Abstract
Maternal modification of offspring sex in birds has strong fitness consequences, however the mechanisms by which female birds can bias sex of their progeny in close concordance with the environment of breeding are not known. In recently established populations of house finches (Carpodacus mexicanus), breeding females lay a sex-biased sequence of eggs when ambient temperature causes early onset of incubation. We studied the mechanisms behind close association of incubation and sex-determination strategies in this species and discovered that pre-ovulation oocytes that produce males and females differed strongly in the temporal patterns of proliferation and growth. In turn, sex-specific exposure of oocytes to maternal secretion of prolactin and androgens produced distinct accumulation of maternal steroids in oocyte yolks in relation to oocyte proliferation order. These findings suggest that sex difference in oocyte growth and egg-laying sequence is an adaptive outcome of hormonal constraints imposed by the overlap of early incubation and oogenesis in this population, and that the close integration of maternal incubation, oocytes' sex-determination and growth might be under control of the same hormonal mechanism. We further document that population establishment and the evolution of these maternal strategies is facilitated by their strong effects on female and offspring fitness in a recently established part of the species range.
Keywords: constraints, incubation, maternal effects, oogenesis, sex ratio
1. Introduction
In species that produce multiple simultaneously growing ova, embryos or neonates, parents need to partition resources among developing offspring without sacrificing their number and quality (Williams 1966). In passerine birds, the growth period of each oocyte is greater than the ovulation interval and multiple ovarian eggs develop inside female during the same time (Johnson 2000; figure 1). When offspring of different sex have different growth costs or requirements, such as exposure to specific hormones, there is a potential conflict between overlap in offspring development and sex-specific partitioning of resources by females (Schwabl et al. 1997; West & Sheldon 2002; Uller 2003; Andersson et al. 2003). This conflict is particularly acute when a female's own reproductive state is regulated by the same hormones (e.g. Williams et al. 2005), thereby reducing the opportunity to accommodate sex-specific hormonal requirements of her offspring. Moreover, the occurrence of the sex-determining first meiotic division after oocyte proliferation and growth in female birds (e.g. Harper 1904; Warren & Scott 1935; reviewed in Pike & Petrie 2003) further constrains the maternal ability for sex-specific allocation of resources and hormones to the eggs.
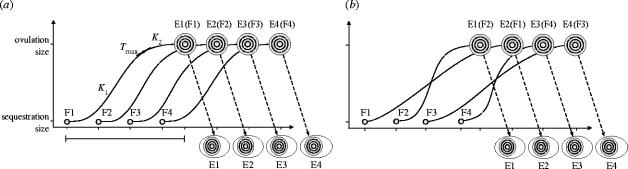
Figure 1.
Schematic illustration of temporal sequence of oocyte RYD phase, ovulation and egg-laying in a bird. (a) Traditional view: hierarchical order of oocyte sequestration (F1–F4) into RYD phase is maintained at ovulation and egg-laying (E1–E4) because of similar RYD growth pattern among oocytes. The sigmoid growth of F1 shows growth parameters measured in this study: K1, initial growth rate; K2, late growth rate; Tmax, time of maximum growth, the horizontal line shows total duration of RYD phase of F1 and is the same for all oocytes. In this example, during its RYD growth, F1 overlaps with growth of F2 for 24 h, with growth of F2+F3 for 24 h, and with growth of F2+F3+F4 for 24 h. (b) Hypothetical view: when RYD growth rate and duration differ among oocytes, the hierarchical order of sequestration (F1–F4) might not be maintained at ovulation (in this example, F2, F1, F4, F3). Growing oocytes would differ in the duration of overlap with RYD phase of other oocytes, and variation in RYD growth patterns could expose growing oocytes to distinct maternal hormonal profiles and lead to accumulation of distinct hormonal concentrations in oocyte yolk prior to ovulation.
In birds, propensity to incubate is regulated by prolactin (PRL), a pituitary hormone that also affects the production and metabolism of steroids by the ovary (Etches et al. 1979; Rozenboim et al. 1993; Tabibzadeh et al. 1995; Sockman et al. 2001; Vleck 2002). Thus, when ambient temperature at the time of egg-laying favours early onset of incubation (Hébert 2002), it results in the overlap between high secretion of PRL and oogenesis, such that high concentrations of PRL, necessary for egg incubation by the female, could constrain the sex-specific steroid requirements of growing follicles (Johnson 1990; Tabibzadeh et al. 1995; Chaudhuri & Maiti 1998; Sockman & Schwabl 1999). Females can overcome this constraint and enable the sex-specific allocation of steroids and resources to male and female eggs within a clutch by adjusting the sequence in which the sexes are produced in the ovary to her hormonal state. Alternatively, exposure of ovarian oocytes to specific concentrations of PRL and steroids and the corresponding accumulation of steroids in growing oocytes (Schwabl 1996) prior to meiosis might bias sex determination in birds in which the female is the heterogametic sex. Both strategies would result in often documented, but unexplained, bias in ovulation order of male and female eggs within a clutch (Ankney 1982; Dijkstra et al. 1990; Krackow 1995a; Cordero et al. 2001; Legge et al. 2001; Blanco et al. 2002; Komdeur & Pen 2002; Komdeur et al. 2002).
In two recently established populations of the house finch (Carpodacus mexicanus), females adjust the order in which male and female eggs are laid into a clutch and this strategy enables population persistence in novel environments (Badyaev et al. 2002a). In both populations, the sex-bias in egg-laying order is closely linked to ambient temperature which induces onset of incubation (Badyaev et al. 2003a,b), and to adaptive patterns of offspring growth and survival (Badyaev et al. 2002a,b). Here we examine the mechanisms behind the association of the onset of incubation and sex-biased ovulation order in a population of house finches breeding at the northern extreme of their recently expanded range. Specifically, we examine growth patterns of pre-ovulation oocytes that will become males or females in relation to environmentally induced onset of incubation and associated hormonal states of mothers. First, we show that male and female oocytes differ in their patterns of growth and exposure to maternal hormones. We then show that frequently documented sex-biased laying order might be a maternal strategy to overcome hormonal constraint imposed by reciprocal interaction of circulating PRL and androgens during overlap between early onset of incubation and egg production. We further examine the evolutionary consequences of this maternal strategy.
2. Material and methods
(a) Study organism
House finches were studied from 1995 to 2003 in a resident, colour banded population in Missoula, Montana, United States (protocols in Badyaev & Martin (2000)). The onset of full incubation was monitored with thermocouples (Badyaev et al. 2003a) which were installed in each nest at the time of nest-building and only nests where incubation started with the first egg were used in this study (except in analyses reported in §2(e), below). All females laid one egg per day, most females between 07:30 and 09:00, but three females as late as 11:00. The eggs were collected sequentially within 20 h after laying, numbered and replaced with freshly laid house finch eggs from different nests as a part of concurrent experiment (Badyaev et al. 2002a). Immediately after collection, eggs were stored at −20 °C.
(b) Oocyte growth measurements
During the rapid yolk deposition (RYD) phase, the type and density of lipids deposited differs between daytime feeding (high-density lipids) and nighttime inanition (low-density) creating distinct layers, each corresponding to approximately 12 h of lipid deposition (Grau 1976; Astheimer & Grau 1990). The shell and albumen of freshly laid frozen eggs were removed, intact frozen yolks were weighed, blastodisk or early embryonic tissues separated for sexing (see §2(c) below), and a 1 mm-thick longitudinal slice was taken from the centre of the yolk. Still frozen yolk slices were placed in 1.5 ml of 4% formalin and incubated in a 60–65 °C water bath for 12 h, then rinsed and placed in 1.5 ml of 6% potassium dichromate and further incubated in a 60–65 °C water bath for 12 h. After staining, yolk slices were rinsed and sliced in half-revealing high-density lipid layers and low-density lipids layers. Yolk slices were photographed under 10× magnification and 7.2 megapixel digital images were analysed with SigmaScan software (SPSS, Inc.). We used thickness and number of daily lipid deposition-layers to calculate the duration and rate of follicle development (fig. 1 in Young & Badyaev 2004). We calculated the overlap among growing follicles of a clutch of eggs using the time when each egg was laid, and the duration of development of all the follicles in the clutch. RYD growth of house finch oocytes is best described by the sigmoid Gompertz curve (Young & Badyaev 2004; fig. 1):
where Wt is the measurement of gain in thickness of an oocyte layer at time t, W0 is the oocyte size at sequestration, K1 is the early growth rate constant and K2 the late growth rate (maturation rate of the exponential decay; Badyaev et al. 2001b). The time at the point of growth curve inflection, Tmax (h), where the incremental oocyte growth is maximum was calculated as:
We report growth parameters for the Gompertz curves in which absolute difference between Sum of Squares of the residuals of successive iterations was <10−4, and the estimated nonlinear regression function provided the highest R2.
(c) Molecular sexing
Early embryo cells of eggs that were incubated for 18–20 h (e.g. 38–40 h post-ovulation) were carefully separated from the surrounding tissues of the whole yolk under 12×magnification. DNA was extracted using standard phenol–chloroform methods. We used PCR primers P8 and P2 (Griffiths et al. 1998) which anneal to conserved exonic regions and amplify across an intron in both CHD1-W and CHD1-Z genes. PCR was carried out in a total volume of 25 μl with the following final reaction conditions: 1.5 mM MgCl2, 200 mM of each dNTP, 200 ng of each primer, 0.5 U of Taq polymerase and 50–200 ng of genomic DNA. PCR amplifications were performed as follows: an initial denaturing step at 94 °C for 2 min followed by 30 cycles of 94 °C for 30 s, 50 °C for 45 s and 70 °C for 30 s, concluding with a final cycle of 48 °C for 1 min and 72 °C for 5 min. The reliability of this molecular sexing method has been established in earlier studies of oocytes and early stage embryos in this species (Badyaev et al. 2002a; Young & Badyaev 2004, for details of validation and the PCR product analysis).
(d) Hormonal sampling and assays
Females were captured and sampled for plasma as they wereleaving their nests after laying each egg (e.g. from 07:30 to 09:00 for most laying females). Pre-laying females were captured and sampled between 07:00 and 09:00 at feeding stations. To minimize disturbance, females were not captured more than three times during each egg-laying cycle (e.g. 15–20 days), with samples of individual females being separated by at least 2 days (mean 4±1.3 days). Upon capture, we collected 150μl of blood from the brachial vein, and immediately separated the plasma by spinning the blood in a portable microhematocrit centrifuge at 2700 rpm. Plasma was stored at −80 °C until radioimmunoassay (RIA) analysis. After oocyte growth was measured for the entire clutch and the oocyte sequence for each female was identified from the oocyte growth and the egg-laying data, each plasma sample was assigned to the corresponding oogenesis stage. For these analyses, we pooled data for the last egg position (4th or 5th egg) and we used population-level hormonal data by compiling the same stage samples from all females (n=82 for PRL samples and n=28 for androgen samples). Our sampling protocol assured a similar number of equally spaced samples of each female and within egg-laying stage variation among females was lower than the variation among egg-laying stages. Hormonal exposure was calculated for each oocyte by aligning the RYD phase for each oocyte with the daily androgen (testosterone and 5α-dihydrotestosterone; T+5α-DHT) and PRL hormonal profiles for the population-wide sample of females. Daily exposure to hormones was an average exposure over 24 h for the duration of RYD of each oocyte.
(i) Female plasma
Plasma PRL levels were determined in duplicate samples using a post-precipitation, double-antibody RIA. The assay used purified chicken PRL as a standard and antisera against chicken PRL derived from rabbits. Dilutions of a plasma pool from house finches demonstrated a high cross-reactivity with the antisera and the slope of the curve for house finch plasma did not differ from the slope of the diluted chicken standard. This indicates that this heterologous RIA can be used to assess relative levels of house finch PRL (Duckworth et al. 2003). Plasma androgen concentrations were measured in a single RIA after extraction of 50–100μl of plasma with 8 ml of diethyl ether/petroleum ether (70 : 30) over Extrelut NT (Merck KGaA) columns. To increase assay sensitivity, extracts were further purified before a standard RIA (Schwabl 1996) employing tritiated T (NET 553, PerkinElmer, Life Sciences Inc.) and a T antibody (Wien Laboratories, Inc.) The antibody cross-reacted with the androgenic T metabolite 5α-DHT. Assay sensitivity for a 100 μl plasma sample was 50 pg T ml−1.
(ii) Yolk androgens
Yolk T and 5α-DHT were quantified using separation protocols (Schwabl 1993) and competitive binding RIA (Wingfield & Farner 1975). Yolks were homogenized and 20–40 mg of the homogenate diluted in 1 ml water was used. Tritiated T (1000 counts min−1) and 5α-DHT (NEN, USA) were added to the yolk samples for the calculation of recoveries. Samples were equilibrated for 30 min after which they were extracted twice with 3 ml diethyl ether and dried under nitrogen. Samples were reconstituted in 10% ethyl acetate in 2,2,4-trimethylpentane and then transferred to chromatography columns containing diatomaceous earth for hormone fraction elution. 5α-DHT was eluted with a concentration of 10% ethyl acetate and T was eluted with a concentration of 20% ethyl acetate. T was measured by RIA, using a specific antibody (Endocrine Sciences, USA). 5α-DHT was assayed using a commercial 125I-labelled radioimmunoassay kit from Diagnostics Systems Laboratories (Webster, TX). Average recovery efficiencies were 59% for 5α-DHT and 87% for T. Combined yolk concentrations of T and 5α-DHT were used in the analyses.
(e) Maternal fitness
We calculated sex bias in egg-laying order as deviation from the average sex-biased egg-laying sequence in the study population over 1995–2003 (Badyaev et al. 2002a). In the study population, strongly sex-biased positions are 1st (female), 2nd (male) and last (male), thus population specific sequence of offspring is female, male, (male or female), (male or female) and male (Badyaev et al. 2002a; 2003a,b). Deviations for each clutch were calculated as the number of egg-laying positions in which sex-bias differed from this pattern (ibid.). For example, a clutch in which the first laid egg was male, the second egg was male and the last egg was female deviated from the population specific sequence by two positions. All females breeding in the study site during their first breeding season were used in this analysis and the onset of incubation was recorded as the proportion of the total number of eggs with which full incubation started (i.e. small values indicate early onset of incubation). All surviving individually marked juveniles were recaptured 40–60 days after they left the nest and offspring recruitment calculated per clutch (both sexes combined). No significant juvenile dispersal takes place before finches are 70–80 days of age (Badyaev et al. 2001a,b).
3. Results and discussion
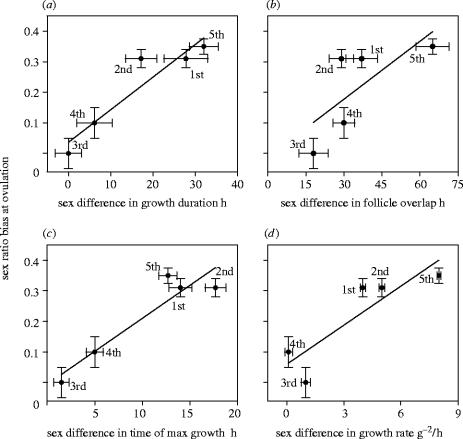
Oocytes that become males or females differed strongly in their pre-ovulation growth (table 1). Oocytes that became males were recruited into the RYD phase earlier, grew faster and overlapped with other growing oocytes for a shorter time than oocytes that became females (table 1). Sex-biased ovulation sequence corresponded to sex differences in oocyte growth patterns, such that the egg-laying positions in which growth of oocytes was the most distinct between the sexes were the most sex-biased in a clutch (figure 2; duration of oocyte growth: standardized regression coefficient (in standard deviation units) bST=0.94, t=4.87, p=0.02; time of maximum growth: bST=0.93, t=4.57, p=0.02; overlap with other growing oocytes: bST=0.72, t=1.89, p=0.10; growth rate: bST=0.87, t=3.16, p=0.05). These results show that the adaptive sex-biased ovulation sequence in this population is an outcome of processes taking place during early follicular development in the ovary.
Table 1.
Growth of pre-ovulation oocytes that became males or females in female house finches that initiated full incubation with the start of egg-laying.
| growth parameter ovulation sequence | males (mean±s.e., n) | females (mean±s.e., n) |
|---|---|---|
| duration of growth, h | ||
| 1st | 66.1±7.1; 10 | 93.4±3.0; 7* |
| 2nd | 82.0±8.2; 6 | 99.0±3.2; 8* |
| 3rd | 94.5±4.1; 6 | 94.7±11.1; 10 |
| 4th | 87.3±8.5; 6 | 93.2±10.1; 4 |
| 5th | 67.3±6.7; 8 | 105.9±13.2; 4* |
| time of max growth, h | ||
| 1st | 5.9±2.6 | 19.9±3.1* |
| 2nd | 6.4±2.1 | 23.5±4.2* |
| 3rd | 15.0±5.7 | 16.5±2.0 |
| 4th | 10.3±3.4 | 15.3±4.1 |
| 5th | 10.9±1.1 | 22.52±2.1* |
| overlap with other follicles, h | ||
| 1st | 98.0±12.3 | 132.0±4.9* |
| 2nd | 144.3±16.3 | 173.2±4.74 |
| 3rd | 154.1±13.7 | 171.6±17.0 |
| 4th | 121.5±18.2 | 154.6±10.5 |
| 5th | 94.8±19.5 | 161.0±27.2* |
| growth rate, K1, g−2 h−1 | ||
| 1st | 0.051±3e−3 | 0.012±5e−3* |
| 2nd | 0.019±2e−3 | 0.071±2e−3* |
| 3rd | 0.067±5e−3 | 0.058±2e−3 |
| 4th | 0.064±4e−3 | 0.048±4e−3 |
| 5th | 0.110±0.01 | 0.030±1.5e−3* |
Note: *p<0.05, for the Kruskal–Wallis test of significant differences between the sexes.
Figure 2.
Relationship between sex-ratio at ovulation (in deviations from 50% males : 50% females for each ovulation position) and sex differences (female minus male value) in (a) duration of oocyte growth (hours), (b) overlap in RYD growth with other RYD oocytes (cumulative hours), (c) time of maximum growth (hours), (d) growth rate (g−2 h−1). Numbers are ovulation order (1–5), standard errors (s.e.) for sex-ratio data are calculated from the among-year deviations during 1995–2003, s.e. for growth parameters are from resampling with replacement, for each ovulation position, of growth data for all oocytes for both sexes (table 1).
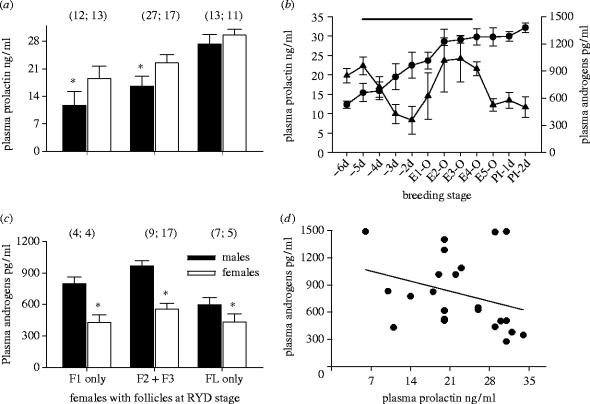
Plasma PRL of breeding females was lower when the RYD oocytes became males compared to when they became females (figure 3a; F=8.42, p<0.01; Waller–Duncan K-ratio test tcric=2.05), except for the females with only the last non-ovulated oocyte (see below; figure 3a). Plasma androgen concentrations of females were higher when their RYD oocytes became males compared to when they became females (figure 3c; F=11.07, p<0.01; tcric=2.19). A subset of females whose hormone levels were measured when they had only the first oocyte at the RYD phase also had lower PRL (figure 3a) and higher androgen concentrations (figure 3c) when this oocyte became a male compared to when it became a female. Females whose hormones were measured when they had only the last oocyte at the RYD stage had higher plasma androgen levels when this oocyte became a male (figure 3c), but their PRL did not differ in relation to the sex of the egg (figure 3a). In addition, females with higher levels of circulating PRL tended to have lower levels of circulating androgens throughout egg-formation (figure 3d; n=24 females; bST=−0.33, t=1.76, p=0.09).
Figure 3.
(a) Circulating plasma prolactin (PRL) of females with oocytes at the RYD phase in relation to oocyte sex and ovulation order. Shown are non-overlapping groups of females that had only one—the first (‘F1- only’) or the last (‘FL-only’) oocyte at the RYD phase at the time of plasma sampling. ‘F2+F3’ were females that had the second and the third oocyte at the RYD stage at the time of sampling. Sample sizes for each follicle and sex are in parentheses. (b) Plasma PRL (circle; n=82 females) and androgen (triangle; n=28) of females that began full incubation with the first egg. Horizontal line shows period of RYD of follicles within a clutch. E1-O indicates the ovulation of the first egg, E2-O is the ovulation of the second egg, laying of the first egg, and the onset of full incubation. PI-1d indicates the first day of the post-egg laying incubation. (c) Circulating plasma androgens (T+5α-DHT) of females with RYD oocytes that became males and females. Asterisk indicates differences between sexes within each group. (d) Relationship between circulating PRL and androgens in females with RYD phase oocytes.
Because female plasma PRL and androgen concentrations change with the progress of egg formation, laying and incubation (figure 3b), temporal variation in male and female follicle sequestration and growth should result in their development in different hormonal milieus. Indeed, earlier recruited and faster growing oocytes that became male eggs were exposed to a higher daily concentration of androgen compared to either oocytes that became females or the average androgen level throughout the period of oogenesis (figure 4a; males versus females; F=3.64, p=0.06; males versus average daily androgen exposure: t=2.88, p<0.05). The daily concentrations of plasma PRL during the proliferation of male and female oocytes did not differ from each other or the average PRL level during oogenesis (figure 4a; males versus females; F=2.04, p=0.16; males versus average daily PRL exposure: t=1.01, p=0.25). Oocytes that developed when their mother's circulating androgens were high accumulated greater amount of androgens (figure 4b; male oocytes: bST=0.68, t=4.23, p=0.004; female oocytes: bST=0.51, t=1.97, P=0.07). Conversely, exposure to higher maternal plasma PRL concentration during oocyte growth correlated with lesser accumulation of androgens in oocytes (figure 4c; male oocytes: bST=−0.54, t=−2.85, p=0.01; female oocytes: bST=−0.59, t=−2.45, p=0.03). Thus, PRL might regulate not only the onset of incubation (figure 3b; Etches et al. 1979; Goldsmith 1982; Crisostomo et al. 1998; Maney et al. 1999), but also, through actions on steroid-metabolizing enzymes, the steroidogenesis in follicles (figure 3d; Silverin 1980; Zadworny et al. 1986; Tabibzadeh et al. 1995; Sockman & Schwabl 1999), the sex-specific growth of developing oocytes (table 1, figure 2) and the concentrations of androgens in the female plasma and the yolks (figure 4b,c).
Figure 4.
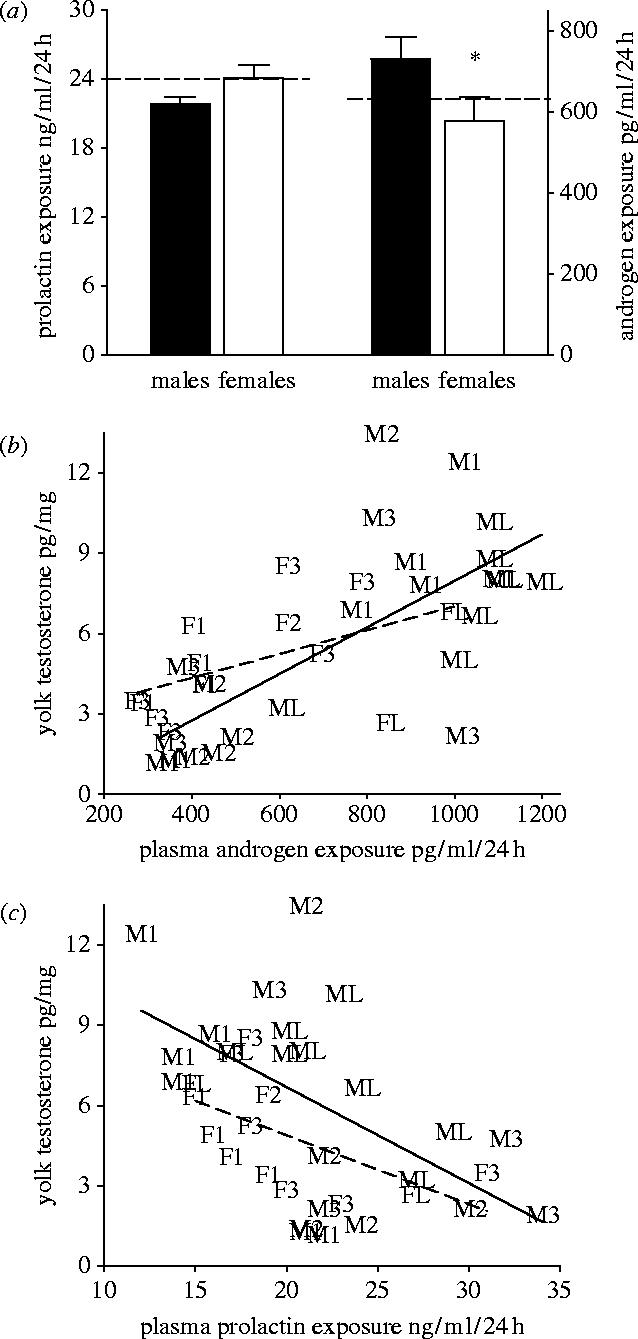
(a) Mean daily concentrations of female plasma PRL and androgen during RYD phase of male (n=36) and female (n=33) oocytes. Dashed line indicates average level of PRL (left) and androgens (right) throughout the oocytes' RYD phase. (b) Relationship between mean daily concentrations of plasma androgens of the mother throughout an oocyte's RYD phase and the total concentration of androgens in the yolk of this oocyte (male oocytes, solid line; female oocytes, dashed line). (c) Relationship between mean daily plasma PRL concentrations throughout an oocyte's RYD phase and total concentrations of androgens in the yolk of this oocyte (male oocytes, solid line; female oocytes, dashed line). Letter followed by number indicates sex (Male or Female) and order of ovulation (1, 2, 3, last).
Sex of an avian egg is determined by the first meiotic division that is thought to occur after the proliferation of the follicle is complete and shortly before the oocyte is ovulated (reviewed in Krackow 1995b; Komdeur et al. 2002; Pike & Petrie 2003). Growth of pre-meiotic oocytes during different hormonal states of females and corresponding accumulation of distinct amounts of steroids in the yolks might influence the cytokinesis as well as the elasticity, position and movement of the meiotic spindles and hence segregation of sex chromosomes during the first meiotic division (Gard 1992; Barton & Goldstein 1996; de Villena & Sapienza 2001). Thus, PRL, through its effects on ovarian steroidogenesis and follicle proliferation might bias oocyte sex determination and at the same time facilitate sex-specific oocyte growth (Bowden et al. 2000; Petrie et al. 2001; Lovern & Wade 2003). Alternatively, male and female oocytes might have different sensitivity to maternal hormones associated with follicle sequestration or ovulation (Ankney 1982; Pike & Petrie 2003). Under either scenario, our results suggest that PRL is a common link integrating the cues from maternal environment (ambient temperature), maternal behavioural strategies (onset of incubation) and modification of male and female follicle growth, resulting in the frequently observed association between the environmentally induced onset of incubation and sex-biased egg-laying order in many bird species (Pike & Petrie 2003).
Maternal modification of the order in which male and female eggs are laid into a clutch results in adaptive patterns of growth and survival in house finches (Badyaev et al. 2002a). This association can evolve either directly, if mothers modify the order in which they produce male and female eggs to enable the precise allocation of growth-enhancing steroids to sons and daughters (Shahabi et al. 1975; Henry & Burke 1999; Petrie et al. 2001; see also Williams et al. 2005) or indirectly, if females that successfully overcome the hormonal constraints (PRL–androgen interaction) imposed by overlap of early incubation and egg production (figure 3d; Mead & Morton 1985; Sockman et al. 2000), have higher fecundity. We found that breeding females that biased the order in which they laid male and female eggs when incubating from the first egg had the highest number of offspring surviving to dispersal age (figure 5; F=11.96, p<0.01; number of deviations in sex-biased order from the population-specific pattern: t=−4.18, p<0.01; onset of incubation: t=−2.54, p=0.01, n=116 nests). Although these results corroborate previous findings that sex-biased laying order is an active female strategy to produce offspring with morphology favoured by local natural selection (Badyaev et al. 2002a), these results also suggest that sex-biased egg-laying order can be adaptive independently of its effects on offspring morphology (figure 5; Badyaev in press). Selection favouring higher fecundity of females that overcome hormonal constraint of early incubation by sex-biasing egg-laying order can produce adaptive maternal effects on offspring growth indirectly, for example by affecting developmental times of sons and daughters (Badyaev et al. 2001a,b; 2003a; 2005). Overall, the strong effects of sex-biased ovulation sequence on maternal and offspring fitness would facilitate its assimilation and rapid evolution in newly established populations of this invasive species.
Figure 5.
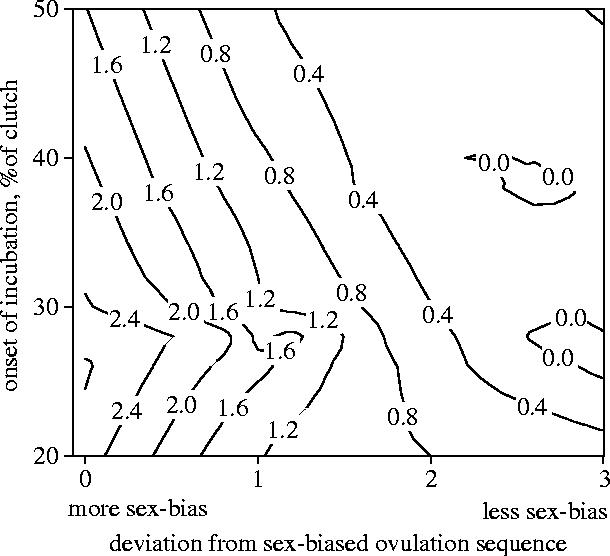
Fitness contour plot of number of offspring surviving to dispersal age in relation to female's onset of incubation and the sex-biased laying order (shown as deviations from the population specific sex-bias in egg-laying sequence) in clutches of 116 females from 1995–2003.
Acknowledgments
This work was supported by NSF grants (DEB-0075388, IBN-0218313, DEB-0077804) and the University of Arizona. We thank many field assistants for help in the field, and P. Weatherhead, K. Sockman, F. Janzen, A. L. Johnson, C. Vleck, T. Williams, R. Marshall, D. Seaman, K. Oh, L. Landeen, and two anonymous reviewers for detailed comments and helpful suggestions.
References
- Andersson M, Wallander J, Oring L, Akst E, Reed J.M, Fleischer R.C. Adaptive seasonal trend in brood sex ratio: test in two sister species with contrasting breeding systems. J. Evol. Biol. 2003;16:510–515. doi: 10.1046/j.1420-9101.2003.00533.x. 10.1046/j.1420-9101.2003.00533.x [DOI] [PubMed] [Google Scholar]
- Ankney C.D. Sex ratio varies with egg sequence in lesser snow geese. Auk. 1982;99:662–666. [Google Scholar]
- Astheimer L.B, Grau C.R. A comparison of yolk growth rates in seabird eggs. Ibis. 1990;132:380–394. [Google Scholar]
- Badyaev, A.V. in press Maternal inheritance and rapid evolution of sexual size dimorphism: passive effects or active strategies? Am. Nat.165 [DOI] [PubMed]
- Badyaev A.V, Martin T.E. Sexual dimorphism in relation to current selection in the house finch. Evolution. 2000;54:987–997. doi: 10.1111/j.0014-3820.2000.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Hill G.E, Whittingham L.A. The evolution of sexual size dimorphism in the house finch: IV population divergence in ontogeny of dimorphism. Evolution. 2001a;55:2534–2549. doi: 10.1111/j.0014-3820.2001.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Whittingham L.A, Hill G.E. The evolution of sexual size dimorphism in the house finch: III developmental basis. Evolution. 2001b;55:176–189. doi: 10.1111/j.0014-3820.2001.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, et al. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002a;295:316–318. doi: 10.1126/science.1066651. 10.1126/science.1066651 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Hill G.E, Whittingham L.A. Population consequences of maternal effects: sex-biased hatching order produces divergence in sexual dimorphism between newly established bird populations. J. Evol. Biol. 2002b;15:997–1003. 10.1046/j.1420-9101.2002.00462.x [Google Scholar]
- Badyaev A.V, Beck M.L, Hill G.E, Whittingham L.A. The evolution of sexual size dimorphism in the house finch: V maternal effects. Evolution. 2003a;57:384–396. doi: 10.1111/j.0014-3820.2003.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Hill G.E, Beck M.L. Interaction between maternal effects: onset of incubation is related to offspring sex in a passerine bird. Oecologia. 2003b;135:386–390. doi: 10.1007/s00442-003-1203-x. [DOI] [PubMed] [Google Scholar]
- Barton N.R, Goldstein L.S.B. Going mobile: microtubule motors and chromosome segregation. Proc. Natl Acad. Sci. USA. 1996;93:1735–1742. doi: 10.1073/pnas.93.5.1735. 10.1073/pnas.93.5.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G, Dávila J.A, López Septiem J.A, Rodríguez R, Martínez F. Sex-biased initial eggs favour sons in the slightly size-dimorphic Scops owl (Otus scops) Biol. J. Linnean Soc. 2002;76:1–7. 10.1046/j.1095-8312.2002.00036.x [Google Scholar]
- Bowden R.M, Ewert M.A, Nelson C.E. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. R. Soc. B. 2000;267:1745–1749. doi: 10.1098/rspb.2000.1205. 10.1098/rspb.2000.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S, Maiti B.R. Effects of gonadotropins and prolactin on ovarian activity of a wild avian species, the tree pie Dendrocitta vagabunda. Ind. J. Exp. Biol. 1998;36:790–795. [PubMed] [Google Scholar]
- Cordero P.J, Vinuela J, Aparicio J.M, Veiga J.P. Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J. Evol. Biol. 2001;14:829–834. 10.1046/j.1420-9101.2001.00320.x [Google Scholar]
- Crisostomo S, Guemene D, Garreau-Mills M, Morvan C, Zadworny D. Prevention of incubation behavior expression in turkey hens by active immunization against prolactin. Theriogenology. 1998;50:675–690. doi: 10.1016/s0093-691x(98)00172-1. 10.1016/S0093-691X(98)00172-1 [DOI] [PubMed] [Google Scholar]
- de Villena F.P.M, Sapienza C. Nonrandom segregation during meiosis: the unfairness of females. Mammalian Genome. 2001;12:331–339. doi: 10.1007/s003350040003. 10.1007/s003350040003 [DOI] [PubMed] [Google Scholar]
- Dijkstra C, Daan S, Buker J.B. Adaptive seasonal variation in the sex ratio of kestrel broods. Funct. Ecol. 1990;4:143–147. [Google Scholar]
- Duckworth R.A, Badyaev A, Parlow A.F. Males with more elaborated sexual ornaments avoid costly parental care in a passerine bird. Behav. Ecol. Sociobiol. 2003;55:176–183. 10.1007/s00265-003-0671-7 [Google Scholar]
- Etches R.J, Gargbutt A, Middleton A.L. Plasma concentrations of prolactin during egg laying and incubation in the ruffed grouse (Bonasa umbellus) Can. J. Zool. 1979;57:1624–1627. [Google Scholar]
- Gard D.L. Microtubule organization during maturation of Xenopus oocytes: assembly and rotation of the meitoic spindles. Dev. Biol. 1992;151:516–530. doi: 10.1016/0012-1606(92)90190-r. 10.1016/0012-1606(92)90190-R [DOI] [PubMed] [Google Scholar]
- Goldsmith A.R. Plasma concentrations of prolactin during incubation and parental feeding throughout repeated breeding cycles in canaries (Serinus canaria) J. Endocrin. 1982;94:51–59. doi: 10.1677/joe.0.0940051. [DOI] [PubMed] [Google Scholar]
- Grau C.R. Ring structure of avian egg yolk. Poul. Sci. 1976;55:1418–1422. [Google Scholar]
- Griffiths R, Double M, Orr K, Dawson R. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1076. doi: 10.1046/j.1365-294x.1998.00389.x. 10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Harper E.H. The fertilization and early development of the Pigeon's egg. Am. J. Anat. 1904;3:349–386. 10.1002/aja.1000030402 [Google Scholar]
- Hébert P.N. Ecological factors affecting initiation of incubation behaviour. In: Deeming D.C, editor. Avian incubation: behaviour, environment, and evolution. Oxford University Press; New York: 2002. pp. 271–279. [Google Scholar]
- Henry M.H, Burke W.H. The effects of in ovo administration of testosterone or an antiandrogen on growth of chick embryos and embryonic muscle characteristics. Poult. Sci. 1999;78:1006–1013. doi: 10.1093/ps/78.7.1006. [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Steroidogenesis and actions of steroids in the hen ovary. Poult. Biol. 1990;2:319–346. [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Whittow G.C, editor. Sturkie's avian physiology. Academic press; San Diego: 2000. pp. 569–596. [Google Scholar]
- Komdeur J, Pen I. Adaptive sex allocation in birds: the complexities of linking theory and practice. Phil. Trans. R. Soc. B. 2002;357:373–380. doi: 10.1098/rstb.2001.0927. 10.1098/rstb.2001.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur J, Magrath M.J.L, Krackow S. Pre-ovulation control of hatchling sex ratio in the Seychelles warbler. Proc. R. Soc. B. 2002;269:1067–1072. doi: 10.1098/rspb.2002.1965. 10.1098/rspb.2002.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krackow S. The developmental asynchrony hypothesis for sex ratios manipulations. J. Theor. Biol. 1995a;176:273–280. doi: 10.1006/jtbi.1995.0197. 10.1006/jtbi.1995.0197 [DOI] [PubMed] [Google Scholar]
- Krackow S. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 1995b;70:225–241. doi: 10.1111/j.1469-185x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Legge S, Hensohn R, Double M, Griffiths R, Cockburn A. Complex sex allocation in the laughing kookaburra. Behav. Ecol. 2001;12:524–533. 10.1093/beheco/12.5.524 [Google Scholar]
- Lovern M, Wade J. Yolk testosterone varies with sex in eggs of the lizard, Anolis carolinensis. J. Exp. Biol. 2003;295A:206–210. doi: 10.1002/jez.a.10225. [DOI] [PubMed] [Google Scholar]
- Maney D.L, Hahn T.P, Schoech S.J, Sharp P.J, Morton M.L, Wingfield J.C. Effects of ambient temperature on photo-induced prolactin secretion in three subspecies of White-crowned sparrow, Zonotrichia Leucophrys. Gen. Comp. Endocrin. 1999;113:445–456. doi: 10.1006/gcen.1998.7219. 10.1006/gcen.1998.7219 [DOI] [PubMed] [Google Scholar]
- Mead P.S, Morton M.L. Hatching asynchrony in the mountain white-crowned sparrow (Zonotrichia leucophyris oriantha): a selected or incidental trait? Auk. 1985;102:781–792. [Google Scholar]
- Petrie M, Schwabl H, Brande-Lavridsen N, Burke T. Sex differences in avian yolk hormone levels. Nature. 2001;412:498. doi: 10.1038/35087652. 10.1038/35087652 [DOI] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. 10.1017/S1464793103006146 [DOI] [PubMed] [Google Scholar]
- Rozenboim I, Tabibzadeh C, Silsby J.L, El Halawani M.E. Effect of ovine prolactin administration on hypothalamic vasoactive intestinal peptide (VIP), gonadotropin releasing hormone I and II content, and anterior pituitary VIP receptors in laying turkey hens. Biol. Reprod. 1993;48:1246–1250. doi: 10.1095/biolreprod48.6.1246. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and its eggs. J. Exp. Zool. 1996;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. 10.1002/(SICI)1097-010X(19961001)276:2%3C157::AID-JEZ9%3E3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Schwabl H, Mock D.W, Gieg J.A. A hormonal mechanism for parental favoritism. Nature. 1997;386:231. 10.1038/386231a0 [Google Scholar]
- Shahabi N.A, Norton H.W, Nalbandov A.V. Steroid levels in follicles and the plasma of hens during the ovulatory cycle. Endocrin. 1975;96:962–968. doi: 10.1210/endo-96-4-962. [DOI] [PubMed] [Google Scholar]
- Silverin B. Effects of prolactin on the gonad and body weight of the male pied flycatcher during the breeding period. Endokrinologie. 1980;76:45–50. [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H. Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen. Comp. Endocrin. 1999;114:257–268. doi: 10.1006/gcen.1999.7252. 10.1006/gcen.1999.7252 [DOI] [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H, Sharp P.J. The role of prolactin in the regulation of clutch size and onset of incubation behavior in the American Kestrel. Horm. Behav. 2000;38:168–178. doi: 10.1006/hbeh.2000.1616. 10.1006/hbeh.2000.1616 [DOI] [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H, Sharp P.J. Regulation of yolk-androgen concentrations by plasma prolactin in the American Kestrel. Horm. Behav. 2001;40:462–471. doi: 10.1006/hbeh.2001.1715. 10.1006/hbeh.2001.1715 [DOI] [PubMed] [Google Scholar]
- Tabibzadeh C, Rozenboim I, Silsby J.L, Pitts G.R, Foster D.N, El Halawani M.E. Modulation of ovarian cytochrome P 450 17a-hydroxylase and cytochrome aromatase messenger ribonucleic acid by prolacin in the domestic turkey. Biol. Reprod. 1995;52:600–608. doi: 10.1095/biolreprod52.3.600. [DOI] [PubMed] [Google Scholar]
- Uller T. Viviparity as a constraint on sex-ratio evolution. Evolution. 2003;57:927–931. doi: 10.1111/j.0014-3820.2003.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Vleck C.M. Hormonal control of incubation behaviour. In: Deeming D.C, editor. Avian Incubation: behaviour, environment, and evolution. Oxford University Press; New York: 2002. pp. 54–62. [Google Scholar]
- Warren D.C, Scott H.M. The time factor in egg formation. Poult. Sci. 1935;14:195–207. [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. 10.1126/science.1069043 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection: a critique of some current evolutionary thought. [Google Scholar]
- Williams T.D, Ames C.E, Kiparissis Y, Wynne-Edwards K.E. Laying-sequence-specific variation in yolk estrogen levels, and relationship to plasma oestogen in female zebra finches (Taenipygia guttata) Proc. R. Soc. B. 2005;272:173–177. doi: 10.1098/rspb.2004.2935. 10.1098/rspb.2004.2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. 10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]
- Young R.L, Badyaev A.V. Evolution of sex-biased maternal effects in birds: I sex-specific resource allocation among simultaneously maturing follicles. J. Evol. Biol. 2004;17:1355–1366. doi: 10.1111/j.1420-9101.2004.00762.x. 10.1111/j.1420-9101.2004.00762.x [DOI] [PubMed] [Google Scholar]
- Zadworny D, Shimada K, Ishida H, Sato K. Gonadotropin-stimulated estradiol production in small ovarian follicles of the hen in suppressed by physiological concentrations of prolactin in vitro. Gen. Comp. Endocrin. 1986;74:468–473. doi: 10.1016/s0016-6480(89)80044-9. [DOI] [PubMed] [Google Scholar]