Abstract
The diurnal hummingbird hawkmoth Macroglossum stellatarum can learn the achromatic (intensity-related) and the chromatic (wavelength-related) aspect of a spectral colour. Free-flying moths learn to discriminate two colours differing in the chromatic aspect of colour fast and with high precision. In contrast, they learn the discrimination of two stimuli differing in the achromatic aspect more slowly and less reliably. When trained to use the chromatic aspect, they disregard the achromatic aspect, and when trained to use the achromatic aspect, they disregard the chromatic aspect, at least to some degree. In a conflicting situation, hummingbird hawkmoths clearly rely on the chromatic aspect of colour. Generally, the moths pay attention to the most reliable cue that allows them to discriminate colours in the learning situation. This is usually the chromatic aspect of the colour but they can learn to attend to the achromatic aspect instead. There is no evidence for relative colour learning, i.e. moths do not learn to choose the longer or shorter of two wavelengths, but it is possible that they learn to choose the darker or brighter shade of a colour, and thereby its relative intensities.
Keywords: sphingids, hawkmoths, Macroglossum stellatarum, chromatic vision, achromatic vision, colour vision
1. Introduction
Visual objects differ in the spectral composition and intensity of the light they reflect. Intensity of light corresponds to the achromatic aspect of colour, while the spectral composition corresponds to the chromatic aspect of colour. The intensity largely depends on and changes with changing illumination whereas the chromatic aspect of colour differs to a lesser degree with the illumination. In bright light, chromatic vision allows the discrimination of a larger number of colours than achromatic vision does (Vorobyev 1997). Many animals including honeybees can use the chromatic and the achromatic aspect of colour to detect and discriminate objects (Lehrer & Bishof 1995; Giurfa et al. 1997) but they learn the chromatic aspect much easier (Frisch 1914). When forced to discriminate stimuli at specific distances, bees use achromatic cues at a large distance (thus with high spatial resolution) and chromatic cues at close distance (and with low resolution; Giurfa et al. 1997). It is largely unknown, however, how honeybees or other insects use both types of information during the approach to a flower, in a natural feeding situation.
At night, when little light is available, chromatic vision becomes less reliable as a result of photon shot noise, and achromatic vision might allow animals to discriminate a larger number of stimuli than chromatic vision does (Vorobyev 1997). We can expect nocturnal animals to rely more on achromatic vision then diurnal animals do. The hummingbird hawkmoth (Macroglossum stellatarum) is a diurnal sphingid but its ancestors were nocturnal or crepuscular moths. Hummingbird hawkmoths have trichromatic colour vision very similar to bees, with receptors sensitive to ultraviolet, blue and green light (Kelber et al. 2003a). It is known that they can discriminate lights of different wavelengths (Kelber 1996; Kelber & Hénique 1999). They choose the training wavelength even when it is brighter or darker by a factor of 10, thus disregarding the achromatic aspect of colour.
Here I ask whether they can also learn to use the achromatic aspect of a colour and which of both aspects they give higher weight in a conflicting situation. I also studied whether hawkmoths learn intensities and wavelengths in an absolute or relative way. Here, relative colour learning implies that an animal learns to choose the shorter or longer of two wavelengths. For instance, wallabies (Macropus eugenii) trained to associate 450 nm with food and 500 nm with no food were later tested with 500 and 550 nm and chose 500 nm which was the shorter of the two wavelengths (Hemmi 1999). Relative colour learning has not yet been shown in any animal other than the wallaby. An animal learns the relative intensity if it learns to choose the dimmer or brighter of two lights, independent of the absolute intensities.
2. Material and methods
Macroglossum stellatarum are bred continuously in the lab. The larvae were fed the native food plant (Galium mollugo L.), and newly eclosed moths were used for the experiment. Training took place in a flight cage, 50×70 cm2 in area, 50 cm high and illuminated from above by four Osram Biolux tubes (resulting in a light intensity of 100 cd m−2). The naive moths were trained to feed from one of two adjacent feeders, 6 cm apart, on a black rectangular disc (20 cm wide and 10 cm high), that was presented vertically in the cage. Each feeder consisted of a plexiglass disc, 20 mm in diameter, with a ring-shaped food reservoir that could be filled from the front. Each feeder disc also served as stimulus. It was illuminated from behind using a Schott ILC 2500 lamp with an 8 mm wide light guide. The end of the light guide was connected to the feeder disc by means of a filter box that held an interference filter, a diffuser and a lens that spread the light evenly over the feeder disc. Light intensities were measured using a radiometer (IL1700) and adjusted in such a way that both stimuli either emitted equal numbers of photons (1016 photons s−1 m−2 sr−1) or differed by a factor of 10. Both lights were brighter than the black background reflecting the cage light but dimmer than a white background would have been. Animals were released individually into the cage where they spontaneously found the food source and fed. Each animal was trained during one feeding session daily, to associate the positive stimulus with a reward of 20% sucrose solution and the negative stimulus with no reward. During one such feeding session, a moth made on average 10 choices.
In three different experiments, three different groups of moths were trained to discriminate two stimuli that differed either in wavelength, in intensity or in both. The training wavelengths of 440 and 470 nm both belong to the range of innately preferred colours, with 440 nm being preferred to 470 nm by naive moths (Kelber 1997). After two days of training, a test was performed prior to each training session. During tests, no sucrose solution was presented, and each time the moth approached and probed a feeder with the proboscis was counted as a choice. As hawkmoths feed on the wing, this is a very clear choice criterion. Ten choices by each animal were registered in each test, this procedure usually lasted between 5 and 10 min. The positions of the rewarded and unrewarded stimulus were swapped in a pseudo-random order, both during training and tests.
3. Results
(a) The chromatic aspect of colour is learned fast and reliably
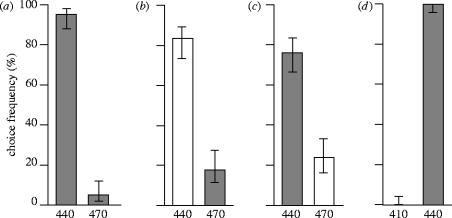
The first group of moths was trained to discriminate between two stimuli that had the same physical intensity but differed by wavelength. In the control test after two days of training, the moths chose the positive stimulus (440 nm, 1016 photons s−1 m−2 sr−1; figure 1a) more frequently than the negative stimulus (470 nm, same intensity as the positive stimulus). They continued to choose the positive wavelength in subsequent tests, where either of the wavelengths was presented with a 10 times higher intensity (figure 1b,c, white bars). The last test was designed to determine whether the moths learned wavelength in an absolute or relative way. When the moths were given the choice between two lights of 410 and 440 nm, they chose the correct training wavelength, 440 nm (figure 1d). Thus, they had learned the absolute wavelength and did not choose the shorter of the two wavelengths.
Figure 1.
Choice frequencies (with 5% confidence intervals) of M. stellatarum trained to discriminate a 440 nm light from a 470 nm light, both with the same intensity (grey bars). Test wavelengths and intensities are given under the abscissa. (a) Control test, 120 choices by 12 moths, G-test, p<0.0001. (b) The light of the rewarded wavelength (white bars) was brighter by a factor 10, 120 choices by 12 animals, G-test, p<0.0001. (c) The light of the unrewarded wavelength was brighter by a factor 10 (white bars), 110 choices by 11 animals, G-test, p<0.0001. (d) Test for relative wavelength learning, 110 choices by 11 animals, G-test, p<0.0001.
(b) The achromatic aspect of colour is learned more slowly
The second group of moths was trained to choose the dimmer of two 440 nm lights that differed in intensity by a factor of 10. Eighteen out of 23 naive animals (78%, binomial test, p<0.05) showed a preference for the stimulus with higher intensity, prior to training. After two days of training, the moths had not yet learned to choose the darker shade of blue (52% correct choices, 140 choices by 14 animals, G-test, n.s.). After six days of training, they chose the low-intensity light (figure 2a, grey bar) more frequently than the high-intensity light (figure 2a, white bar). This was the case even in the test two days later, where they were given the choice between the training wavelength, at high intensity, and a 470 nm light, at low intensity (figure 2b). In this test, the achromatic aspect of colour was obviously more important for their choice than the chromatic aspect. In the same test, moths that were trained to the same positive stimulus but 470 nm as negative stimulus, chose differently (see previous paragraph and figure 1b).
Figure 2.
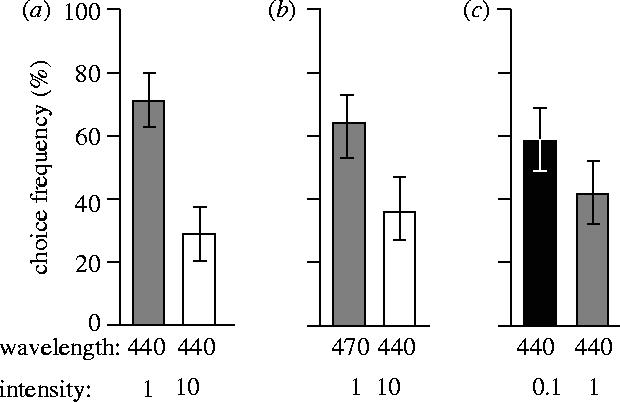
Choice frequencies (with 5% confidence intervals) of M. stellatarum trained to discriminate a dimmer (grey bars) from a brighter (white bars) light of 440 nm. (a) Control test after six days of training, 130 choices by 13 animals, G-test, p<0.001. (b) The dim light is chosen even if it has changed wavelength, 470 nm; 130 choices by 13 animals, G-test, p<0.005. (c) Test for relative intensity learning, black bars indicate choices for a light 10 times dimmer than the positive training intensity (grey bars); 110 choices by 11 animals, G-test, n.s.
When given the choice between a 440 nm light of the training intensity and another light of the same wavelength that was dimmer by a factor of 10, the result was not clear. Some animals chose the dimmer light more frequently, others chose the correct absolute intensity more frequently and still others chose randomly. Taken together, the choices in this test do not allow one to decide whether the moths had learned the absolute or relative intensity (figure 2c).
(c) Chromatic versus achromatic aspect of colour
A third group of moths was trained to associate a dim stimulus of 440 nm with food and a bright stimulus of 470 nm with the absence of food. They chose correctly in the control test with the training stimuli (figure 3a). When the correct wavelength was presented with the incorrect intensity, and vice versa, moths preferentially chose the correct colour and incorrect intensity (figure 3b). In this conflicting situation, they clearly relied on the chromatic aspect of colour and disregarded intensity.
Figure 3.
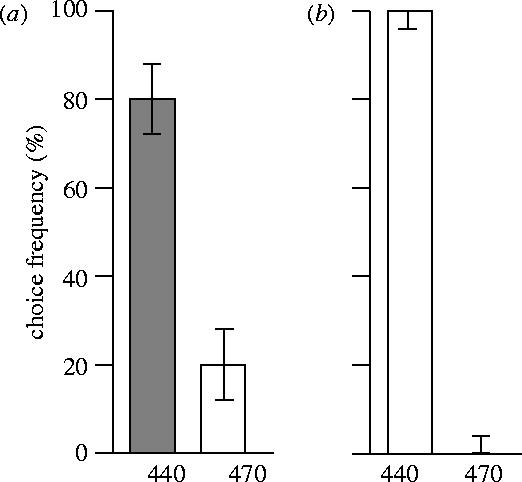
Choice frequencies (with 5% confidence intervals) of M. stellatarum trained to discriminate two stimuli differing in both intensity and wavelength. (a) Control test with the training stimuli; 130 choices by 13 animals, G-test, p<0.0001. (b) When the correct intensity was paired with the incorrect wavelength and vice versa, moths chose the correct wavelength; 130 choices by 13 animals, G-test, p<0.0001.
4. Discussion
(a) Moths can use the achromatic and chromatic aspect of colour: a matter of attention?
I conclude that hummingbird hawkmoths can learn to associate both, the chromatic and the achromatic aspect of colour, with a reward. After training to the same positive stimulus (a dim light of 440 nm), two groups of moth made different choices depending on the negative stimulus present during training (compare figures 1b and 2b): moths trained to choose between a dim light of 440 and 470 nm preferred a bright light of 440 nm (correct wavelength) to a dim light of 470 nm (correct intensity). Moths trained to discriminate the dim light of 440 nm from a bright light of the same wavelength preferred the dim light of 470 nm (correct intensity) to the bright light of 440 nm (correct wavelength). The result is so clear (100% choices) that it is unlikely to be only due to the fact that 440 nm is the more attractive wavelength.
These results strongly indicate that the learning situation determines the learning result. The moths learn to use the cue that contains relevant information. When two colours differ in wavelength only, they learn to use the chromatic aspect. The reason may be that they direct their attention only to this cue (Zentall & Riley 2000). This is consistent with results of earlier experiments using many different combinations of positive and negative training wavelengths (Kelber & Hénique 1999). In a situation where two stimuli differed in intensity only the moths learned to rely on the achromatic aspect.
Why did it take the moths much longer time to learn this discrimination than the wavelength discrimination? One possible explanation is that the moths usually direct their attention towards the chromatic aspect of colour and change this only after several days. Earlier experiments (Balkenius & Kelber 2004) found that colour had a strong influence on place learning. When trained to discriminate colours such as blue and yellow, moths did not pay any attention to feeder position but with similar colours, they paid attention to the position of the rewarded feeder. It is possible that moths pay more attention to achromatic cues if they are combined with wavelengths different from their innate preference.
This would easily explain the results of an earlier experiment (Kelber & Hénique 1999) where moths were trained to discriminate two long-wavelength stimuli (590 and 630 nm). Both stimuli had equal physical intensities—however, as a result of its spectral sensitivity, the moths' green receptor captures many more photons from the 590 nm stimulus than from the 630 nm stimulus. The moths learned to discriminate both colours in the training situation but when the 630 nm stimulus was presented with a higher intensity than the 590 nm stimulus, they were unable to choose the correct colour. This result clearly indicated that the moths do not possess a second long-wavelength receptor besides the green receptor. The discrimination must have been based on the achromatic difference between both stimuli. The moths continued to choose the stimulus that resulted in a higher photon catch, when physical intensities were changed. The same moths continued to choose the stimulus with the higher intensity, for several days, in a second discrimination task where they were able to use colour (630 versus 470 nm; Kelber & Hénique 1999). They had learned to direct their attention to the achromatic aspect of colour, and it took them several days to change and use the chromatic aspect of colour.
Attention has been suggested as an explanation for differences in discrimination performance in bees: honeybees and bumble-bees discriminate very similar colours only if they see both colours during training (Dyer & Chittka 2004a; Giurfa 2004). Possibly, paying attention to the small colour difference is essential for learning (Giurfa 2004). Increased attention may also explain the trade-off between speed and accuracy in bumble-bees trained in difficult colour discrimination tasks (Dyer & Chittka 2004b).
(b) Moths give more weight to the chromatic aspect of colour
The chromatic aspect of colour is learned faster and more reliably (95% correct choices after two days of training; figure 1) than the achromatic aspect (52% correct choices after two days and 70% correct choices after six days of training; figure 2). Similar studies have not been preformed with other Lepidoptera, but the results can be compared to those obtained from honeybees and bumble-bees. In a similar way to the hawkmoth, honeybees learn the achromatic aspect of colour so much more slowly that Frisch (1914) concluded they did not learn it at all. Later experiments proved that bees can learn both aspects of colour but that the achromatic and chromatic pathways have different spatial resolution (Giurfa et al. 1997). The achromatic pathway is based on the green receptors (Lehrer 1993) and the chromatic pathway involves two opponent interactions between all three receptor types (Brandt & Vorobyev 1997). In controlled y-maze experiments, bees learn the chromatic aspect of objects larger than 15° in visual angle, and the achromatic aspect of objects smaller than that (Giurfa et al. 1997). Similar to the moths, bees need many trials to reliably learn stimuli that do not offer chromatic contrast (Lehrer & Bishof 1995). With high contrasts, bees can even detect large targets by means of their achromatic contrast (Hempel de Ibarra et al. 2000).
Little is known about the attention honeybees give to chromatic and achromatic contrast in a situation where both cues are available. However, there are indications that the chromatic aspect of colour is given more attention by bees as well. In the natural situation where a bee flies unrestricted over a meadow, any flower is small from a distance. The achromatic contrast should therefore be seen (and possibly be used) first and the chromatic contrast should only become visible when the bee is closer to the flower. Spaethe et al. (2001) performed an experiment somewhat closer to the natural situation than the y-maze experiments: bumble-bees were trained to find artificial flowers on an artificial miniature meadow. When they were trained to find large flowers, their search times were correlated with the chromatic contrast of the flower to the background. Only when the flowers were very small (8 mm in diameter), search times correlated with their achromatic contrast to the background (Spaethe et al. 2001). Thus, just as the moths, bees may prefer to pay more attention to one or the other stimulus, depending on the learning situation, and they may prefer the chromatic signal when it is available. In the hummingbird hawkmoth, the chromatic channel is based on three receptor types in a similar way as in the honeybee (Kelber & Hénique 1999) but the neuronal basis of the achromatic channel and the spatial resolution of both channels are unknown.
Generally, achromatic contrast is considered a less reliable cue for object recognition than chromatic contrast (Kelber et al. 2003b). High achromatic contrasts can be caused by shadows just as well as by objects. Moreover, achromatic contrast is less reliable under changing light conditions. A blue flower that is darker than the background of green leaves, for an insect green receptor at noon, may easily become brighter during late twilight (Campenhausen 1986; own unpublished data). The diurnal hummingbird hawkmoth has evolved from crepuscular or nocturnal ancestors. It is therefore not surprising that it prefers to use the chromatic aspect of colour for flower detection and possesses good colour constancy (Balkenius & Kelber 2004). In nocturnal hawkmoths, only chromatic vision has been tested so far. The nocturnal elephant hawkmoth (Deilephila elpenor) learns flower colours independent of intensity (Kelber et al. 2002) and possesses colour constancy (Balkenius & Kelber 2004). However, most nocturnal hawkmoth-pollinated flowers are white (without reflecting ultraviolet light; e.g. Vogel 1954; White et al. 1994) and thus present a high and reliable achromatic contrast to the dark background of leaves. It seems likely that nocturnal moths use this cue to a larger degree than diurnal animals do.
(c) Relative versus absolute learning
As already indicated by earlier results (Balkenius & Kelber 2004), hawkmoths learn colour in an absolute way (figure 1d). The only animal that has been shown to learn wavelengths in a relative way is the wallaby—a marsupial with dichromatic colour vision (Hemmi 1999). In dichromats, a single interaction between two receptor types describes the chromatic aspect of colour and the colours are arranged from short to long wavelengths. It therefore seems reasonable that a dichromat learns relative positions of colours rather than absolute wavelengths. In trichromatic animals, colour vision is based on two independent chromatic interactions between three receptors (Kelber et al. 2003b) and relative wavelength is not an obvious concept for them. A possible explanation is that moths learned the contrast of the stimuli to the black background and were confused when that changed. The intensity difference used in the experiments was large—a factor of 10 resulting in a contrast of 82%—which may also have contributed to the result. More experiments are necessary to understand whether and when moths learn relative or absolute intensities.
Acknowledgments
I am grateful to Michael Pfaff for sharing his immense knowledge on the ‘flying teddy bears’, to Maja Tarka for skilled help with training them, to Anna Balkenius for discussions on the subject, and to the anonymous referees for constructive suggestions on the text. The generous financial support by the Swedish Research Council in Stockholm and the Crafoord Foundation in Lund is gratefully acknowledged.
References
- Balkenius A, Kelber A. Colour constancy in hawkmoths. J. Exp. Biol. 2004;207:3307–3316. doi: 10.1242/jeb.01158. 10.1242/jeb.01158 [DOI] [PubMed] [Google Scholar]
- Brandt R, Vorobyev M. Metric analysis of threshold spectral sensitivity in the honeybee. Vis. Res. 1997;37:425–437. doi: 10.1016/s0042-6989(96)00195-2. [DOI] [PubMed] [Google Scholar]
- Campenhausen C.v. Photoreceptors, lightness constancy and colour vision. Naturwissenschaften. 1986;73:674–675. doi: 10.1007/BF00366692. [DOI] [PubMed] [Google Scholar]
- Dyer A, Chittka L. Fine color discrimination requires differential conditioning in bumblebees. Naturwissenschaften. 2004a;91:224–227. doi: 10.1007/s00114-004-0508-x. 10.1007/s00359-003-0475-2 [DOI] [PubMed] [Google Scholar]
- Dyer A, Chittka L. Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. J. Comp. Physiol. A. 2004b;190:759–763. doi: 10.1007/s00359-004-0547-y. [DOI] [PubMed] [Google Scholar]
- Frisch K.v. Der Farbensinn und Formensinn der Biene. Zool. Jahrb. Abt. Allg. Zool. Physiol. Tiere. 1914;35:1–188. [Google Scholar]
- Giurfa M. Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften. 2004;91:228–231. doi: 10.1007/s00114-004-0530-z. 10.1007/s00114-004-0530-z [DOI] [PubMed] [Google Scholar]
- Giurfa M, Vorobyev M, Brandt R, Posner B, Menzel R. Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic stimuli. J. Comp. Physiol. A. 1997;180:235–243. 10.1007/s003590050044 [Google Scholar]
- Hemmi J. Dichromatic colour vision in an Australian marsupial, the tammar wallaby. J. Comp. Physiol. A. 1999;185:509–515. doi: 10.1007/s003590050411. 10.1007/s003590050411 [DOI] [PubMed] [Google Scholar]
- Hempel de Ibarra N, Vorobyev M, Brandt R, Giurfa M. Detection of dim and bright colours by honeybees. J. Exp. Biol. 2000;203:3289–3298. doi: 10.1242/jeb.203.21.3289. [DOI] [PubMed] [Google Scholar]
- Kelber A. Colour learning in the hawkmoth Macroglossum stellatarum. J. Exp. Biol. 1996;199:1127–1131. doi: 10.1242/jeb.199.5.1127. [DOI] [PubMed] [Google Scholar]
- Kelber A. Innate preferences for flower features in the hawkmoth Macroglossum stellatarum. J. Exp. Biol. 1997;200:826–835. doi: 10.1242/jeb.200.4.827. [DOI] [PubMed] [Google Scholar]
- Kelber A, Hénique U. Trichromatic colour vision in the hummingbird hawkmoth, Macroglossum stellatarum. J. Comp. Physiol. A. 1999;184:535–541. 10.1007/s003590050353 [Google Scholar]
- Kelber A, Balkenius A, Warrant E.J. Scotopic colour vision in nocturnal hawkmoths. Nature. 2002;419:922–925. doi: 10.1038/nature01065. 10.1038/nature01065 [DOI] [PubMed] [Google Scholar]
- Kelber A, Balkenius A, Warrant E.J. Colour vision in diurnal and nocturnal hawkmoths. Integr. Comp. Biol. 2003a;43:571–579. doi: 10.1093/icb/43.4.571. [DOI] [PubMed] [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. 2003b;78:81–118. doi: 10.1017/s1464793102005985. 10.1017/S1464793102005985 [DOI] [PubMed] [Google Scholar]
- Lehrer M. Spatial vision in the honeybee: the use of different cues in different tasks. Vis. Res. 1993;34:2363–2385. doi: 10.1016/0042-6989(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Lehrer M, Bishof S. Detection of model flowers by honeybees: the role of chromatic and achromatic contrast. Naturwissenschaften. 1995;82:145–147. [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl Acad. Sci. USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. 10.1073/pnas.071053098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. G. Fischer; Jena: 1954. Blütenbiologische Typen als Elemente der Sippengliederung. [Google Scholar]
- Vorobyev M. Costs and benefits of increasing the dimensionality of colour vision system. In: Taddei-Ferretti C, editor. Biophysics of photoreception: molecular and phototransductive events. World Scientific; Singapore: 1997. pp. 280–289. [Google Scholar]
- White R.H, Stevenson R.D, Bennett R.R, Cutler D.E, Haber W.A. Wavelength discrimination and the role of ultraviolet vision in the feeding behavior of hawkmoths. Biotropica. 1994;26:427–435. [Google Scholar]
- Zentall T.R, Riley D.A. Selective attention in animal discrimination learning. J. Gen. Psychol. 2000;127:45–66. doi: 10.1080/00221300009598570. [DOI] [PubMed] [Google Scholar]