Abstract
Many ecological dynamics occur over time-scales that are well beyond the duration of conventional experiments or observations. One useful approach to overcome this problem is extrapolation of temporal dynamics from spatial variation. We review two complementary variants of this approach that have been of late increasingly employed: the use of natural gradients to infer anthropogenic effects and the use of anthropogenic gradients to infer natural dynamics. Recent studies have considered a variety of naturally occurring gradients associated with climate, CO2, disturbance and biodiversity gradients, as well as anthropogenic gradients such as those created by biological invasions, habitat fragmentation and land abandonment. These studies show that natural gradients are useful in predicting long-term consequences of human-induced environmental changes, whereas anthropogenic gradients are helpful in inferring the mechanisms behind natural dynamics because covarying factors are often more clearly understood in anthropogenic gradients than in natural gradients. We classify these studies into several categories, each with different strengths and weaknesses, and outline how the limitations can be overcome by combining the gradient-based approach with other approaches. Overall, studies reviewed here demonstrate that the development of basic ecological concepts and the application of these concepts to environmental problems can be more effective when conducted complementarily than when pursued separately.
Keywords: biodiversity, biological invasions, climate change, ecological restoration, gradient analysis, space-for-time substitution
1. Introduction
Most ecological systems are highly dynamic rather than static in terms of both their species composition and abundances and their functioning at a variety of spatial scales. Understanding what drives this composition and functioning requires consideration of community and ecosystem dynamics over multiple generations of individual species. These dynamics often occur over decades, centuries or millennia, particularly when long-lived organisms, for example trees, clonal shrubs and large vertebrates are the dominant components of the ecological community. Although long-term observational studies are making significant contributions to the understanding of long-term dynamics (e.g. Kratz et al. 2003), these time-frames are well beyond the duration of conventional experiments or observations, and this presents a major challenge to both basic and applied ecology.
One approach that has proven useful in understanding long-term ecological dynamics is the use of spatial variation in ecological processes across sites that vary in environmental conditions. This approach, which has a long tradition in ecology (e.g. Cowles 1899; Jenny 1941; Whittaker 1975), extrapolates temporal dynamics by comparing multiple sites in a region. This is apparent in gradient analyses (“comparisons [of community and ecosystem processes] carried out along gradients of an underlying controlling factor,” Vitousek & Matson 1991, p. 287), space-for-time substitution studies (Pickett 1989), investigations of chronosequences (Stevens & Walker 1970) and ‘natural experiments’ (“[experiments] in which the experimenter does not establish the perturbation but instead selects sites where the perturbation is already running or has run,” Diamond 1986, p. 12). Such analyses have been long recognized as having potential for understanding community dynamics (e.g. Whittaker & Niering 1965; Beals 1969) and, to a lesser extent, ecosystem processes (Crocker & Major 1955; Odum 1969). Gradients that may be useful for this approach can be either anthropogenic or natural in origin. For example, several studies have used gradients created by human land use to investigate long-term ecological consequences of habitat modification by humans (e.g. Lawton et al. 1998; Yeates et al. 2000), whereas other studies have used natural gradients across sites differing in certain species' densities to infer the long-term role of the species in ecosystems (e.g. Schoener & Toft 1983; Polis & Hurd 1996; Wardle et al. 1997a).
Two complementary variants of this approach have been increasingly employed in recent studies: the use of natural gradients to infer potential effects of anthropogenic factors, and the use of anthropogenic gradients to infer natural dynamics. In this paper, we review these recent uses of gradient analyses, which we will collectively call complementary gradient analyses. We note that these analyses are not necessarily the same as gradient analyses in a statistical sense; our emphasis here is conceptual not statistical. Focusing primarily on community-level and ecosystem-level dynamics in terrestrial systems, we begin by presenting examples of complementary gradient analyses that have used natural gradients and then those that have used anthropogenically derived gradients. Next, we categorize these studies to identify potential limitations associated with different types of complementary gradient analyses. We also discuss how these limitations can be overcome when gradient analyses are used in combination with other approaches. In doing this we aim to show how complementary usages of natural and anthropogenic gradients can offer unique opportunities for understanding the drivers of long-term ecological dynamics in both natural and modified landscapes.
2. Using natural gradients to infer anthropogenic dynamics
There is an increasing need for scientific predictions regarding long-term consequences of anthropogenic influences on the functioning of ecosystems. A challenge in accomplishing this goal is to predict what may happen over decades, centuries or millennia, following anthropogenic changes that have only become apparent relatively recently. However, a variety of natural gradients of environmental and biotic conditions encapsulate the extent of change in ecological drivers that human activity is causing or is foreseen to cause (table 1). We discuss four representative types of natural gradients that have been used to predict anthropogenic impacts on ecological dynamics.
Table 1.
Examples of gradients used in complementary gradient analyses.
| type of gradient | space-for-time substitution | biotic or abiotic gradient | representative reference |
|---|---|---|---|
| natural | |||
| CO2 | indirect | abiotic | Miglietta & Raschi (1993) |
| climatic | indirect | abiotic | Berg et al. (1993) |
| disturbance | direct | abiotic | Wardle et al. (2003a) |
| biodiversity | direct or indirecta | biotic | Wardle et al. (1997a) |
| anthropogenic | |||
| biological invasions: role of species in ecosystems | none | biotic | Vitousek & Walker (1989) |
| biological invasions: community assembly rules | none | biotic | Gotelli & Arnett (2000) |
| biological invasions: diversity–stability relationship | direct or nonea | biotic | Wiser et al. (1998) |
| biological invasions: niche breadth and disturbance | none | biotic | Vázquez & Simberloff (2002) |
| habitat fragmentation | direct or noneb | abiotic | Terborgh et al. (2001) |
| land abandonment and ecological restoration | direct | abiotic | Garnier et al. (2004) |
If the biodiversity gradient is caused by spatial variation in site age (successional gradient), direct space-for-time substitution is involved. If the biodiversity gradient is caused by spatial variation not related to site age (e.g. physical barrier to dispersal), indirect space-for-time substitution is involved. However, we suggest that most biodiversity gradients probably fall into the former category.
If habitat fragments differing in age are used to create a successional gradient, direct space-for-time substitution is involved. If habitat fragments differing in size or the degree of isolation but not in age are used to create a size or isolation gradient, no space-for-time substitution is involved.
(a) CO2 gradients
Much of the recent interest in global change ecology has focused on how projected anthropogenic increases in atmospheric CO2 may impact upon community and ecosystem processes. Although CO2 enrichment experiments have enhanced our understanding of the mechanistic basis of pulsed CO2 enrichment effects over months or years (e.g. Körner & Arnone 1992; Díaz et al. 1993; Oren et al. 2001; Schlesinger & Lichter 2001), anthropogenic CO2 enrichment will occur gradually over decades or centuries. There is mounting evidence that experimental studies overestimate the ecological effects of CO2 enrichment that occur more gradually and over longer durations than simulated experimentally (Luo & Reynolds 1999; Klironomos et al. 2005). However, long-term effects of CO2 enrichment can be studied by using natural springs that create gradients of CO2 gas, and which are at least several decades old. First employed in Italy by Miglietta & Raschi (1993), there has been a steady increase in the use of this approach around the world (Raschi et al. 1997). For example, a series of springs in New Zealand has been used to show that in a multi-decadal time-frame, increased atmospheric CO2 greatly increases ecosystem storage of C and N (Ross et al. 2000), promotes plant allocation of carbon to plant roots and root mycorrhizal colonization (Rillig et al. 2000) and influences plant litter quality and decomposition rates (Ross et al. 2002, 2003). Such studies provide critical validation for predictions about long-term effects of CO2 enrichment that otherwise can only be arrived at by extrapolation from shorter-term experiments.
(b) Climatic gradients
There are many natural gradients of climate that can be used to represent the types of climate changes that are predicted to occur due to human burning of fossil fuels. For example, the small but positive effects of temperature on soil decomposition and nutrient mineralization processes (and hence negative effects on soil C sequestration) that are predicted to occur through global warming have been demonstrated by using gradients of both latitude (e.g. Berg et al. 1993; Gholz et al. 2000; but see Giardina & Ryan 2000) and elevation (Vitousek et al. 1994; Nakatsubo et al. 1997). Positive effects of precipitation on ecosystem productivity and decomposition have also been demonstrated by the use of natural gradients of rainfall regime (e.g. Moore et al. 1999; Austin 2002). These studies provide long-term insights into community and ecosystem responses that are not possible through short-term experiments in which climatic factors are varied as treatments. This type of space-for-time substitution is particularly valuable when it is possible to examine ecosystem processes across climate gradients for which geology and plant species are constant (e.g. Vitousek et al. 1994; Austin 2002; Vitousek 2004). Gradient studies such as these are valuable not necessarily because they represent typical ecosystems, but rather because there are few confounding factors that influence ecosystem processes, thus making it easier to infer causal relationships.
(c) Disturbance gradients
Natural gradients can also be used to study long-term effects of changes in disturbance regimes resulting from human land use. (In this article we follow Walker & del Moral (2003) to define disturbance as “a relatively discrete event in time and space that alters habitat structure and often involves a loss of biomass.”) For example, widespread wildfire suppression has occurred over the past two centuries because an increasing number of people have been inhabiting regions subjected to natural fire regimes. Space-for-time substitution studies in ecosystems showing natural variation in fire frequency can provide useful information on long-term effects of anthropogenically altered fire regime on both community and ecosystem properties (Larocque et al. 2000; DeLuca et al. 2002). For example, in a study of lake islands in Sweden in which time since last fire varies naturally from a few decades to over 6000 years, it was shown that fire suppression impaired several ecosystem processes both above- and below-ground, leading to substantial C accumulation, although these effects only became evident after several hundred years without fire (Wardle et al. 1997a, 2003a, 2004). Space-for-time substitution such as this enables identification of long-term ecological effects of wildfire, and the likely impacts of this on the global carbon cycle (e.g. Wardle et al. 2003b), which would be impossible to identify through short-term experiments in which wildfire is varied as an experimental treatment.
(d) Biodiversity gradients
Several studies have used natural gradients of species diversity to infer how species extinction may affect ecosystem functioning, particularly productivity, in the long term (e.g. Wardle et al. 1997a; Troumbis & Memtsas 2000; Enquist & Niklas 2001). This type of approach has sometimes been criticized because many confounding factors covary with diversity (e.g. Tilman et al. 1997; Naeem 1999). Nevertheless, most observational studies using natural biodiversity gradients do not find evidence for a positive correlation between diversity and ecosystem function, and this may suggest that whatever positive role biodiversity may have in nature is of sufficiently minor importance to be overridden by other drivers of community and ecosystem functioning (Wardle et al. 1997b). Moreover, because species are expected to be lost from ecosystems in a non-random order (Wardle 1999; Fukami et al. 2001; Solan et al. 2004; Zavaleta & Hulvey 2004), natural gradients may offer insights that are more closely related to what real ecosystems experience over a long term than do experiments that use randomly assembled communities (Díaz et al. 2003).
3. Using anthropogenic gradients to infer natural dynamics
When multiple factors influence the behaviour of dynamics, which is almost always the case in ecology, experimental manipulation is often necessary to ascertain the role of suspected factors. Anthropogenic gradients are essentially long-term experiments at large spatial scales, despite not being designed as such. This is why anthropogenic gradients have helped to deepen the conceptual understanding of natural ecological dynamics (table 1). Here we discuss three types of anthropogenic gradients that have been used for this purpose. It is important to note that human influence on natural landscapes has been so pervasive that it is sometimes difficult to distinguish between ‘natural’ and anthropogenically induced dynamics. Here we use the word natural in a broad sense, referring to any phenomena that can occur in the absence of obvious human influences and are not tied to any particular applied problems.
(a) Biological invasions
Increasingly researchers have compared areas invaded and uninvaded by exotic species to develop basic concepts about long-term ecological dynamics (Cadotte et al. 2005; Sax et al. 2005). Invasions on islands are particularly suited for addressing ecosystem impacts of species (Courchamp et al. 2003). One such study involves offshore islands in New Zealand, several of which have been invaded by exotic rats and several of which have not. This system has been used to show adverse effects of rat introduction about 800 years ago on current vegetation (Campbell & Atkinson 1999, 2002). Further, on these islands, native seabirds serve as ecosystem drivers by transporting nutrients from the ocean and by cultivating soil through burrowing. Rats devastate seabird populations through predation. Comparison of rat-free islands with rat-invaded islands helps to clarify the role of seabirds as ecosystem engineers (see www.landcareresearch.co.nz/research/biodiversity/forest/rasp/). Another related situation involves the Aleutian Islands of Alaska, in which seabirds have important effects on vegetation and nutrient properties of some islands, while predation of seabirds by introduced foxes has mitigated these seabird effects on other islands (Croll et al. 2005).
Gradient analyses can also be performed on much larger landmasses. For example, the effects of biotic disturbance on the operation of community assembly rules have been revealed by comparison of native ant communities at adjacent continental sites that have been invaded and uninvaded by invasive ants (Gotelli & Arnett 2000; Sanders et al. 2003). Effects (or lack thereof) of species diversity, relative to various environmental conditions, on community stability have been studied by examining whether native communities varying in species richness differ in the probability of invasions (Wiser et al. 1998) or the number of exotic species that have invaded (Stohlgren et al. 1999; Brown & Peet 2003). The relative importance of local and regional processes on species diversity has been tested by studying biotic interchanges of previously isolated fishes (Smith et al. 2004). The hypothesis that more specialized species are more susceptible to disturbance and therefore prone to extinction has been tested with plants and pollinators in comparable sites invaded or uninvaded by introduced ungulates that disturb the system by trampling and grazing (Vázquez & Simberloff 2002). Effects of N-fixing species on ecosystem-level properties have been studied by comparing adjacent areas in natural forests in Hawaii with and without an invasive actinorhizal shrub (Vitousek & Walker 1989).
(b) Habitat fragmentation
Habitat fragmentation through human land use offers opportunities to test fundamental ecological concepts, such as how trophic cascades and other community dynamics vary over large scales (e.g. at least several km2). For example, in a series of islands of varying sizes created by hydroelectric development in Venezuela, it was shown that habitat size adversely affected densities of top predatory taxa, which led to cascading effects through the aboveground food web, ultimately affecting regeneration of canopy tree species and soil nutrients (Terborgh et al. 2001; Lambert et al. 2003; Feeley & Terborgh 2005). Studies utilizing habitat fragments of varying sizes created by human activity can also contribute to the understanding of biotic drivers of community and ecosystem properties in the long term. For example, fragments of grassland in Germany that differ in size and proximity to each other have proven useful for understanding effects of landscape configuration of habitats on communities of plants (Krauss et al. 2004) and invertebrates (Tscharntke et al. 2002). These studies newly found, among other things, that a mixture of small and large fragments would support more invertebrate species than would either many small fragments or a few large fragments of the same total area (Tscharntke et al. 2002).
(c) Land abandonment and ecological restoration
Former agricultural sites that have been abandoned and allocated for ecological restoration also offer opportunities for complementary gradient analyses. For example, the understanding of the role of functional groups in determining ecosystem properties over time has been advanced by comparison of sites differing in the time since land abandonment (Garnier et al. 2004). Long-term ecosystem impacts of an invasive grass were revealed by comparing their effects among sites of different abandonment age (Christian & Wilson 1999). Many other abandoned sites have the potential to be utilized for space-for-time substitution studies to test succession theory (Cairns & Heckman 1996; Palmer et al. 1997). These opportunities may have traditionally been under-utilized, but this situation may improve owing to increased efforts of coordination between restoration managers and the research community (Michener 1997; Young 2000; Temperton et al. 2004). Young et al. (2005) identified several basic ecological issues that could be addressed effectively in future research if we make use of restoration projects. These questions include understanding Allee effects, population viability analyses, gap analysis, metapopulation and metacommunity dynamics, ontogenetic niche shifts, and historical and stochastic effects.
4. Types and limitations of complementary gradient analyses
Those studies that have used complementary gradient analyses can be broadly categorized in two ways: (i) whether the analyses involve direct, indirect or no space-for-time substitution; and (ii) whether the gradient under consideration is abiotic or biotic. These categorizations help to clarify the limitations of complementary gradient analyses, as each type has a different kind of potential limitation (table 2).
Table 2.
Some potential limitations of complementary gradient analyses associated with different types of approaches (see §4 for details).
| type of approach | potential limitation | approaches that help to overcome limitation |
|---|---|---|
| direct space-for-time substitution | unknown site history | a, b |
| indirect space-for-time substitution | ecological and evolutionary time lags | c |
| biotic gradients | extra covarying factors | c, d |
| biological invasions | evolutionarily novel perturbation | b, c |
| common to all types | covarying factors | a, b, c, d, e |
Approaches that help to overcome limitation include: a, long-term observation; b, multi-region and multi-species comparison; c, field experiments; d, structural equation modelling; e, microbial microcosm experiments (see §5 for details).
(a) Direct, indirect, or no space-for-time substitution
We propose that there are three types of gradient analyses based on the use of space-for-time substitution. The first type involves direct space-for-time substitution, in which temporal dynamics are inferred by studying multiple sites of differing age (e.g. Vitousek 2004; see figure 1a). An example is the use of sites that differ in the time since last fire to infer ecosystem development in boreal forests (e.g., Zackrisson et al. 2004). Pickett (1989) provided a thorough evaluation of the potential limitations of direct space-for-time substitution. One of the most serious potential limitations is the effect that unknown variation in site history may have had on long-term community dynamics (Pickett 1989; Tilman 1989; Johnson et al. 1994; Fastie 1995). For example, Larocque et al. (2000) found, using palynology and anthracology, that vegetation dynamics after the last fire depended on the interval between consecutive fires in boreal forests. Therefore, space-for-time substitution studies that use only the time since the last fire could result in misleading inferences.
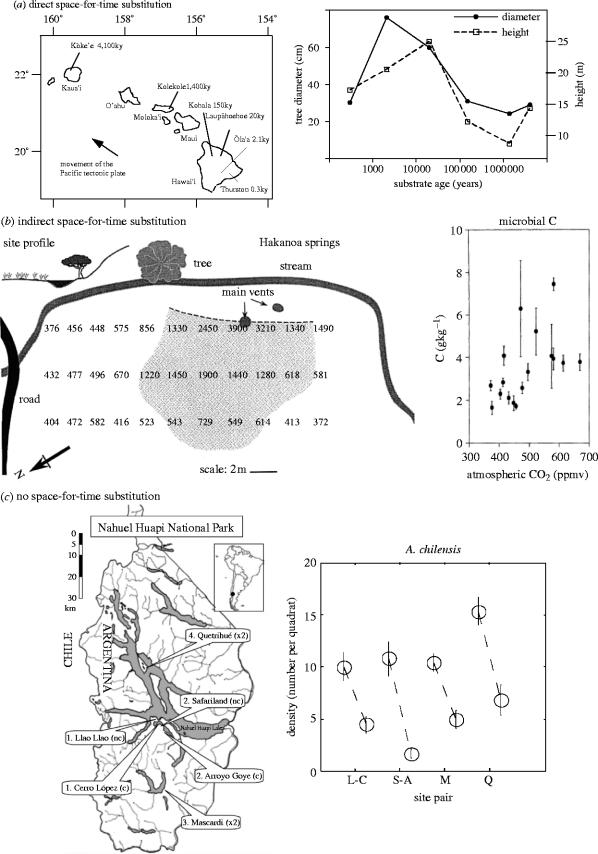
Figure 1.
Examples of gradient analyses involving (a) direct, (b) indirect or (c) no space-for-time substitution. In (a), a gradient of site age across the Hawaiian Islands (left panel) was used to infer long-term changes in the diameter and height of the dominant tree species (right panel) and other community and ecosystem properties (adapted from Vitousek 2004). In (b), a gradient of atmospheric CO2 concentrations created by a natural CO2 spring in New Zealand (left panel) was used to infer long-term changes in microbial C (right panel) and other community and ecosystem properties (adapted from Ross et al. 2000). In (c), a gradient of the density (presence or absence) of introduced ungulates in Argentina (left panel) was used to infer long-term effects on plant densities (right panel), pollination and reproduction (adapted from Vázquez & Simberloff 2004). L-C, S-A, M and Q in the right panel are the initial letters of the site names shown in the left panel.
A second type is indirect space-for-time substitution, in which temporal dynamics are inferred by studying multiple sites that differ in abiotic conditions not directly related to site age (e.g. Ross et al. 2000; see figure 1b). An example is the use of sites that differ in temperature owing to elevational variation (e.g. Nakatsubo et al. 1997). A main potential limitation of this type of gradient analysis is that it relies on the assumption that communities and ecosystems will respond to changes in the focal variable (e.g. increases in temperature) over time in the same way that they will over space (Dunne et al. 2004). The response of communities can be slower than inferred by indirect space-for-time substitution for both ecological and evolutionary reasons. Ecologically, time lags occur especially when species are limited in dispersal (Wiser et al. 1998; Fukami 2004) and when indirect species interactions significantly influence communities resulting in long-term transient dynamics (Tilman 1989; Tilman et al. 1994; Hastings 2004; Cadotte & Fukami 2005). Evolutionarily, spatial variation in community structure along a natural gradient can be a result of adaptation to local conditions (cf. Gillespie & Roderick 2002). Anthropogenic changes in the environment may proceed too rapidly to allow comparable evolutionary adaptation. Thus, it is possible that communities respond quite differently to anthropogenic changes than they do to natural gradients (cf. Denslow 1980; Hayes & Holl 2003), potentially limiting the power of natural gradients for understanding the consequences of anthropogenic factors.
The third type of gradient analyses involves no space-for-time substitution (e.g. Vázquez & Simberloff 2004; see figure 1c). In these analyses, spatial gradients are used as treatments, as in formal experiments. For example, sites with and without N-fixing exotic species are used as treatments without a need for space-for-time substitution (e.g. Vitousek & Walker 1989). These studies share potential limitations common to all gradient analysis, i.e. that confounding factors that vary along the gradient may themselves affect the response variables of interest.
(b) Abiotic or biotic gradients
Gradient analyses can also be categorized according to whether the gradients are abiotic or biotic. Many gradients are abiotic, including those involving CO2, temperature, precipitation, disturbance intensity and habitat fragment size, but other gradients are biotic, including those of species diversity, species composition and the density of biological invaders. Although abiotic and biotic components of an ecosystem can interact to affect each other, biotic components are obligatorily dependent upon abiotic factors, but not vice versa. This asymmetrical reliance means that biotic gradients may be more greatly affected by additional confounding factors, since they are usually governed by underlying abiotic gradients. Therefore, compared to studies using abiotic gradients, those using biotic gradients can have extra burden of proof to ensure that other factors are constant or do not covary across gradients.
Additional potential problems emerge with biotic gradients involving invasive species because such species are often evolutionarily novel to the system being invaded. For example, a recent meta-analysis indicated that the impact of biological invaders is greater when the invaders are taxonomically more distinct (Ricciardi & Atkinson 2004), and therefore more evolutionarily ‘unfamiliar,’ to the native community being invaded (but see Duncan & Williams 2002). A limitation here is that it may not always be easy to quantify the evolutionary and ecological novelty of the biological invaders, which may make it difficult to compare results of different gradient analyses for generalization.
5. Overcoming limitations by combining other approaches
Each limitation of complementary gradient analyses discussed above can be overcome, at least partially, by employing additional approaches in combination with complementary gradient analyses (table 2). We discuss several such approaches.
(a) Long-term observations
Conducting long-term observations (Hobbie 2003; Kratz et al. 2003), or natural trajectory experiments (Diamond 1986), on the same site to which gradient analyses are applied, helps us to understand the extent to which inferences from gradient analyses may be affected by unknown site history. This is the case even if the observations cannot run as long as the duration covered by gradient analyses (Pickett 1989; Michener et al. 1997; Fleming 1999). When direct observations are not possible, in some cases it is possible to reconstruct past ecological dynamics through historical evidence such as tree rings (Fastie 1995), pollen records (Larocque et al. 2000) and other paleo-ecological data to examine whether site history has affected patterns inferred through gradient analyses. These studies suggest that there are sometimes major flaws in using space-for-time substitution to infer long-term ecological dynamics (Johnson et al. 1994). Molnar & Botta-Dukat (1998) demonstrated the utility of ‘improved space-for-time substitution,’ which controls for present and past abiotic conditions. Long-term observations can provide insights even for gradient analyses that do not involve space-for-time substitution. For example, Wiser et al. (1998) combined long-term monitoring and gradient analysis to show that the relative contribution of abiotic conditions and native species composition to invasion resistance changed during long-term regional spread of an invasive plant.
(b) Multi-region and multi-species comparisons
Most complementary gradient analyses of community and ecosystem dynamics have been applied to a single region. However, gradient analyses employed simultaneously on multiple comparable regions (as opposed to multiple habitat types; Warming 1895) can be powerful for discovering dynamics that are general across different regions. For example, Wardle et al. (2004) found that ecosystem dynamics were consistent among six long-term soil chronosequences in various regions ranging from tropical to boreal zones (but see also Kitayama 2005; Wardle et al. 2005). Gradient analyses that investigate different species in a community can also be useful for generalization. For example, Richardson et al. (2005) found that changes in leaf and litter nutrient concentrations along a long-term chronosequence are remarkably consistent across species of contrasting growth forms. While neither Wardle et al. (2004) nor Richardson et al. (2005) involved natural-to-anthropogenic or anthropogenic-to-natural inferences, there is no reason why similar approaches cannot be used for complementary gradient analyses. We believe that there is considerable scope for studies that use comparable gradient analyses conducted in multiple regions. When gradient analyses find consistent trends in multiple regions, such results would strongly suggest the existence of general processes that are robust to variation in abiotic and biotic site history (see also meta-analysis by Wan et al. 2001). Conversely, multi-region comparisons can be equally useful for identifying the systems and processes sensitive to historical contingency (Scheffer et al. 2001).
(c) Field experiments
Field experiments, though limited in the temporal and spatial scales that they can encompass, are a useful tool for disentangling causal relationships suggested by gradient analyses. Several studies have explicitly employed a combination of complementary gradient analyses and field experiments. Some of the best examples concern effects of predicted changes in climate and atmospheric CO2 concentration on ecosystem properties. Dunne et al. (2004) combined a complementary gradient analysis and a field experiment within a single system to show how the two approaches could complement each other to overcome each other's limitations. They found that the experimentally induced response of soil carbon dynamics to global warming differed from a trend inferred from a natural gradient analysis, indicating that gradient analysis alone would produce a limited understanding of long-term consequences of global warming. However, they also found through the same combined approach that the response of flowering phenology to global warming was consistent between the gradient and experimental studies. Newton et al. (2001) conducted transplant experiments across atmospheric CO2 concentration gradients at a natural CO2 spring. Their results confirmed that qualitative differences existed between short-term (transient) and long-term (equilibrium) responses of plant communities to CO2 increase (see also Marchi et al. 2004). Field experiments have also been combined with gradient analyses to investigate nutrient limitation and biotic interactions in tropical (Cleveland et al. 2002; Vitousek 2004) and boreal (DeLuca et al. 2002; Wardle & Zackrisson 2005) forests (see also Mitchell & Chandler 1939).
(d) Structural equation modelling
Another technique that helps to make stronger inferences about causality is structural equation modelling, a multivariate statistical technique that can be used for disentangling complex causal links in ecological dynamics (Wootton 1994; Pugesek et al. 2002). Ultimately, experimental tests would strengthen conclusions reached from gradient analyses, but as we have discussed, experimental approaches are often not feasible for studying very long-term dynamics. Structural equation modelling is particularly useful when combined with gradient analyses, because it can help to establish causal relations from observational data in the absence of formal experimental manipulation. For example, Vázquez & Simberloff (2004) used path analysis, a special type of structural equation modelling, on observational data from four pairs of grazed and ungrazed sites to reveal the indirect effects that introduced ungulates had on pollination and reproduction of native plants.
(e) Microbial microcosm experiments
Finally, experiments with microbial model organisms can make an important contribution to the conceptual understanding of long-term ecological dynamics (Jessup et al. 2004; Srivastava et al. 2004). In these systems, it is possible to conduct experiments over tens or hundreds of generations within the time-frame of several days or weeks (e.g. Rainey & Travisano 1998; Warren et al. 2003; Cadotte & Fukami 2005). Although uncritical extrapolation must be avoided (Carpenter 1996), microbial systems allow for rigorous control over community assembly and environmental conditions (Fukami & Morin 2003), which helps to identify plausible mechanisms (including abiotic and biotic history) of the dynamics inferred from gradient analyses. For example, microcosm experiments have recently been used to suggest mechanisms for long-term community dynamics that arise through invasion (Jiang & Morin 2004) and adaptive radiation (Rainey & Travisano 1998; MacLean & Bell 2003).
6. Conclusions
Collectively, recent complementary gradient studies reviewed here demonstrate that the development of basic concepts on long-term ecological dynamics and the application of these concepts to environmental issues can be more effective when conducted in a complementary manner than when pursued separately. Traditionally, basic and applied ecology have been considered largely distinct from each other, as apparent in such pairs of journals as Ecology and Ecological Applications, and Journal of Ecology and Journal of Applied Ecology. However, this distinction is increasingly becoming blurred. As we have shown here, many recent studies have used complementary gradient analyses to address a wide range of long-term ecological dynamics—those that are naturally occurring and those that are anthropogenically induced. Natural gradients are useful in forming empirically based predictions about long-term consequences of human induced environmental changes that have begun relatively recently. Meanwhile, anthropogenic gradients are helpful in inferring the mechanisms responsible for natural dynamics, because covarying factors are often more clearly understood in anthropogenic gradients than in natural gradients.
We emphasize that there are several types of approaches that can be utilized for addressing questions about how community and ecosystem properties respond to natural and anthropogenic factors, and that different approaches are best suited for addressing different questions or issues; these are best considered on a case-by-case basis. Natural experiments (and observational studies based on these) are clearly most appropriate in situations where the driving variable of interest varies significantly over space without major covarying confounding factors, such as gradients of natural fire regime, climate or CO2 concentration. In other cases some caution is required in interpretation, for example where the driving variable of interest varies over space but not independently of other driving factors; this is often apparent for using spatial gradients in studying the ecological consequences of plant diversity for which plant species or functional group composition and soil fertility may covary with diversity. Some other ecological drivers can only be effectively studied by formal experimentation and, therefore, in the short term, especially when there are difficulties in finding systems in which that driver varies over space in an unambiguous manner.
However, our review of gradient studies has also indicated that the understanding of long-term ecological dynamics would progress most rapidly with a coordinated use of multiple methodologies (e.g. Diamond 1986; Drake et al. 1996; Resetarits & Bernardo 1998). As with any other approaches in ecology, complementary gradient analyses have potential limitations, and we have pointed out that different types of complementary gradient analyses are constrained by a different set of potential limitations. However, the studies reviewed here show that many of these limitations can be overcome by integrating various other types of methods. Using multiple-region comparisons and combining field experiments with gradient analysis are two ways that we believe would be particularly helpful in future research. When used in these and other integrated manners, complementary gradient analyses provide unique opportunities to investigate long-term dynamics of communities and ecosystems over time-frames that cannot be studied with other approaches.
Acknowledgments
We thank Duane Peltzer and two anonymous reviewers for helpful comments and Charles Godfray for inviting us to write this article. The Royal Society of New Zealand (Marsden Fund) and the Japan Society for the Promotion of Science (Research Fellowship for Young Scientists) supported this work.
References
- Austin A.T. Differential effects of precipitation on production and decomposition along a rainfall gradient in Hawaii. Ecology. 2002;83:328–338. [Google Scholar]
- Beals E.W. Vegetational change along altitudinal gradients. Science. 1969;165:981–985. doi: 10.1126/science.165.3897.981. [DOI] [PubMed] [Google Scholar]
- Berg B, et al. Litter mass loss rates in pine forests of Europe and eastern United States: some relationships with climate and litter quality. Biogeochemistry. 1993;20:127–159. [Google Scholar]
- Brown R.L, Peet R.K. Diversity and invasibility of southern Appalachian plant communities. Ecology. 2003;84:32–39. [Google Scholar]
- Cadotte M.W, Fukami T. Dispersal, spatial scale, and species diversity in a hierarchically structured experimental landscape. Ecol. Lett. 2005;8:548–557. doi: 10.1111/j.1461-0248.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- Cadotte M.W, McMahon S.M, Fukami T, editors. Conceptual ecology and invasions biology: reciprocal approaches to nature. Springer; Dordrecht, The Netherlands: 2005. [Google Scholar]
- Cairns J, Heckman J.R. Restoration ecology: the state of an emerging field. Annu. Rev. Energ. Env. 1996;21:167–189. doi:10.1146/annurev.energy.21.1.167 [Google Scholar]
- Campbell D.J, Atkinson I.A.E. Effect of kiore (Rattus exulans Peale) on recruitment of indigenous coastal trees on northern offshore islands of New Zealand. J. R. Soc. NZ. 1999;29:265–290. [Google Scholar]
- Campbell D.J, Atkinson I.A.E. Depression of tree recruitment by the Pacific rat (Rattus exulans Peale) on New Zealand's northern offshore islands. Biol. Conserv. 2002;107:19–35. doi:10.1016/S0006-3207(02)00039-3 [Google Scholar]
- Carpenter S.R. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology. 1996;77:677–680. [Google Scholar]
- Christian J.M, Wilson S.D. Long-term ecosystem impacts of an introduced grass in the northern Great Plains. Ecology. 1999;80:2397–2407. [Google Scholar]
- Cleveland C.C, Townsend A.R, Schmidt S.K. Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems. 2002;5:680–691. [Google Scholar]
- Courchamp F, Chapuis J.-L, Pascal M. Mammal invaders on islands: impact, control and control impact. Biol. Rev. 2003;78:347–383. doi: 10.1017/s1464793102006061. doi:10.1017/S1464793102006061 [DOI] [PubMed] [Google Scholar]
- Cowles H.C. The ecological relations of the vegetation on the sand dunes of Lake Michigan. Bot. Gaz. 1899;27:95–117. doi:10.1086/327796 (See also pp. 167–202, 281–308, 361–369.) [Google Scholar]
- Crocker R.L, Major J. Soil development in relation to vegetation and surface age at Glacier Bay. Alaska J. Ecol. 1955;43:427–448. [Google Scholar]
- Croll D.A, Maron J.L, Estes J.A, Danner E.M, Boyd G.V. Introduced predators transform subarctic islands from grasslands to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. doi:10.1126/science.1108485 [DOI] [PubMed] [Google Scholar]
- DeLuca T.H, Nilsson M.-C, Zackrisson O. Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia. 2002;133:206–214. doi: 10.1007/s00442-002-1025-2. doi:10.1007/s00442-002-1025-2 [DOI] [PubMed] [Google Scholar]
- Denslow J.S. Patterns of plant species diversity during succession under different disturbance regimes. Oecologia. 1980;46:18–21. doi: 10.1007/BF00346960. doi:10.1007/BF00346960 [DOI] [PubMed] [Google Scholar]
- Diamond J. Overview: laboratory experiments, field experiments, and natural experiments. In: Diamond J, Case T.J, editors. Community ecology. Harper & Row; New York: 1986. pp. 3–22. [Google Scholar]
- Díaz S, Grime J.P, Harris J, McPherson E. Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide. Nature. 1993;364:616–617. doi:10.1038/364616a0 [Google Scholar]
- Díaz S, Chapin F.S, III, Symstad A, Wardle D.A, Huennecke L. Functional diversity revealed through removal experiments. Trends Ecol. Evol. 2003;18:140–146. doi:10.1016/S0169-5347(03)00007-7 [Google Scholar]
- Drake J.A, Huxel G.R, Hewitt C.L. Microcosms as models for generating and testing community theory. Ecology. 1996;77:670–677. [Google Scholar]
- Duncan R.P, Williams P.A. Darwin's naturalization hypothesis challenged. Nature. 2002;417:608–609. doi: 10.1038/417608a. doi:10.1038/417608a [DOI] [PubMed] [Google Scholar]
- Dunne J.A, Saleska S.R, Fischer M.L, Harte J. Integrating experimental and gradient methods in ecological climate change research. Ecology. 2004;85:904–916. [Google Scholar]
- Enquist B.J, Niklas K.J. Invariant scaling relationships across tree-dominated communities. Nature. 2001;410:685–690. doi: 10.1038/35070500. doi:10.1038/35070500 [DOI] [PubMed] [Google Scholar]
- Fastie C.L. Causes and ecosystem consequences of multiple pathways of primary succession at Glacier Bay, Alaska. Ecology. 1995;76:1899–1916. [Google Scholar]
- Feeley K.J, Terborgh J.W. The effects of herbivore density on soil nutrients and tree growth in tropical forest fragments. Ecology. 2005;86:116–124. [Google Scholar]
- Fleming R.A. Statistical advantages in, and characteristics of, data from long-term research. Forestry Chronicle. 1999;75:487–489. [Google Scholar]
- Fukami T. Community assembly along a species pool gradient: implications for multiple-scale patterns of species diversity. Popul. Ecol. 2004;46:137–147. doi:10.1007/s10144-004-0182-z [Google Scholar]
- Fukami T, Morin P.J. Productivity–biodiversity relationships depend on the history of community assembly. Nature. 2003;424:423–426. doi: 10.1038/nature01785. doi:10.1038/nature01785 [DOI] [PubMed] [Google Scholar]
- Fukami T, Naeem S, Wardle D.A. On similarity among local communities in biodiversity experiments. Oikos. 2001;95:340–348. doi:10.1034/j.1600-0706.2001.950216.x [Google Scholar]
- Garnier E, et al. Ecology. Vol. 85. 2004. Plant functional markers capture ecosystem properties during secondary succession. [Google Scholar]
- Gholz H.L, Wedin D, Smitherman S, Harmon M.E, Parton W.J. Long term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biol. 2000;6:751–755. doi:10.1046/j.1365-2486.2000.00349.x [Google Scholar]
- Giardina C.P, Ryan M.G. Evidence that decomposition rates of organic matter in mineral soil do not vary with temperature. Nature. 2000;404:858–861. doi: 10.1038/35009076. doi:10.1038/35009076 [DOI] [PubMed] [Google Scholar]
- Gillespie R.G, Roderick G.K. Arthropods on islands: colonization, speciation, and conservation. Annu. Rev. Entomol. 2002;47:595–632. doi: 10.1146/annurev.ento.47.091201.145244. doi:10.1146/annurev.ento.47.091201.145244 [DOI] [PubMed] [Google Scholar]
- Gotelli N.J, Arnett A.E. Biogeographic effects of red ant invasion. Ecol. Lett. 2000;3:257–261. doi:10.1046/j.1461-0248.2000.00138.x [Google Scholar]
- Hastings A. Transients: the key to long-term ecological understanding? Trends Ecol. Evol. 2004;19:39–45. doi: 10.1016/j.tree.2003.09.007. doi:10.1016/j.tree.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Hayes G.F, Holl K.D. Cattle grazing impacts on annual forbs and vegetation composition of mesic grasslands in California. Conserv. Biol. 2003;17:1694–1702. doi:10.1111/j.1523-1739.2003.00281.x [Google Scholar]
- Hobbie J.E. Scientific accomplishments of the long-term ecological research program: an introduction. BioScience. 2003;53:17–20. [Google Scholar]
- Jenny H. McGraw-Hill; New York: 1941. Factors of soil formation. [Google Scholar]
- Jessup C.M, Kassen R, Forde S.E, Kerr B, Buckling A, Rainey P.B, Bohannan B.J.M. Big questions, small worlds: microbial model systems in ecology. Trends Ecol. Evol. 2004;19:189–197. doi: 10.1016/j.tree.2004.01.008. doi:10.1016/j.tree.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Jiang L, Morin P.J. Productivity gradients cause positive diversity–invasibility relationships in microbial communities. Ecol. Lett. 2004;7:1047–1057. doi:10.1111/j.1461-0248.2004.00660.x [Google Scholar]
- Johnson E.A, Miyanishi K, Kleb H. The hazards of interpretation of static age structures as shown by stand reconstructions in a Pinus contorta–Picea engelmannii forest. J. Ecol. 1994;82:923–931. [Google Scholar]
- Kitayama K. Comment on “ecosystem properties and forest declines in contrasting long-term chronosequences”. Science. 2005;308:633b. doi: 10.1126/science.308.5722.633a. doi:10.1126/science.1109537 [DOI] [PubMed] [Google Scholar]
- Klironomos J.N, Allen M.F, Rillig M.C, Piotrowski J, Makvandi-Nejad S, Wolfe B.E, Powell J.R. Abrupt rise in atmospheric CO2 overestimates community response in a model plant–soil system. Nature. 2005;433:621–624. doi: 10.1038/nature03268. doi:10.1038/nature03268 [DOI] [PubMed] [Google Scholar]
- Körner C, Arnone J.A., III Response to elevated carbon dioxide in artificial tropical ecosystems. Science. 1992;257:1672–1675. doi: 10.1126/science.257.5077.1672. [DOI] [PubMed] [Google Scholar]
- Kratz T.K, Deegan L.A, Harmon M.E, Lauenroth W.K. Ecological variability in space and time: insights gained from the US LTER program. BioScience. 2003;53:57–67. [Google Scholar]
- Krauss J, Klein A.M, Steffan-Dewenter I, Tscharntke T. Effects of habitat area, isolation, and landscape diversity on plant species richness of calcareous grasslands. Biodiv. Conserv. 2004;13:1427–1439. doi:10.1023/B:BIOC.0000021323.18165.58 [Google Scholar]
- Larocque I, Bergeron Y, Campbell I.D, Bradshaw R.H.W. Vegetation changes through time on islands of Lake Duparquet, Abitibi, Canada. Can. J. For. Res. 2000;30:179–190. doi:10.1139/cjfr-30-2-179 [Google Scholar]
- Lambert T.D, Adler G.H, Riveros C.M, Lopez L, Ascanio R, Terborgh J. Rodents on tropical land-bridge islands. J. Zool. 2003;260:179–187. doi:10.1017/S0952836903003637 [Google Scholar]
- Lawton J.H, et al. Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forests. Nature. 1998;391:72–76. doi:10.1038/34166 [Google Scholar]
- Luo Y.Q, Reynolds J.F. Validity of extrapolating field CO2 experiments to predict carbon sequestration in natural ecosystems. Ecology. 1999;80:1568–1583. [Google Scholar]
- MacLean R.C, Bell G. Divergent evolution during an experimental adaptive radiation. Proc. R. Soc. B. 2003;270:1645–1650. doi: 10.1098/rspb.2003.2408. doi:10.1098/rspb.2003.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Tognetti R, Vaccari F.P, Lanini M, Kaligaric M, Miglietta F, Raschi A. Physiological and morphological responses of grassland species to elevated atmospheric CO2 concentrations in FACE-systems and natural CO2 springs. Funct. Plant Biol. 2004;31:181–194. doi: 10.1071/FP03140. doi:10.1071/FP03140 [DOI] [PubMed] [Google Scholar]
- Michener W.K. Quantitatively evaluating restoration experiments: research design, statistical analysis, and data management considerations. Restor. Ecol. 1997;5:324–337. doi:10.1046/j.1526-100X.1997.00546.x [Google Scholar]
- Michener W.K, Blood E.R, Bildstein K.L, Brinson M.M, Gardner L.R. Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol. Appl. 1997;7:770–801. [Google Scholar]
- Miglietta F, Raschi A. Studying the effects of elevated CO2 in the open in a naturally enriched environment in Central Italy. Vegetatio. 1993;104/105:391–400. doi:10.1007/BF00048168 [Google Scholar]
- Mitchell H.F, Chandler R.F. Black Rock Forest Bulletin No. 11. Cornwall Press; Cornwall: 1939. The nitrogen nutrition and growth of certain deciduous trees of the northeastern United States. [Google Scholar]
- Molnar Z, Botta-Dukat Z. Improved space-for-time substitution for hypothesis generation: secondary grasslands with documented site history in SE-Hungary. Phytocoenologia. 1998;28:1–29. [Google Scholar]
- Moore T.R, et al. Litter decomposition rates in Canadian forests. Global Change Biol. 1999;5:75–82. doi:10.1046/j.1365-2486.1998.00224.x [Google Scholar]
- Naeem S. Power behind diversity's throne. Nature. 1999;401:653–654. doi:10.1038/44297 [Google Scholar]
- Nakatsubo T, Uchida M, Horikoshi T, Nakane K. Comparative study of the mass loss rate of moss litter in boreal and subalpine forests in relation to temperature. Ecol. Res. 1997;12:47–54. [Google Scholar]
- Newton P.C.D, Clark H, Edwards G.R, Ross D.J. Experimental confirmation of ecosystem model predictions comparing transient and equilibrium plant responses to elevated atmospheric CO2. Ecol. Lett. 2001;4:344–347. doi:10.1046/j.1461-0248.2001.00233.x [Google Scholar]
- Odum E.P. The strategy of ecosystem development. Science. 1969;164:262–270. doi: 10.1126/science.164.3877.262. [DOI] [PubMed] [Google Scholar]
- Oren R, et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature. 2001;411:469–472. doi: 10.1038/35078064. doi:10.1038/35078064 [DOI] [PubMed] [Google Scholar]
- Palmer M.A, Ambrose R.F, Poff N.L. Ecological theory and community restoration ecology. Restor. Ecol. 1997;5:291–300. doi:10.1046/j.1526-100X.1997.00543.x [Google Scholar]
- Pickett S.T.A. Space-for-time substitution as an alternative to long-term studies. In: Likens G.E, editor. Long-term studies in ecology: approaches and alternatives. Springer-Verlag; New York: 1989. pp. 110–135. [Google Scholar]
- Polis G.A, Hurd S.D. Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 1996;147:396–423. doi:10.1086/285858 [Google Scholar]
- Pugesek B, Tomer A, von Eye A, editors. Structural equation modeling: applications in ecological and evolutionary biology research. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Rainey P.B, Travisano M. Adaptive radiation in a heterogeneous environment. Nature. 1998;394:69–72. doi: 10.1038/27900. doi:10.1038/27900 [DOI] [PubMed] [Google Scholar]
- Raschi A, Miglietta F, Tognetti R, van Gardingen P, editors. Plant responses to elevated carbon dioxide: evidence from natural springs. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- Resetarits W.J, Bernardo J, editors. Experimental ecology: issues and perspectives. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- Ricciardi A, Atkinson S.K. Distinctiveness magnifies the impact of biological invaders in aquatic ecosystems. Ecol. Lett. 2004;7:781–784. doi:10.1111/j.1461-0248.2004.00642.x [Google Scholar]
- Richardson S.J, Peltzer D.A, Allen R.B, McGlone M.S. Resorption proficiency along a chronosequence: responses among communities and within species. Ecology. 2005;86:20–25. [Google Scholar]
- Rillig M.C, Hernandez G.Y, Newton P.C.D. Arbuscular mycorrhizae respond to elevated atmospheric CO2 after long term exposure: evidence from a CO2 spring in New Zealand supports the resource balance model. Ecol. Lett. 2000;3:475–478. doi:10.1046/j.1461-0248.2000.00178.x [Google Scholar]
- Ross D.J, Tate K.R, Newton P.C.D, Wilde R.H, Clark H. Carbon and nitrogen pools and mineralization in a grassland gley soil under elevated carbon dioxide at a natural CO2 spring. Global Change Biol. 2000;6:779–790. doi:10.1046/j.1365-2486.2000.00357.x [Google Scholar]
- Ross D.J, Tate K.R, Newton P.C.D, Clark H. Decomposability of C3 and C4 grass litter sampled under different concentrations of atmospheric carbon dioxide at a natural CO2 spring. Plant Soil. 2002;240:275–286. doi:10.1023/A:1015779431271 [Google Scholar]
- Ross D.J, Tate K.R, Newton P.C.D, Clark H. Carbon mineralization in an organic soil, with and without grass litter, from a high CO2 environment at a carbon dioxide spring. Soil Biol. Biochem. 2003;35:1705–1709. doi:10.1016/j.soilbio.2003.08.008 [Google Scholar]
- Sanders N.J, Gotelli N.J, Heller N.E, Gordon D.M. Community disassembly by an invasive species. Proc. Natl Acad. Sci. USA. 2003;100:2474–2477. doi: 10.1073/pnas.0437913100. doi:10.1073/pnas.0437913100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax D.F, Stachowicz J.J, Gaines S.D. Sinauer; Sunderland: 2005. Species invasions: insights into ecology, evolution, and biogeography. [Google Scholar]
- Schlesinger W.H, Lichter J. Limited carbon storage in soil and litter of experimental forest plots under elevated atmospheric CO2. Nature. 2001;411:466–469. doi: 10.1038/35078060. doi:10.1038/35078060 [DOI] [PubMed] [Google Scholar]
- Scheffer M, Carpenter S, Foley J.A, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. doi:10.1038/35098000 [DOI] [PubMed] [Google Scholar]
- Schoener T.W, Toft C.A. Spider populations: extraordinarily high densities on islands without top predators. Science. 1983;219:1353–1355. doi: 10.1126/science.219.4590.1353. [DOI] [PubMed] [Google Scholar]
- Smith S.A, Bell G, Bermingham E. Cross-Cordillera exchange mediated by the Panama Canal increased the species richness of local freshwater fish assemblages. Proc. R. Soc. B. 2004;271:1889–1896. doi: 10.1098/rspb.2004.2796. doi:10.1098/rspb.2004.2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan M, Cardinale B.J, Downing A.L, Engelhardt K.A.M, Ruesink J.L, Srivastava D.S. Extinction and ecosystem function in the marine benthos. Science. 2004;306:1177–1180. doi: 10.1126/science.1103960. doi:10.1126/science.1103960 [DOI] [PubMed] [Google Scholar]
- Srivastava D.S, et al. Are natural microcosms useful model systems for ecology? Trends Ecol. Evol. 2004;19:379–384. doi: 10.1016/j.tree.2004.04.010. doi:10.1016/j.tree.2004.04.010 [DOI] [PubMed] [Google Scholar]
- Stevens P.R, Walker T.W. The chronosequence concept and soil formation. Q. Rev. Biol. 1970;45:333–350. doi:10.1086/406646 [Google Scholar]
- Stohlgren T.J, et al. Exotic plant species invade hot spots of native plant diversity. Ecol. Monogr. 1999;69:25–46. [Google Scholar]
- Temperton V.M, Hobbs R.J, Nuttle T, Halle S, editors. Assembly rules and restoration ecology: bridging the gap between theory and practice. Island Press; Washington, DC: 2004. [Google Scholar]
- Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. doi:10.1126/science.1064397 [DOI] [PubMed] [Google Scholar]
- Tilman D. Ecological experimentation: strengths and conceptual problems. In: Likens G.E, editor. Long-term studies in ecology: approaches and alternatives. Springer-Verlag; New York: 1989. pp. 136–157. [Google Scholar]
- Tilman D, May R.M, Lehman C.L, Nowak M.A. Habitat destruction and the extinction debt. Nature. 1994;371:65–66. doi:10.1038/371065a0 [Google Scholar]
- Tilman D, Naeem S, Knops J, Reich P, Siemann E, Wedin D, Ritchie M, Lawton J.H. Biodiversity and ecosystem properties. Science. 1997;278:1866–1867. doi:10.1126/science.278.5345.1865c [Google Scholar]
- Troumbis A.Y, Memtsas D. Observational evidence that diversity may increase productivity in Mediterranean shrublands. Oecologia. 2000;125:101–108. doi: 10.1007/PL00008880. [DOI] [PubMed] [Google Scholar]
- Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C. Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol. Appl. 2002;12:354–363. [Google Scholar]
- Vázquez D.P, Simberloff D. Ecological specialization and susceptibility to disturbance: conjectures and refutations. Am. Nat. 2002;159:606–623. doi: 10.1086/339991. doi:10.1086/339991 [DOI] [PubMed] [Google Scholar]
- Vázquez D.P, Simberloff D. Indirect effects of an introduced ungulate on pollination and plant reproduction. Ecol. Monogr. 2004;74:281–308. [Google Scholar]
- Vitousek P.M. Nutrient cycling and limitation: Hawai'i as a model system. Princeton University Press; Princeton, NJ: 2004. [Google Scholar]
- Vitousek P.M, Matson P.A. Gradient analysis of ecosystems. In: Cole J.J, Lovett G, Findlay S, editors. Comparative analysis of ecosystems: patterns, mechanisms, and theories. Springer-Verlag; New York: 1991. pp. 287–298. [Google Scholar]
- Vitousek P.M, Walker L.R. Biological invasion by Myrica faya in Hawai'i: plant demography, nitrogen fixation, ecosystem effects. Ecol. Monogr. 1989;59:247–265. [Google Scholar]
- Vitousek P.M, Turner D.R, Parton W.J, Sanford R.L. Litter decomposition on the Mauna Loa environmental matrix, Hawai'i: patterns, mechanisms and models. Ecology. 1994;75:418–429. [Google Scholar]
- Walker L.R, del Moral R. Primary succession and ecosystem rehabilitation. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Wan S.Q, Hui D.F, Luo Y.Q. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: a meta-analysis. Ecol. Appl. 2001;11:1349–1365. [Google Scholar]
- Wardle D.A. Is “sampling effect” a problem for experiments investigating biodiversity–ecosystem function relationships? Oikos. 1999;87:403–407. [Google Scholar]
- Wardle D.A, Zackrisson O. Effects of species and functional group loss on island ecosystem properties. Nature. 2005;435:806–810. doi: 10.1038/nature03611. doi:10.1038/nature03611 [DOI] [PubMed] [Google Scholar]
- Wardle D.A, Zackrisson O, Hörnberg G, Gallet C. Influence of island area on ecosystem properties. Science. 1997;277:1296–1299. doi:10.1126/science.277.5330.1296 [Google Scholar]
- Wardle D.A, Zackrisson O, Hörnberg G, Gallet C. Biodiversity and ecosystem properties. Science. 1997;278:1867–1869. [Google Scholar]
- Wardle D.A, Hörnberg G, Zackrisson O, Kalela-Brundin M, Coomes D.A. Long term effects of wildfire on ecosystem properties across an island area gradient. Science. 2003;300:972–975. doi: 10.1126/science.1082709. doi:10.1126/science.1082709 [DOI] [PubMed] [Google Scholar]
- Wardle D.A, Yeates G.W, Barker G.M, Bellingham P.J, Bonner K.I, Williamson W. Island biology and ecosystem functioning in epiphytic soil communities. Science. 2003;301:1717–1720. doi: 10.1126/science.1087809. doi:10.1126/science.1087809 [DOI] [PubMed] [Google Scholar]
- Wardle D.A, Walker L.R, Bardgett R.D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science. 2004;305:509–513. doi: 10.1126/science.1098778. doi:10.1126/science.1098778 [DOI] [PubMed] [Google Scholar]
- Wardle D.A, Walker L.R, Bardgett R.D. Response to comment on ecosystem properties and forest decline in contrasting long-term chronosequences. Science. 2005;308:633c. doi: 10.1126/science.1098778. doi:10.1126/science.1109723 [DOI] [PubMed] [Google Scholar]
- Warming E. Plantesamfund: Grundträk af den Ökologiska Plantegoegrafi. Philipsen; Copenhagen: 1895. [Google Scholar]
- Warren P.H, Law R, Weatherby A.J. Mapping the assembly of protist communities in microcosms. Ecology. 2003;84:1001–1011. [Google Scholar]
- Whittaker R.H. 2nd edn. MacMillan; New York: 1975. Communities and ecosystems. [Google Scholar]
- Whittaker R.H, Niering W.A. Vegetation of the Santa Catalina mountains, Arizona. II. A gradient analysis of the south slope. Ecology. 1965;46:429–452. [Google Scholar]
- Wiser S.K, Allen R.B, Clinton P.W, Platt K.H. Community structure and forest invasion by an exotic herb over 23 years. Ecology. 1998;79:2071–2081. [Google Scholar]
- Wootton J.T. Predicting direct and indirect effects—an integrated approach using experiments and path-analysis. Ecology. 1994;75:151–165. [Google Scholar]
- Yeates G.W, Hawke M.F, Rijkse W.C. Changes in soil fauna and soil conditions under Pinus radiata agroforestry regimes during a 25-year rotation. Biol. Fertil. Soil. 2000;31:391–406. doi:10.1007/s003749900186 [Google Scholar]
- Young T.P. Restoration ecology and conservation biology. Biol. Conserv. 2000;92:73–83. doi:10.1016/S0006-3207(99)00057-9 [Google Scholar]
- Young T.P, Petersen D.A, Clary J.J. The ecology of restoration: historical links, emerging issues and unexplored realms. Ecol. Lett. 2005;8:662–673. doi:10.1111/j.1461-0248.2005.00764.x [Google Scholar]
- Zackrisson O, DeLuca T.H, Nilsson M.-C, Sellstedt A, Berglund L.M. Nitrogen fixation increases with successional age in boreal forests. Ecology. 2004;85:3327–3334. [Google Scholar]
- Zavaleta E.S, Hulvey K.B. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science. 2004;306:1175–1177. doi: 10.1126/science.1102643. doi:10.1126/science.1102643 [DOI] [PubMed] [Google Scholar]