Abstract
Environmental energy availability can explain much of the spatial variation in species richness. Such species–energy relationships encompass a diverse range of forms, and there is intense debate concerning which of these predominate, and the factors promoting this diversity. Despite this there has been relatively little investigation of whether the form, and relative strength, of species–energy relationships varies with (i) the currency of energy availability that is used, and (ii) the ecological characteristics of the constituent species. Such investigations can, however, shed light on the causal mechanisms underlying species–energy relationships. We illustrate this using the British breeding avifauna. The strength of the species–energy relationship is dependent on the energy metric used, with species richness being more closely correlated with temperature than the Normalized Difference Vegetation Index, which is a strong correlate of net primary productivity. We find little evidence, however, for the thermoregulatory load hypothesis that high temperatures enable individuals to invest in growth and reproduction, rather than thermoregulation, increasing population sizes that buffer species from extinction. High levels of productive energy may also elevate population size, which is related to extinction risk by a negative decelerating function. Therefore, the rarest species should exhibit the strongest species–energy relationship. We find evidence to the contrary, together with little support for suggestions that high-energy availability elevates species richness by increasing the numbers of specialists or predators.
Keywords: NDVI, more individuals hypothesis (MIH), niche breadth, niche position, species–energy relationships, temperature
1. Introduction
Local and regional species richness vary by orders of magnitude across the globe (Gaston & Spicer 2004). Understanding the factors controlling this variation is one of ecology's most important challenges (Hutchinson 1959; Brown 1981; Rosenzweig 1995; Gaston 2000). There is now a growing consensus that much of the pattern can be explained by differences in environmental energy availability (Hawkins et al. 2003; Pimm & Brown 2004); such species–energy relationships have been described for a range of taxa, habitats and, when using latitude as a crude surrogate for energy, geological time periods (e.g. Currie 1991; Roy et al. 1998; Crame 2001; Hawkins et al. 2003). These relationships exhibit a variety of forms, which has stimulated much debate regarding which predominate and the factors that give rise to this diversity (Waide et al. 1999; Mittelbach et al. 2001; Mittelbach, Scheiner & Steiner 2003; Whittaker & Heegaard 2003). Much of the debate has focused on the influence of spatial scale on the form of the species–energy relationship. Generally, studies conducted at small spatial grains, the unit of investigation, document unimodal species–energy relationships; studies that use larger grain sizes, scattered across one or more regions, find an increasing proportion of monotonic positive species–energy relationships. Although species–energy relationships are typically strong their relative strengths may vary considerably (see papers reviewed by Waide et al. 1999; Mittelbach et al. 2001), yet the causes of this variation are seldom discussed.
The form of species–energy relationships, and their underlying causal mechanisms, may depend on how energy availability is measured. Previous studies have used a number of currencies, which can be divided into two main categories (Evans et al. 2005c). First, solar energy metrics, such as temperature or ultra-violet radiation (UV), record the amount of solar energy falling upon the earth's surface. Broadly speaking, solar energy metrics may give rise to species–energy relationships through two pathways. According to the evolutionary rates hypothesis (Rohde 1978, 1992), high temperatures and/or UV may increase the mutation rate, leading to accelerated rates of evolution and speciation, thus high-energy areas may be species rich because more species evolved there. Alternatively, the thermoregulatory load hypothesis suggests that high temperature may enable endotherms to switch investment from keeping warm to growth and reproduction, thus promoting larger populations that are less vulnerable to extinction (Turner et al. 1988). Other things being equal, small bodied endotherm species are more vulnerable to heat loss (James 1970), thus the smallest endotherm species may exhibit the strongest species–energy relationships (Cousins 1989).
Second, productive-energy metrics record the amount of resources available for consumers to turn into biomass. This can be measured as net primary productivity, or its correlates, such as the Normalized Difference Vegetation Index (NDVI), which is a satellite derived measure of the greenness of vegetation (Boelman et al. 2003; Kerr & Ostrovosky, 2003). The most frequently cited pathway linking productive energy to species richness is the more individuals hypothesis (MIH); this suggests that in areas with high plant productivity consumers may be able to maintain larger populations that reduce their extinction risk, thus elevating species richness (Wright 1983).
Both the evolutionary rates and thermoregulatory load hypotheses predict that solar energy metrics will be a better predictor of species richness than productive energy metrics. A recent review found that this was the case at high northern latitudes, but that the converse was true in other areas (Hawkins et al. 2003). In contrast, Kaspari et al. (2004) found that solar energy was the superior predictor of ant species richness across a large latitudinal range that spanned the tropics. Relatively few studies have, however, explicitly investigated how the use of different energy metrics influences the shape of the species–energy relationship, or have constructed species–energy relationships that simultaneously use solar and productive energy metrics as predictors (but see Diniz-Filho & Bini 2005).
Discussion of the diversity of forms and relative strengths of species–energy relationships has largely been framed in the context of entire assemblages. Consideration of how the identity and associated characteristics of the species contributing to species–energy relationships influence their form and strength has been relatively muted, despite the fact that this may provide important information on the underlying causal mechanisms. The one exception to this concerns comparisons of species–energy relationships in abundant and widespread species, relative to rarer and more localized ones (Jetz & Rahbek 2002; Ruggiero & Kitzberger 2004; Vázquez & Gaston 2004; Evans et al. 2005c). Species vary, however, in a number of other traits which, depending on the causal mechanisms that drive species–energy relationships, may influence how species richness responds to energy availability (Evans et al. 2005b,c).
First, in areas with high plant productivity resources may be sufficiently abundant for species to specialize on a few resource types, generating narrower niche breadths that promote coexistence and elevate species richness (see Vázquez & Stevens 2004; Evans et al. 2005b,c). Similarly, high-energy areas may be the only ones that contain relatively scarce resources in sufficient abundance to maintain viable populations of the niche position specialists (sensu Shugart & Patten 1972) that use them (Abrams 1995; Evans et al. 2005b,c). Specialist species, defined in terms of narrow niche breadths and use of scarce resources, may therefore respond more strongly to energy availability than less specialized ones. Second, the transfer of energy between trophic levels is inefficient and thus the number of trophic levels may be regulated by the amount of energy at the base of the food chain (Oksanen et al. 1981; Fretwell 1987; but see Post 2002). The longer food chains in highly productive areas may thus enable greater numbers of predatory species to occur. Species–energy relationships, constructed for different trophic levels, may thus vary in their form and strength. Third, migratory species may be able to exploit seasonal flushes in resource availability more fully than residents and thus exhibit stronger species–energy relationships (Rabenold 1979, 1993). Fourth, species–energy relationships comprising taxa that have undergone marked population declines or range contractions in response to human activities, or those that occupy habitats markedly altered by humans, may also differ in their form relative to species that are comparatively uninfluenced by anthropogenic factors (Gaston 2004; Evans & Gaston 2005). More generally, the inheritance of ecological traits through the sharing of common ancestors may introduce taxonomic bias into the form of species–energy relationships.
Here, we provide one of the first comprehensive assessments of how the form and relative strength of the species–energy relationship depends on the type of energy metric used and the characteristics of the constituent species. We use the breeding avifauna of Britain as a case study. We construct species–energy relationships, using annual and seasonal measures of solar and productive energy, across the entire assemblage and groups of species classified by specialization, trophic level, population size, body size, habitat type, threat status (based on population trends), migratory status and taxonomy.
2. Material and Methods
(a) Avian data
We used the summer (breeding) distribution of the British avifauna recorded in April–July 1988–1991, shown in the second BTO/SOC/IW atlas of breeding birds (Gibbons et al. 1993). These data record species presence/absence at a resolution of 10 km×10 km quadrats on a continuous grid. Fieldwork was coordinated by a network of regional organisers and undertaken by experienced volunteer ornithologists. Data are based on timed visits, of 2 h duration, to at least eight 2 km×2 km quadrats within each 10 km quadrat and supplemented with additional records collated over the four survey years. For most quadrats very few species are likely to have been unrecorded and we thus consider our data to be free of significant concerns regarding under-sampling. These constitute one of the best sets of distributional data for any assemblage and have been successfully used in numerous macroecological studies (e.g. Turner et al. 1988; Gaston et al. 1997; Lennon et al. 2000; Donald & Greenwood 2001). We excluded marine species and vagrants (species recorded as a few individuals typically in only one or two quadrats), but retained the more naturalized introductions, giving a total of 189 species. Some initial filtering was performed on the distributional data; 10 km quadrats (100 km2) that contained less than 50% land were excluded, leaving a total of 2262 quadrats.
Data on breeding population size and body mass were taken from the compilation in Gaston & Blackburn (2000), with additional data, for Columba livia, from Greenwood et al. (1996), and species were grouped into quartiles of population abundance and body mass. Niche breadth and niche position data were derived, for 85 species, from a canonical correspondence analysis based on avian abundance data and environmental variables (Gregory & Gaston 2000). We divided species into those with niche positions below the median, which use relatively common resources (e.g. Parus caerulus) and those with high niche positions that use relatively scarce resources (e.g. Carduelis spinus). Species were classified into two groups of high and low niche breadths in the same manner.
Migratory status was categorized in two ways. First, species were categorized as long-distance migrants if most of their breeding populations wintered outside Britain, in sub-Saharan Africa for most species, with others classed as long-distance residents. Second, species were classed as short-distance migrants if most of their breeding population wintered in an area different to that in which they bred, even if they remained in Britain, with the remainder being classified as short-distance residents. Thus species, such as Falco columbarius, that breed on moorland and winter on the coast were classified as residents under the first definition, but migrants according to the second definition.
Data on the major food items in each species' diet were obtained from Cramp et al. (1977–1994) and species were classified as herbivores, omnivores, invertebrate predators and vertebrate predators, with 32, 50, 79 and 28 species in each of these respective categories. For a small number of species (for example some grouse, buntings and finches), adults are herbivores whilst chicks feed on invertebrates for the first few days of their life; these species were classified on the basis of both their adult and chick diets, i.e. as omnivores rather than herbivores, but classifying them as the latter does not markedly change our results.
Species were classified by their main habitat type (farmland, woodland, and other) according to the categorization provided in Gibbons et al. (1993), with 28, 49 and 112 species in the three habitat types respectively. This classification separates habitats by the extent to which they have been modified in recent decades. Avian population trend data indicate extensive modification of farmland; intermediate levels, on average, of habitat alteration in woodlands (with many species exhibiting stable or increasing population trends, but some having declining population trends); and relatively little modification elsewhere (Crick et al. 2004). Finally, species were classified by their threat status based upon Gregory et al. (2002). This uses a number of criteria, including population or range declines, extent of European conservation concern for the species, and the extent to which populations are concentrated into a few sites, to list species as red (threatened), amber (moderately threatened) and green (unthreatened). We only used information relating to the magnitude of historic and recent population declines, and range contractions, in assigning species to threat status. Therefore, species that are naturally rare but are listed as moderately threatened on the basis of European conservation concern, such as Alcedo atthis, were considered to be unthreatened for the purposes of this analysis.
(b) Energy metrics
We used two measures of energy availability in our analyses. First, we used mean monthly temperature data that were derived from meteorological recording station readings for the period 1961-90 using surface interpolation techniques (Barrow et al. 1993). Second, we obtained NDVI data from the NOAA/NASA Pathfinder AVHRR Land Data Set (see http://www.ciesin.org/). Note that NDVI and net primary productivity have been found to be strongly positively correlated at latitudes and habitat types similar to those that occur in Britain (Boelman et al. 2003; Kerr & Ostrovosky 2003). The NDVI data were collected between 1981 and 2001 at a spatial resolution of a 0.1° latitude/longitude grid, approximately equivalent to an 8 km quadrat (64 km2) in the UK. Daily readings are converted to maximum values for each 10-day period, which markedly reduces the effects of cloud cover. From these we calculated mean monthly NDVI values and then used GIS to re-project these data at a 10 km resolution which was compatible with our avian distribution data. For both temperature and NDVI we calculated a mean annual measure of energy availability and a mean summer value calculated from the monthly averages for May, June and July.
(c) Analyses
All analyses were conducted in SAS (Version 8.2). Spatial autocorrelation may invalidate the assumption of independent errors, distorting classical tests of association and rendering correlation coefficients, regression slopes and associated significance tests misleading (Cressie 1991; Legendre 1993; Lennon 2000; Legendre et al. 2002). To avoid this, analyses were conducted using the PROC MIXED procedure to implement spatial correlation models that fit a spatial covariance matrix to the data and use this to adjust test statistics accordingly (Littell et al. 1996). Spatial null models, i.e. ones which lacked predictor variables, which assumed exponential spatial covariance structures fitted the data significantly better than independent error null models (in all cases likelihood ratio tests p<0.0001) and also gave a better fit than spatial models that specified alternative covariance structures (spherical, Gaussian, linear, linear log and power).
We constructed multiple regression models that included both temperature and NDVI, and their square terms, as predictors. We used one measure of temperature, and one of NDVI, selecting the seasonal measure that gave the best fit to our data in models confined to a single measure of energy (see table 1). A full set of models containing all possible combinations of our predictors (temperature, NDVI and their square terms) was constructed and we used Akaike's Information Criteria (AIC) to compare the fit of competing models (Akaike 1973). The use of AIC in ecological research is increasingly recommended (Burnham & Anderson 2001; Ginzburg & Jensen 2004, Johnson & Omland 2004). The AIC estimates the Kullback–Leibler information lost by approximating full reality with the fitted model; computation entails terms representing lack of fit and a bias correction factor related to model complexity. Following Johnson & Omland (2004), we calculated the difference between each model's AIC value and that of the best fitting model, the one with the smallest AIC, and used these data to calculate the weight of each model, the probability that it provides the best fit to the data. In order to investigate the influence of taking spatial autocorrelation into account we also conducted analyses that assumed independent errors by constructing general linear models (GLMs) using a stepwise selection procedure, with p<0.05 being adopted as the threshold for retaining a term in the minimum adequate model (see Electronic Appendix).
Table 1.
Akaike Information Criteria (AIC) values for regressions of species richness that use a single seasonal measure of either temperature or the Normalized Difference Vegetation Index (NDVI).
| response | summer temperature | summer temperature & summer temperature2 | annual temperature | annual temperature & annual temperature2 | r2 best temperature model (%) | summer NDVI | summer NDVI & summer NDVI2 | annual NDVI | annual NDVI & annual NDVI2 | r2 best NDVI model (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| all species | 17522.1 | 17396.3* | 17545.3 | 17445.6 | 24.8 | 17686.3 | 17683.4 | 17684.2 | 17673.2 | 14.9 |
| non-passerines | 15329.2 | 15285.6* | 15340.5 | 15300.5 | 8.2 | 15435.6 | 15433.9 | 15434.9 | 15422.3 | 9.5 |
| passerines | 14382.1 | 14185.3* | 14409.7 | 14253.8 | 37.8 | 14528.5 | 14530.5 | 14526.7 | 14523.6 | 22.4 |
| long distance resident | 16364.8 | 16227.8* | 16379.3 | 16275.9 | 22.1 | 16514.6 | 16508.3 | 16513.1 | 16495.6 | 14.9 |
| long distance migrant | 11888.0 | 11834.0* | 11930.5 | 11878.7 | 26.4 | 12022.7 | 12031.4 | 12022.9 | 12029.4 | 13.3 |
| partial resident | 15776.0 | 15650.9* | 15809.7 | 15720.0 | 40.3 | 16028.5 | 16026.4 | 16025.5 | 16016.7 | 18.5 |
| partial migrant | 13151.1 | 13065.3* | 13155.5 | 13069.8 | 3.2 | 13165.2 | 13169.0 | 13162.1 | 13156.7 | 4.4 |
| red list | 9944.3 | 9859.9* | 10011.7 | 9929.0 | 47.9 | 10146.3 | 10153.4 | 10146.2 | 10149.7 | 6.9 |
| amber list | 11534.7 | 11418.1* | 11535.9 | 11423.9 | 8.5 | 11531.0 | 11526.7 | 11529.6 | 11513.2 | 6.7 |
| green list | 15654.2 | 15554.5* | 15678.4 | 15610.6 | 29.5 | 15867.2 | 15868.5 | 15863.6 | 15858.8 | 14.3 |
| 1st population quartile | 12711.3 | 12443.5* | 12740.8 | 12532.5 | 56.0 | 13077.0 | 13080.5 | 13074.5 | 13071.1 | 27.8 |
| 2nd population quartile | 13497.9 | 13369.3* | 13509.8 | 13403.1 | 14.6 | 13538.3 | 13545.3 | 13538.9 | 13536.9 | 11.3 |
| 3rd population quartile | 12870.5* | 12873.8 | 12878.5 | 12879.9 | 3.5 | 12911.0 | 12904.2 | 12911.1 | 12902.0 | 4.8 |
| 4th population quartile | 8405.0 | 8410.5 | 8403.5 | 8406.9 | 0.6 | 8378.5* | 8383.4 | 8392.1 | 8397.6 | 7.4 |
| 1st bodymass quartile | 12775.2 | 12657.1* | 12811.2 | 12717.5 | 36.8 | 12949.7 | 12952.1 | 12945.4 | 12944.9 | 17.3 |
| 2nd bodymass quartile | 11231.1 | 11096.5* | 11248.6 | 11143.2 | 16.8 | 11292.2 | 11295.0 | 11295.2 | 11286.5 | 11.9 |
| 3rd bodymass quartile | 12070.1 | 12031.6* | 12116.9 | 12079.1 | 35.2 | 12250.9 | 12257.7 | 12250.1 | 12252.5 | 4.2 |
| 4th bodymass quartile | 11776.0 | 11701.7* | 11760.5 | 11709.6 | 9.6 | 11790.4 | 11789.5 | 11788.4 | 11780.8 | 2.2 |
| farmland | 10064.9 | 10007.9* | 10114.5 | 10058.3 | 69.2 | 10447.3 | 10457.7 | 10444.2 | 10453.7 | 8.6 |
| woodland | 13405.5 | 13232.8* | 13442.7 | 13273.8 | 34.3 | 13495.8 | 13499.7 | 13492.9 | 13489.4 | 21.3 |
| other habitats | 15594.2 | 15543.5* | 15582.3 | 15547.5 | 7.2 | 15605.0 | 15604.2 | 15606.3 | 15600.5 | 2.8 |
| niche breadth (broad) | 12570.2 | 12483.4* | 12631.8 | 12559.0 | 76.0 | 13180.1 | 13189.1 | 13176.3 | 13181.7 | 13.7 |
| niche breadth (narrow) | 12791.7 | 12553.4* | 12783.9 | 12614.1 | 23.4 | 12793.7 | 12796.0 | 12784.2 | 12776.1 | 6.7 |
| niche position (low) | 12145.7 | 11924.7* | 12179.7 | 12014.9 | 56.4 | 12542.6 | 12547.0 | 12534.0 | 12535.4 | 20.3 |
| niche position (high) | 12876.4 | 12744.8* | 12909.6 | 12818.3 | 38.2 | 13091.7 | 13097.9 | 13099.9 | 13097.8 | 18.5 |
| herbivores | 8599.8 | 8556.7* | 8670.5 | 8635.9 | 46.7 | 8879.9 | 8886.5 | 8877.0 | 8879.0 | 4.4 |
| omnivores | 12550.2 | 12460.3* | 12605.8 | 12525.2 | 44.4 | 12771.8 | 12770.0 | 12772.9 | 12765.2 | 16.8 |
| predators—inverts | 14396.1 | 14281.2* | 14395.9 | 14312.6 | 13.9 | 14494.7 | 14499.2 | 14491.8 | 14489.6 | 8.8 |
| predators—verts | 10453.2 | 10381.4* | 10453.4 | 10392.6 | 8.2 | 10456.3 | 10460.6 | 10450.0 | 10450.0 | 0.04 |
Summer energy metrics are calculated as the mean value during May, June and July. Smaller AIC values indicate a better model fit. Bold type indicates if summer or annual measures of temperature and NDVI gave the best fit to the data. The best overall single predictor of species richness is indicated by*. Explanatory power is indicated by the r2 values from general linear models.
3. Results and discussion
When predictors are confined to a single seasonal measure of either temperature or NDVI the former is a better predictor of species richness for all our species groups, except that comprising the rarest species (table 1). Species richness generally increases with temperature along a positive decelerating curve, although the rarest and moderately rare species exhibit positive linear relationships these have low explanatory power (tables 1 and 2, figure 1). The general form of the species–temperature relationship remains unchanged when annual, instead of summer, temperature is used as a predictor (table 1). These results confirm those of earlier work (Hawkins et al. 2003) that at high northern latitudes, such as Britain, temperature is a better predictor of species richness than metrics that combine temperature and water availability, such as measures of plant productivity.
Table 2.
Multiple regression models of species–energy relationships that take spatial autocorrelation into account.
| number of species | response | temperature | temperature2 | NDVI | NDVI2 | model weight | r2 (%) |
|---|---|---|---|---|---|---|---|
| 189 | all species | F1,2257=137.9++++ | F1,2257=111.9−−−− | F1,2257=19.7++++ | F1,2257=17.9−−−− | 0.987 | 30.4 |
| 107 | non-passerines | F1,2257=57.1++++ | F1,2257=45.2−−−− | F1,2257=18.1++++ | F1,2257=19.2−−−− | 0.946 | 16.0 |
| 82 | passerines | F1,2257=205.8++++ | F1,2257=170.3−−−− | F1,2257=13.4+++ | F1,2257=9.7−− | 0.562 | 43.1 |
| 82 | passerines | F1,2258=219.2++++ | F1,2258=20.9++++ | F1,2258=14.0−−− | 0.438 | 38.9 | |
| 141 | long distance resident | F1,2257=151.6++++ | F1,2257=126.3−−−− | F1,2257=26.3++++ | F1,2257=24.7−−−− | 0.999 | 28.4 |
| 48 | long distance migrant | F1,2258=60.8++++ | F1,2258=45.1−−−− | F1,2258=9.6++ | 0.839 | 29.2 | |
| 116 | partial resident | F1,2257=144.9++++ | F1,2257=110.5−−−− | F1,2257=24.8++++ | F1,2257=21.9−−−− | 0.998 | 45.6 |
| 73 | partial migrant | F1,2259=98.3++++ | F1,2259=91.1−−−− | 0.846 | 3.2 | ||
| 35 | red list | F1,2257=95.0++++ | F1,2257=67.6−−−− | F1,2257=20.4++++ | F1,2257=17.8−−−− | 0.896 | 53.0 |
| 35 | amber list | F1,2257=110.6++++ | F1,2257=169.7−−−− | F1,2257=23.2++++ | F1,2257=22.7−−−− | 0.953 | 15.1 |
| 119 | green list | F1,2257=115.3++++ | F1,2257=89.6−−−− | F1,2257=14.9+++ | F1,2257=13.3−−− | 0.857 | 33.2 |
| 47 | 1st population quartile | F1,2257=336.0++++ | F1,2257=262.4−−−− | F1,2257=16.8++++ | F1,2257=12.6−−− | 0.760 | 62.2 |
| 47 | 1st population quartile | F1,2258=340.7++++ | F1,2258=265.7−−−− | F1,2258=26.1++++ | 0.241 | 58.2 | |
| 47 | 2nd population quartile | F1,2258=132.9++++ | F1,2258=118.8−−−− | F1,2258=5.6+ | 0.472 | 15.4 | |
| 47 | 2nd population quartile | F1,2259=156.8++++ | F1,2259=139.0−−−− | 0.316 | 14.6 | ||
| 47 | 2nd population quartile | F1,2257=130.1++++ | F1,2257=116.5−−−− | F1,2257=9.4+++ | F1,2257=8.0−− | 0.212 | 18.4 |
| 47 | 3rd population quartile | F1,2258=39.9++++ | F1,2258=18.7+++ | F1,2258=19.9−−− | 0.782 | 8.3 | |
| 48 | 4th population quartile | F1,2259=4.9+ | F1,2259=37.0−−−− | 0.391 | 7.4 | ||
| 48 | 4th population quartile | F1,2257=45.5++++ | 0.290 | 0.7 | |||
| 48 | 4th population quartile | F1,2258=6.2+ | F1,2258=4.8− | F1,2258=39.2−−−− | 0.237 | 7.6 | |
| 47 | 1st mass quartile | F1,2258=127.2++++ | F1,2258=98.5−−−− | F1,2258=24.4++++ | 0.599 | 39.3 | |
| 47 | 1st mass quartile | F1,2257=123.2++++ | F1,2257=95.1−−−− | F1,2257=12.3+++ | F1,2257=9.0−− | 0.401 | 40.6 |
| 47 | 2nd mass quartile | F1,2259=165.1++++ | F1,2259=144.6−−−− | 0.746 | 16.8 | ||
| 48 | 3rd mass quartile | F1,2259=63.6++++ | F1,2259=42.72−−−− | 0.660 | 35.2 | ||
| 48 | 3rd mass quartile | F1,2257=53.3++++ | F1,2257=35.3−−−− | F1,2257=13.2+++ | F1,2257=12.4−−− | 0.231 | 42.5 |
| 47 | 4th mass quartile | F1,2259=86.6++++ | F1,2259=82.0−−−− | 0.508 | 9.6 | ||
| 47 | 4th mass quartile | F1,2257=84.1++++ | F1,2257=80.7−−−− | F1,2257=12.6+++ | F1,2257=13.8−−− | 0.416 | 72.7 |
| 28 | farmland | F1,2258=93.3++++ | F1,2258=52.1−−−− | F1,2258=9.3++ | 0.841 | 69.3 | |
| 49 | woodland | F1,2258=175.8++++ | F1,2258=145.7−−−− | F1,2258=33.9++++ | 0.525 | 39.0 | |
| 49 | woodland | F1,2257=168.3++++ | F1,2257=139.0−−−− | F1,2257=13.7+++ | F1,2257=9.4−− | 0.475 | 40.8 |
| 112 | other habitats | F1,2257=63.3++++ | F1,2257=60.7−−−− | F1,2257=9.8++ | F1,2257=11.5−−− | 0.757 | 10.8 |
| 43 | niche breadth (broad) | F1,2258=141.2++++ | F1,2258=76.3−−−− | F1,2258=20.0++++ | 0.954 | 76.7 | |
| 42 | niche breadth (narrow) | F1,2257=245.5++++ | F1,2257=244.9−−−− | F1,2257=15.9+++ | F1,2257=14.5−− | 0.743 | 27.2 |
| 43 | niche position (low) | F1,2258=288.8++++ | F1,2258=218.8−−−− | F1,2258=20.8++++ | 0.785 | 58.7 | |
| 43 | niche position (low) | F1,2257=284.8++++ | F1,2257=215.9−−−− | F1,2257=10.7++++ | F1,2257=7.7−−−− | 0.214 | 62.3 |
| 42 | niche position (high) | F1,2258=148.0++++ | F1,2258=117.1−−−− | F1,2258=15.5++++ | 0.869 | 40.4 | |
| 16 | herbivores | F1,2257=64.7++++ | F1,2257=40.8−−−− | F1,2257=27.5++++ | F1,2257=26.7−−−− | 0.958 | 52.1 |
| 64 | Omnivores | F1,2257=102.7++++ | F1,2257=73.3−−−− | F1,2257=27.4++++ | F1,2257=24.2−−−− | 0.998 | 49.7 |
| 81 | Predators—inverts | F1,2259=148.4++++ | F1,2259=126.4−−−− | 0.770 | 13.9 | ||
| 28 | Predators—verts | F1,2259=76.0++++ | F1,2259=77.9−−−− | 0.968 | 8.2 |
Model fit was assessed using the Akaike Information Criteria (AIC), smaller values indicate a better fit. The model weight is the probability that the model provides the best fit to the data; we present all models with weights greater than 0.2. Explanatory power is indicated by the r2 values from general linear models. Models use either summer or annual measures of temperature and NDVI according to which measure provided the best fit to the data in tests that used a single energy metric as a predictor (see table 1). ++++p<0.0001, +++p<0.001, ++p<0.01, +p<0.05; Negative effects −−−−p<0.0001, −−−p<0.001, −−p< 0.01, − p<0.05.
Figure 1.
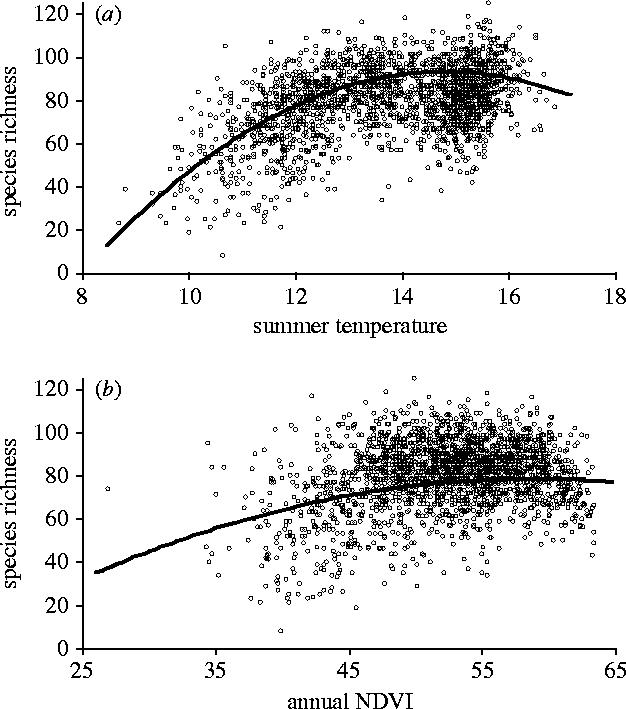
Relationships between species richness and (a) temperature (b) NDVI across the entire assemblage of British breeding birds. Open circles represent raw data and lines represent the predicted relationship from models that take into account spatial autocorrelation and use a single measure of energy, the seasonal measure that gives the best fit to the data (see table 1).
Kaspari et al. (2004) also found that the species richness of ant assemblages was more closely related to temperature than to plant productivity and suggested that such a pattern provided support for Rohde's (1978, 1992) evolutionary rates hypothesis. This states that higher levels of solar radiation increase mutation rates, promoting faster molecular evolution and greater speciation, so more species occur in high-energy areas because more evolve there. The British avifauna contains one endemic bird species, Loxia scotica, although its taxonomic status is debated (Summers et al. 2002). We are not aware of any other suggestions that species of birds breeding in Britain evolved there. Indeed, this seems highly unlikely. First, Britain has been subject to frequent glaciation periods during which most species would have been displaced further south. Moreover, speciation in Britain could only have occurred during the interglacial periods and these were typically much shorter, approximately 25,000 years (Adams et al. 1999), than the time typically required for avian speciation, which has been estimated at between 250,000 and two million years (Avise et al. 1998; Johnson & Cicero 2004). Second, most species breeding in Britain have large geographic ranges covering Europe, and often parts of Asia and/or Africa (Gregory & Blackburn 1998); it would be rather remarkable if the majority of these species evolved in the same small area of their distribution. In addition, established exotic avian species in Britain exhibit a strong–species energy relationship (Evans et al. 2005d), which cannot arise through a relationship between energy and speciation rates, suggesting that other mechanisms must play a role. It thus seems highly unlikely that the evolutionary rates mechanism can explain the species–energy relationship in British birds.
The thermoregulatory load hypothesis (Turner et al. 1988) also predicts that species richness will respond more strongly to temperature than NDVI. Limited support for this hypothesis is provided by the fact that breeding species richness responds to summer, rather than annual, temperature (table 1); these two variables are, however, strongly correlated (r2=92%). Small bodied species are more vulnerable to heat loss than larger ones (James 1970), thus the thermoregulatory load hypothesis predicts that smaller bodied species will exhibit the strongest species–temperature relationships, as is the case in our data (table 1). The strength of the species–temperature relationship does not, however, decline consistently across species grouped into quartiles of increasing mass. Therefore, whilst our data are partly consistent with the thermoregulatory load hypothesis they do not provide conclusive support for it. Lennon et al. (2000) found that seasonal changes in temperature gradients across Britain are not reflected in seasonal changes in gradients of species richness, and also concluded that the thermoregulatory load hypothesis does not generate species–energy relationships in the avifauna.
Given the lack of support for causal pathways that link species richness to temperature it is unclear why the latter is a better predictor of species richness than NDVI. One possibility is that in highly modified regions such as Britain, which are dominated by intensive agriculture, NDVI is an imperfect measure of the amount of plant productivity that is available to free-living consumers. It has been estimated that in developed countries the proportion of net primary productivity that is acquired by humans (HANPP) may reach 50% (Haberl et al. 2002; Imhoff et al. 2004). If this proportion varies spatially then this may disrupt the apparent relationship between productive energy and species richness. Unfortunately, data on spatial variation in HANPP across Britain are not available and thus this hypothesis cannot yet be tested. Whilst temperature is a better predictor of species richness than NDVI in models restricted to a single measure of energy availability, it is important to note that temperature and NDVI are correlated (in GLMs summer NDVI=−132.79+28.49 summer temp−1.03 summer temp2, r2=31.6%; annual NDVI=−20.96+7.10 annual temp−0.39 annual temp2, r2=10.2%). Moreover, NDVI measures are retained in most of the best fitting multiple spatial regression models (table 2), the exceptions being models of predator species richness and those of all but the smallest species, and all their non-spatial equivalents (Electronic Appendix). This indicates that species richness does respond to productive energy availability. This comparison is also compatible with the suggestion that GLS is more sensitive to correlation between predictor variables than OLS (Diniz-Filho et al. 2003), but a full investigation of this issue is beyond the scope of this paper.
For most groups of the British avifauna, species richness is related to NDVI along a positive decelerating curve (tables 1 and 2, figure 1). However, one third of species groups (10 out of 29) exhibit a positive linear species–NDVI relationship (tables 1 and 2). Similarly, in 11 of our species groups, assessment of whether linear models provide a better fit to the data than ones containing a square term depends on whether annual or summer NDVI measures are used (table 1). The form of the species–energy relationship thus seems to be more sensitive to the seasonal nature of NDVI measures than those of temperature; this is expected given the closer correlation between annual and summer temperature (r2=92%) than between summer and annual NDVI (r2=73%).
Across the entire assemblage the species–energy relationship was moderately strong (r2=30%); however, our species groups exhibited much variation in the strength of their species–energy relationships, with pseudo r2 values ranging from less than 5% to over 75%, and this variation is not related to differences in the number of species comprising each grouping (table 2). Species–energy relationships were much stronger in passerines than non-passerine orders (tables 1 and 2). The latter comprise a range of evolutionary groups of varying levels of relatedness. Other studies have reported strong species–energy relationships in non-passerines, such as in South American owls (Diniz-Filho et al. 2004) and established exotic birds in Britain (Evans et al. 2005d), and a link between taxonomy and the strength of species–energy relationships is unlikely to be simple. The difference that we find does, however, suggest that biases in the distribution of ecological traits between taxonomic groups may result in the latter exhibiting species–energy relationships of varying strength. The dataset that we analyse, however, contains insufficient species to justify analyses conducted on smaller taxonomic groups, such as families.
Long distance migrants and residents exhibited species–energy relationships of similar strengths. In contrast to most species groups, the species richness of long distance migrants was more closely related to summer rather than annual NDVI (table 1), and whilst the difference in fit is relatively small this is compatible with Rabenold's (1979, 1993) suggestion that long-distance migrants may be able to respond better to seasonal flushes of productive energy than long-distance residents. A similar trend was not, however, apparent in short-distance migrants (table 1), a group which had a weak species–energy relationship (table 2). This weak relationship may be, in part, because this group comprised many species that breed in high altitude moorland regions with low temperatures and plant productivity.
Species with relatively broad niches exhibited stronger species–energy relationships than those with narrower ones (tables 1 and 2). There was a similar trend, albeit less noticeable, for species that used more widespread resources, i.e. have low niche positions, to have stronger species–energy relationships than those that used relatively scarce resources (tables 1 and 2). Although we lack niche breadth and position data for many of the species that we consider, our data incorporate a wide range of values and it appears unlikely that the conclusions are influenced by data availability. These patterns are not consistent with suggestions that high-energy areas contain more species because increased energy availability promotes the occurrence of viable populations of specialized species (Abrams 1995; also see Vázquez & Stevens 2004; Evans et al. 2005c).
Trophic position exerts a marked influence on the strength of the species–energy relationship, with herbivores and omnivores exhibiting similar relationships to each other that were much stronger than those of invertebrate and vertebrate predators (tables 1 and 2). These patterns are not those predicted by the suggestion that high energy levels promote species richness by increasing the number of trophic levels in an assemblage (Oksanen et al. 1981; Fretwell 1987).
Species groups that have undergone marked population declines (red-listed species) or occupy highly modified habitats (farmland species) exhibit species–energy relationships that are as strong as those with stable population trends (green listed species) or those occupying moderately modified habitats (such as woodland). However, species characteristic of other even less modified habitats, or that have experienced moderate population declines, have much weaker species–energy relationships. These findings suggest that there is no simple correlation between habitat modification/population trends and the strength of the species–energy relationship.
Species–energy relationships were strongest in the commonest species and their strength declined consistently across quartiles of species abundances. This confirms the results of previous work, which used temperature in isolation as a measure of energy availability, reporting stronger species–energy relationships in the most abundant species (Evans et al. 2005c). Generally, there is a negative correlation between body-size and population size (Damuth 1981), and such a pattern has been demonstrated in British birds (Blackburn et al. 1996); this may contribute to the finding that the smaller bodied species exhibit the strongest species–energy relationship. Similarly, British bird species with a low niche position, but not those with a broad niche breadth, tend to be common (Gregory & Gaston 2000); and, although this correlation is noisy, this may partly explain why species with a low niche position exhibit the strongest species–energy relationships.
The more individuals hypothesis (MIH; Wright 1983) is one of the most frequently cited explanations for species–energy relationships. It states that high-energy availability increases resource abundance enabling species to maintain larger populations, which are thus buffered from extinction, consequentially promoting species richness. Extinction risk is linked to population size by a negative decelerating function (Lande 1993), thus the MIH predicts that the rarest species should exhibit the strongest species–energy relationship. Our data do not support this pattern, concurring with other work that questions the extent to which the MIH acts as a general and sole driver of species–energy relationships in British birds (Evans et al. 2005a,b), and more widely (Currie et al. 2004).
In summary, we present the first comprehensive analysis that dissects the species–energy relationship into its component parts, on the basis of the ecological and taxonomic characteristics of its constituent species, whilst using a range of energy metrics. Doing so enables us to test hypotheses relating to the poorly known underlying causal mechanisms of species–energy relationships (Currie et al. 2004; Evans et al. 2005b,c). Our data are not compatible with suggestions that high levels of energy availability increase species richness by increasing population sizes, or the numbers of predatory and specialist species. Other causal explanations of species–energy relationships have been proposed. The range limitation hypothesis suggests that more species may occur in high-energy areas, particularly warm ones, as more species are physiologically able to maintain viable populations in such conditions (Kerr et al. 1998; Evans et al. 2005b,c). In addition, the dynamic equilibrium hypothesis suggests that high levels of productive energy may enable species populations to recover more rapidly from disturbances which, depending on the frequency of disturbance events, may generate positive species–energy relationships (Huston 1979; Evans et al. 2005c). These alternative hypotheses would merit testing in future investigations of the mechanisms driving the species–energy relationship amongst British breeding birds.
Acknowledgements
We thank the thousands of volunteers who collected the ornithological data, which was supplied by the British Trust for Ornithology. G.P. and S.G.R. helped with the GIS data; R.A.F. commented on an earlier version of this manuscript. This work was supported by The Leverhulme Trust.
Supplementary Material
References
- Abrams P.A. Monotonic or unimodal diversity-productivity gradients, what does competition theory predict? Ecology. 1995;76:2019–2027. [Google Scholar]
- Adams J, Maslin M, Thomas E. Sudden climate transitions during the Quaternary. Prog. Phys. Geog. 1999;23:1–36. 10.1191/030913399670425018 [Google Scholar]
- Akaike H. Information theory as an extension of the maximum likelihood principle. In: Petrov B.N, Csaki F, editors. In second international symposium on information theory. Akademiai Kiado; Kiado: 1973. pp. 267–281. [Google Scholar]
- Avise J.C, Walker D, Johns G.C. Speciation durations and Pleistocene effects on vertebrate phylogeography. Proc. R. Soc. B. 1998;265:1707–1712. doi: 10.1098/rspb.1998.0492. 10.1098/rspb.1998.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow E, Hulme M, Jiang T. University of East Anglia Climatic Research Unit; Norwich: 1993. A 1961–1990 baseline climatology and future climatic change scenarios for Great Britain and Europe. Part 1: 1961–1990 Great Britain baseline climatology. [Google Scholar]
- Blackburn T.M, Lawton J.H, Gregory R.D. Relationships between abundances and life histories of British birds. J. Anim. Ecol. 1996;65:52–62. [Google Scholar]
- Boelman N.T, Stieglitz M, Rueth H.M, Sommerkorn M, Griffin K.L, Shaver G.R, Gamon J.A. Response of NDVI, biomass, and ecosystem gas exchange to long-term warming and fertilization in wet sedge tundra. Oecologia. 2003;135:414–421. doi: 10.1007/s00442-003-1198-3. [DOI] [PubMed] [Google Scholar]
- Brown J.H. Two decades of homage to Santa Rosalia: toward a general theory of diversity. Am. Zool. 1981;21:877–888. [Google Scholar]
- Burnham K.P, Anderson D.R. Kullback–Leibler information as a basis for strong inference in ecological studies. Wildl. Res. 2001;28:111–119. 10.1071/WR99107 [Google Scholar]
- Cousins S.H. Species richness and the energy theory. Nature. 1989;340:350–351. 10.1038/340350b0 [Google Scholar]
- Crame J.A. Taxonomic diversity gradients through geological time. Divers. Distrib. 2001;7:175–189. [Google Scholar]
- Cramp S, Simmons K.E.L, Perrins C.M. Handbook of the birds of Europe, the middle east and North Africa. vol. 1–9. Oxford University Press; 1977–1994. [Google Scholar]
- Cressie N. Statistics for spatial data. Wiley; New York: 1991. [Google Scholar]
- Crick, H. Q. P. et al 2004 Breeding Birds in the Wider Countryside: their conservation status 2003. BTO Research Report No. 353. BTO, Thetford (http://www.bto.org/birdtrends).
- Currie D.J. Energy and large-scale patterns of animal and plant species richness. Am. Nat. 1991;137:27–49. 10.1086/285144 [Google Scholar]
- Currie D.J, et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004;7:1121–1134. 10.1111/j.1461-0248.2004.00671.x [Google Scholar]
- Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. 10.1038/290699a0 [Google Scholar]
- Diniz-Filho J.A, Bini L.M. Modelling geographical patterns in species richness using eigenvector-based spatial filters. Global Ecol. Biogeogr. 2005;14:177–185. 10.1111/j.1466-822X.2005.00147.x [Google Scholar]
- Diniz-Filho J.A.F, Bini L.M, Hawkins B.A. Spatial autocorrelation and red herrings in geographical ecology. Global Ecol. Biogeogr. 2003;12:53–64. 10.1046/j.1466-822X.2003.00322.x [Google Scholar]
- Diniz-Filho J.A.F, Rangel T.F.L.V.B, Hawkins B.A. A test of multiple hypotheses for the species richness gradient of South American owls. Oecologia. 2004;140:633–638. doi: 10.1007/s00442-004-1577-4. 10.1007/s00442-004-1577-4 [DOI] [PubMed] [Google Scholar]
- Donald P.F, Greenwood J.J.D. Spatial patterns of range contraction on British breeding birds. Ibis. 2001;143:593–601. [Google Scholar]
- Evans K.L, Gaston K.J. People, energy and avian species richness. Global Ecol. Biogeogr. 2005;14:187–196. 10.1111/j.1466-822X.2004.00139.x [Google Scholar]
- Evans K.L, Greenwood J.J.D, Gaston K.J. Relative contribution of abundant and rare species to species–energy relationships. Biol. Lett. 2005a;1:87–90. doi: 10.1098/rsbl.2004.0251. 10.1098/rsbl.2004.0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K.L, Greenwood J.J.D, Gaston K.J. The roles of extinction and colonisation in generating species–energy relationships. J. Anim. Ecol. 2005b;74:498–507. [Google Scholar]
- Evans K.L, Warren P.H, Gaston K.J. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. 2005c;80:1–25. doi: 10.1017/s1464793104006517. 10.1017/S1464793104006517 [DOI] [PubMed] [Google Scholar]
- Evans K.L, Warren P.H, Gaston K.J. Does energy availability influence classical patterns of spatial variation in exotic species richness? Global Ecol. Biogeogr. 2005d;14:57–65. 10.1111/j.1466-822X.2004.00134.x [Google Scholar]
- Fretwell S.D. Food-chain dynamics—the central theory of ecology. Oikos. 1987;50:291–301. [Google Scholar]
- Gaston K.J. Global patterns in biodiversity. Nature. 2000;405:220–227. doi: 10.1038/35012228. 10.1038/35012228 [DOI] [PubMed] [Google Scholar]
- Gaston K.J. Macroecology and people. Basic Appl. Ecol. 2004;5:303–307. 10.1016/j.baae.2004.05.001 [Google Scholar]
- Gaston K.J, Blackburn T.M. Blackwell Science Ltd; Oxford: 2000. Pattern and process in macroecology. [Google Scholar]
- Gaston K.J, Spicer J.I. second edn. Blackwell publishing; Oxford: 2004. Biodiversity: an introduction. [Google Scholar]
- Gaston K.J, Blackburn T.M, Gregory R.D. Abundance-range size relationships of breeding and wintering birds in Britain: a comparative analysis. Ecography. 1997;20:569–579. [Google Scholar]
- Gibbons D.W, Reid J.B, Chapman R.A. T., A.D. Poyser; London: 1993. The new atlas of breeding birds in Britain and Ireland: 1988–1991. BTO/SWC/IWC. [Google Scholar]
- Ginzburg L.R, Jensen C.X.J. Rules of thumb for judging ecological theories. Trends Ecol. Evol. 2004;19:121–126. doi: 10.1016/j.tree.2003.11.004. 10.1016/j.tree.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Greenwood J.J.D, Gregory R.D, Harris S, Morris P.A, Yalden D.W. Relations between abundance, body size and species number in British birds and mammals. Phil. Trans. R. Soc. B. 1996;351:265–278. [Google Scholar]
- Gregory R.D, Blackburn T.M. Macroecological patterns in British breeding birds: covariation of species geographical range sizes at differing spatial scales. Ecography. 1998;21:527–534. [Google Scholar]
- Gregory R.D, Gaston K.J. Explanations of commonness and rarity in British breeding birds, separating resource use and resource availability. Oikos. 2000;88:5515–5526. 10.1034/j.1600-0706.2000.880307.x [Google Scholar]
- Gregory R.D, Wilkinson N.I, Noble D.G, Robinson R.A, Brown A.F, Hughes J, Procter D.A, Gibbons D.W, Galbraith C.A. The population status of birds in the United Kingdom, Channel Islands and Isle of Man: an analysis of conservation concern 2002–2007. British Birds. 2002;95:410–450. [Google Scholar]
- Haberl H, Krausmann F, Erb K.H, Schulz N.B, Rojstaczer S, Sterling S.M, Moore N. Human appropriation of net primary productivity. Science. 2002;296:1968–1969. doi: 10.1126/science.296.5575.1968. 10.1126/science.296.5575.1968 [DOI] [PubMed] [Google Scholar]
- Hawkins B.A, Field R, Cornell H.V, Currie D.J, Guégan J-F, Kaufman D.M, Kerr J.T, Mittelbach G.G, Oberdorff T, O'Brien E.M, Porter E.E, Turner J.R.G. Energy, water and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- Huston M. A general hypothesis of species diversity. Am. Nat. 1979;113:81–101. 10.1086/283366 [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalia, or why there are so many kinds of animals. Am. Nat. 1959;93:145–149. 10.1086/282070 [Google Scholar]
- Imhoff M.L, Bounoua L, Ricketts T.H, Loucks C, Harriss R, Lawrence W.T. Global patterns in human consumption of net primary production. Nature. 2004;429:870–873. doi: 10.1038/nature02619. 10.1038/nature02619 [DOI] [PubMed] [Google Scholar]
- James F.C. Geographic size variation in birds and its relationship to climate. Ecology. 1970;51:365–390. [Google Scholar]
- Jetz W, Rahbek C. Geographic range size and determinants of avian species richness. Science. 2002;297:1548–1551. doi: 10.1126/science.1072779. 10.1126/science.1072779 [DOI] [PubMed] [Google Scholar]
- Johnson J.B, Omland K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004;18:101–108. doi: 10.1016/j.tree.2003.10.013. 10.1016/j.tree.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Johnson N.K, Cicero C. New mitochondrial DNA data affirm the importance of Pleistocene speciation in North American birds. Evolution. 2004;58:1122–1130. doi: 10.1111/j.0014-3820.2004.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Kaspari M, Ward P.S, Yuan M. Energy gradients and the geographic distribution of local ant diversity. Oecologia. 2004;140:407–413. doi: 10.1007/s00442-004-1607-2. 10.1007/s00442-004-1607-2 [DOI] [PubMed] [Google Scholar]
- Kerr J.T, Ostrovsky M. From space to species: ecological applications for remote sensing. Trends Ecol. Evol. 2003;18:299–305. 10.1016/S0169-5347(03)00071-5 [Google Scholar]
- Kerr J.T, Vincent R, Currie D.J. Lepidopteran richness patterns in North America. Ecoscience. 1998;5:446–553. [Google Scholar]
- Lande R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 1993;142:911–927. doi: 10.1086/285580. 10.1086/285580 [DOI] [PubMed] [Google Scholar]
- Legendre P. Spatial autocorrelation: trouble or new paradigm? Ecology. 1993;74:1659–1673. [Google Scholar]
- Legendre P, Dale M.R.T, Fortin M.J, Gurevitch J, Hohn M, Myers D. The consequences of spatial structure for design and analysis of ecological field surveys. Ecography. 2002;25:601–615. 10.1034/j.1600-0587.2002.250508.x [Google Scholar]
- Lennon J.J. Red-shifts and red herrings in geographical ecology. Ecography. 2000;23:101–113. 10.1034/j.1600-0587.2000.230111.x [Google Scholar]
- Lennon J.J, Greenwood J.J.D, Turner J.R.G. Bird diversity and environmental gradients in Britain: a test of the species–energy hypothesis. J. Anim. Ecol. 2000;69:581–598. 10.1046/j.1365-2656.2000.00418.x [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute Inc; Cary NC: 1996. SAS® System for mixed models. [Google Scholar]
- Mittelbach G.C, Steiner C.F, Scheiner S.M, Gross K.L, Reynolds H.L, Waide R.B, Willig M.R, Dodson S.I, Gough L. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. [Google Scholar]
- Mittelbach G.C, Steiner C.F, Scheiner S.M. What is the observed relationship between species richness and productivity? Reply. Ecology. 2003;84:3390–3395. [Google Scholar]
- Oksanen J, Fretwell S.D, Arruda J, Niemelä P. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 1981;118:240–261. 10.1086/283817 [Google Scholar]
- Pimm S.L, Brown J.H. Domains of diversity. Science. 2004;304:831–833. doi: 10.1126/science.1095332. 10.1126/science.1095332 [DOI] [PubMed] [Google Scholar]
- Post D.M. The long and short of food chain length. Trends Ecol. Evol. 2002;17:269–277. 10.1016/S0169-5347(02)02455-2 [Google Scholar]
- Rabenold K.N. A reversed latitudinal diversity gradient in avian communities of eastern deciduous forests. Am. Nat. 1979;114:275–286. 10.1086/283474 [Google Scholar]
- Rabenold K.N. Latitudinal gradients in avian species diversity and the role of long-distance migration. Curr. Ornith. 1993;10:247–274. [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity and their causes II. Marine parasitological evidence for a time hypothesis. Biol. Zbl. 1978;97:405–418. [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity, the search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- Rosenzweig M.L. Cambridge University Press; Cambridge, UK: 1995. Species diversity in space and time. [Google Scholar]
- Roy K, Jablonski D, Valentine J.W, Rosenberg G. Marine latitudinal diversity gradients: tests of causal hypotheses. Proc. Natl Acad. Sci. USA. 1998;95:3699–3702. doi: 10.1073/pnas.95.7.3699. 10.1073/pnas.95.7.3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero A, Kitzberger T. Environmental correlates of mammal species richness in South America: effects of spatial structure, taxonomy and geographic range. Ecography. 2004;27:401–416. 10.1111/j.0906-7590.2004.03801.x [Google Scholar]
- Shugart H.H, Patten B.C. Niche quantification and the concept of niche pattern. In: Patten H.H, editor. Systems analysis and simulation ecology. Academic Press; New York: 1972. pp. 283–327. [Google Scholar]
- Summers R.W, Jardine D.C, Marquiss M, Rae R. The distribution and habitats of crossbills Loxia spp. in Britain, with special reference to the Scottish Crossbill Loxia scotica. Ibis. 2002;144:393–410. 10.1046/j.1474-919X.2002.00064.x [Google Scholar]
- Turner J.R.G, Lennon J.J, Lawrenson J.A. British bird distributions and the energy theory. Nature. 1988;335:539–541. 10.1038/335539a0 [Google Scholar]
- Vázquez L.B, Gaston K.J. Rarity, commonness, and patterns of species richness: the mammals of Mexico. Global Ecol. Biogeogr. 2004;13:535–542. 10.1111/j.1466-822X.2004.00126.x [Google Scholar]
- Vázquez D.P, Stevens R.D. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 2004;164:E1–E19. doi: 10.1086/421445. 10.1086/421445 [DOI] [PubMed] [Google Scholar]
- Waide R.B, Willig M.R, Steiner C.F, Mittelbach G.C, Gough L, Dodson S.I, Juday G.P, Parmenter R. The relationship between net primary productivity and species richness. Annu. Rev. Ecol. Syst. 1999;30:257–300. 10.1146/annurev.ecolsys.30.1.257 [Google Scholar]
- Whittaker R.J, Heegaard E. What is the observed relationship between species richness and productivity? Comment. Ecology. 2003;84:3384–3390. [Google Scholar]
- Wright D.H. Species–energy theory, an extension of species-area theory. Oikos. 1983;41:496–506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


