:171–182. [Google Scholar]
- Brooks R. Negative genetic correlation between male sexual attractiveness and survival. Nature. 2000;406:67–70. doi: 10.1038/35017552. 10.1038/35017552 [DOI] [PubMed] [Google Scholar]
- Carroll M, Hanlon A, Hanlon T, Zangerl A.R, Berenbaum M.R. Behavioral effects of carotenoid sequestration by the parsnip webworm Depressaria pastinacella. J. Chem. Ecol. 1997;23:2707–2719. [Google Scholar]
- Darwin C. The descent of man, and selection in relation to sex. J. Murray; London: 1871. [Google Scholar]
- Endler J.A. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Endler J.A. Signals, signal conditions, and the direction of evolution. Am. Nat. 1992;139:1–27. 10.1086/285308 [Google Scholar]
- Endler J.A, Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. 10.1016/S0169-5347(98)01471-2 [DOI] [PubMed] [Google Scholar]
- Endler J.A, Houde A.E. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49:456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Evans J.P, Kelley J.L, Bisazza A, Finazzo E, Pilastro A. Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. B. 2004;271:2035–2042. doi: 10.1098/rspb.2004.2815. 10.1098/rspb.2004.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre B, Gregoire A, Preault M, Cezilly F, Sorci G. Immune activation rapidly mirrored in a secondary sexual trait. Science. 2003;300:103. doi: 10.1126/science.1081802. 10.1126/science.1081802 [DOI] [PubMed] [Google Scholar]
- Goodwin T.W. Chapman & Hall; London: 1984. The biochemistry of the carotenoids. [Google Scholar]
- Grether G.F. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata) Evolution. 2000;54:1712–1724. doi: 10.1111/j.0014-3820.2000.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Grether G.F, Hudon J, Millie D.F. Carotenoid limitation of sexual coloration along an environmental gradient in guppies. Proc. R. Soc. B. 1999;266:1317–1322. 10.1098/rspb.1999.0781 [Google Scholar]
- Grether G.F, Hudon J, Endler J.A. Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies (Poecilia reticulata) Proc. R. Soc. B. 2001a;268:1245–1253. doi: 10.1098/rspb.2001.1624. 10.1098/rspb.2001.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether G.F, Millie D.F, Bryant M.J, Reznick D.N, Mayea W. Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology. 2001b;82:1546–1559. [Google Scholar]
- Grether G.F, Kasahara S, Kolluru G.R, Cooper E.L. Sex-specific effects of carotenoid intake on the immunological response to allografts in guppies (Poecilia reticulata) Proc. R. Soc. B. 2004;271:45–49. doi: 10.1098/rspb.2003.2526. 10.1098/rspb.2003.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G.E. Plumage coloration is a sexually selected indicator of male quality. Nature. 1991;350:337–339. 10.1038/350337a0 [Google Scholar]
- Holland B, Rice W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. 10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde A.E. The effects of female choice and male–male competition on the mating success of male guppies. Anim. Behav. 1988;36:888–896. [Google Scholar]
- Houde A.E. Effect of artificial selection on male colour patterns on mating preference of female guppies. Proc. R. Soc. B. 1994;256:125–130. [Google Scholar]
- Houde A.E. Princeton University Press; Princeton, NJ: 1997. Sex, color, and mate choice in guppies. [Google Scholar]
- Houde A.E, Endler J.A. Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science. 1990;248:1405–1408. doi: 10.1126/science.248.4961.1405. [DOI] [PubMed] [Google Scholar]
- Houde A.E, Torio A.J. Effect of parasitic infection on male color pattern and female choice in guppies. Behav. Ecol. 1992;3:346–351. [Google Scholar]
- Hunt J, Bussiere L.F, Jennions M.D, Brooks R. What is genetic quality? Trends Ecol. Evol. 2004;19:329–333. doi: 10.1016/j.tree.2004.03.035. 10.1016/j.tree.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A. Good parent and good genes models of handicap evolution. J. Theor. Biol. 1999;200:97–109. doi: 10.1006/jtbi.1999.0979. 10.1006/jtbi.1999.0979 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Philos. Soc. 1997;72:283–327. doi: 10.1017/s0006323196005014. 10.1017/S0006323196005014 [DOI] [PubMed] [Google Scholar]
- Kennedy C.E.J, Endler J.A, Poynton S.L, McMinn H. Parasite load predicts mate choice in guppies. Behav. Ecol. Sociobiol. 1987;21:291–296. 10.1007/BF00299966 [Google Scholar]
- Kirkpatrick M. Sexual selection by female choice in polygynous animals. Ann. Rev. Ecol. Syst. 1987;18:43–70. 10.1146/annurev.es.18.110187.000355 [Google Scholar]
- Kodric-Brown A. Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav. Ecol. Sociobiol. 1989;25:393–401. 10.1007/BF00300185 [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. 10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesna I, Sabelis M.W. Diet-dependent female choice for males with ‘good genes’ in a soil predatory mite. Nature. 1999;401:581–584. 10.1038/44125 [Google Scholar]
- Lopez S. Acquired resistance affects male sexual display and female choice in guppies. Proc. R. Soc. B. 1998;265:717–723. 10.1098/rspb.1998.0352 [Google Scholar]
- Lopez S. Parasitized female guppies do not prefer showy males. Anim. Behav. 1999;57:1129–1134. doi: 10.1006/anbe.1998.1064. 10.1006/anbe.1998.1064 [DOI] [PubMed] [Google Scholar]
- Lozano G.A. Carotenoids, parasites, and sexual selection. Oikos. 1994;70:309–311. [Google Scholar]
- Magurran A.E, Seghers B.H. Sexual conflict as a consequence of ecology: evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc. R. Soc. B. 1994;255:31–36. [Google Scholar]
- McGraw K.J, Ardia D.R. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 2003;162:704–712. doi: 10.1086/378904. 10.1086/378904 [DOI] [PubMed] [Google Scholar]
- Mead L.S, Arnold S.J. Quantitative genetic models of sexual selection. Trends Ecol. Evol. 2004;19:264–271. doi: 10.1016/j.tree.2004.03.003. 10.1016/j.tree.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Milinski M, Bakker T.C.M. Costs influence sequential mate choice in sticklebacks, Gasterosteus aculeatus. Proc. R. Soc. B. 1993;250:229–233. [Google Scholar]
- Møller A.P, Biard C, Blount J.D, Houston D.C, Ninni P, Saino N, Surai P.F. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult. Biol. Rev. 2000;11:137–159. [Google Scholar]
- Olson V.A, Owens I.P.F. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. 10.1016/S0169-5347(98)01484-0 [DOI] [PubMed] [Google Scholar]
- Payne R.J.H, Pagel M. Inferring the origins of state-dependent courtship traits. Am. Nat. 2001;157:42–50. doi: 10.1086/317007. 10.1086/317007 [DOI] [PubMed] [Google Scholar]
- Proctor H.C. Sensory exploitation and the evolution of male mating behaviour—a cladistic test using water mites (Acari, Parasitengona) Anim. Behav. 1992;44:745–752. [Google Scholar]
- Qvarnstrom A, Part T, Sheldon B.C. Adaptive plasticity in mate preference linked to differences in reproductive effort. Nature. 2000;405:344–347. doi: 10.1038/35012605. [DOI] [PubMed] [Google Scholar]
- Rodd F.H, Hughes K.A, Grether G.F, Baril C.T. A possible non-sexual origin of a mate preference: are male guppies mimicking fruit? Proc. R. Soc. B. 2002;269:475–481. doi: 10.1098/rspb.2001.1891. 10.1098/rspb.2001.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.L, Snedden W.A. On the functional design of mate preferences and receiver biases. Anim. Behav. 2004;68:427–432. 10.1016/j.anbehav.2003.08.031 [Google Scholar]
- Rosenqvist G, Houde A. Prior exposure to male phenotypes influences mate choice in the guppy, Poecilia reticulata. Behav. Ecol. 1997;8:194–198. [Google Scholar]
- Ryan M.J. Sexual selection, sensory systems, and sensory exploitation. In: Futuyma D, Antonovics J, editors. Oxford surveys in evolutionary biology. vol. 7. Oxford University Press; Oxford, UK: 1990. pp. 157–195. [Google Scholar]
- Ryan M.J, Fox J.H, Wilczynski W, Rand A.S. Sexual selection for sensory exploitation in the frog Physalaemus pustlosus. Nature. 1990;343:66–67. doi: 10.1038/343066a0. 10.1038/343066a0 [DOI] [PubMed] [Google Scholar]
- Schaefer H.M, Schaefer V, Levey D.J. How plant–animal interactions signal new insights in communication. Trends Ecol. Evol. 2004;19:577–584. 10.1016/j.tree.2004.08.003 [Google Scholar]
- Schluter D, Price T. Honesty, perception and population divergence in sexually selection traits. Proc. R. Soc. B. 1993;253:117–122. doi: 10.1098/rspb.1993.0089. [DOI] [PubMed] [Google Scholar]
- Smith C, Barber I, Wootton R.J, Chittka L. A receiver bias in the origin of three-spined stickleback mate choice. Proc. R. Soc. B. 2004;271:949–955. doi: 10.1098/rspb.2004.2690. 10.1098/rspb.2004.2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard M.J. Sexual selection, competitive communication and species-specific signals in insects. In: Lewis T, editor. Insect communication. Academic; New York: 1984. [Google Scholar]
- Widemo F, Saether S.A. Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol. Evol. 1999;14:26–31. doi: 10.1016/s0169-5347(98)01531-6. 10.1016/S0169-5347(98)01531-6 [DOI] [PubMed] [Google Scholar]


