Abstract
Individual specialization underpins the division of labour within ant societies, but only in a small minority do morphological specialists, or physical castes, exist in the workforce. The genetic conditions that allow such castes to evolve are well understood, but the ecological pressures that select for them are not. We provide compelling evidence that the task of transporting novel prey selected for an exaggerated transport caste, or ‘submajor’, in the army ant Eciton burchellii. This species is the only Eciton that preys upon large arthropods as well as ants, the ancestral prey type, and by comparing load-transport among Eciton species and within E. burchellii, we show that this mixed diet significantly constrains transport efficiency. Crucially, however, we also show that E. burchellii submajors are highly specialized on transporting non-ant prey, and we demonstrate experientially that it is specifically this prey type that constrains prey-transport efficiency. Our study also suggests that phylogenetic constraints associated with the Eciton lifestyle intensified selection for the exaggerated submajor of E. burchellii. Thus, we propose that a novel task may only select for a special caste when phylogenetic constraints preclude the evolution of alternative solutions. This identifies a new and potentially general scenario for the evolution of physical castes.
Keywords: army ants, Eciton, caste, morphology, transport
1. Introduction
The great evolutionary and ecological success of ant societies (Hölldobler & Wilson 1990) can be attributed to the efficiencies that accrue from the division of labour within their colonies (Oster & Wilson 1978; Hölldobler & Wilson 1990). This organisational system is underpinned by individual specialization, but paradoxically, morphologically distinct specialists within the general workforce are rare (Oster & Wilson 1978; Baroni Urbani 1998). While the genetic conditions that allow such ‘physical castes’ to evolve are well understood (reviewed in Bourke & Franks 1995), the ecological pressures that select for them are not (reviewed in Oster & Wilson 1978; Bourke & Franks 1995). Thus, under what conditions specialised physical castes (hereafter castes) evolve remains a key issue in the study of social evolution, and it is one that must be addressed for a broader understanding of the selective forces that shape the organisation of animal societies.
Oster & Wilson (1978) theorized that the efficiency benefits of a division of labour would drive the proliferation of worker castes within a species towards a theoretical optimum of one caste for each task, but that this process would be impeded by various constraints. One of the important ecological constraints that they proposed was ‘task overlap’, which suggests that caste evolution can be constrained when the morphological characteristics needed to perform different tasks overlap closely (Oster & Wilson 1978). Thus, within the theoretical framework of Oster & Wilson (1978), the task overlap hypothesis provides one possible explanation for why special castes rarely evolve. Building on the logic of this hypothesis, here we ask a related but new question: can a novel task associated with the invasion of a new niche actually select for a special caste? Stated another way, can differences in the degree of caste evolution between a species and its close relatives be explained by selection related to relatively small differences in their ecology?
Worker morphology varies considerably within and among species in the army ant genus Eciton (Rettenmeyer 1963; Franks 1985; Powell & Franks submitted), making this group well suited for studying caste evolution. One important difference among species is the presence of a distinct ‘submajor’ caste in some species but not in others (Powell & Franks submitted). Second in overall size to the defensive major caste, submajors have legs, head, and grasping mandibles that are disproportionately large, compared to standard workers (Franks 1985; Powell & Franks submitted). The presence of this caste seems to be a derived characteristic with the genus, and it may have evolved more than once (Powell & Franks submitted). Of particular interest here, however, is that in E. burchellii (formerly Eciton burchelli see Bolton 1995), submajor morphology is significantly more exaggerated than in any other species known to have this caste (Powell & Franks submitted). Has an important novel task selected for the exaggerated submajor of E. burchellii?
Submajors constitute only 3% of the worker population of an E. burchellii colony, but play a disproportionately important role in individual and ‘team’ prey-transport (Franks 1985; Franks et al. 2001), suggesting their evolution is linked to load-transport. Moreover, selection for efficient load-transport of both prey and larvae is likely to be particularly strong in Eciton because colonies capture vast quantities of prey and routinely migrate to new nest sites (Schneirla 1971). However, all New World army ants are nomadic and conduct group-raids, so why is a distinct submajor caste found only in some Eciton, and why has it reached such an exaggerated form in E. burchellii? The answer may lie with key differences between Eciton ecology and that of other New World army ants, and with important dietary differences among Eciton species.
Eciton species are notably different from other New World army ants because they retrieve prey and migrate above ground, whereas members of the other genera are partly or exclusively subterranean (Rettenmeyer 1963). This frees the evolution of Eciton morphology from constraints associated with routinely running through tunnels, and it also makes their lifestyle uniquely time-limited. Surface-activity exposes the prey of Eciton species to scavengers during prey-transport and their queen and larvae to predators during migrations, and this vulnerability puts a premium on completing both transport tasks quickly. Accordingly, Eciton retrieve prey at high speed (Feener et al. 1988; Franks et al. 1999) and relocate their large colonies in fast, orderly migrations completed in one night (Rettenmeyer 1963; Franks & Fletcher 1983). Moreover, Eciton species have a strict cyclical schedule of raids and migrations (Rettenmeyer 1963), which brings further time-limitations (Franks et al. 1999). By contrast, Labidus species exemplify the considerably less exposed and less time-limited lifestyle of other New world army ants because although they often raid above ground, they retrieve prey and migrate underground or through constructed soil tunnels, raid at various times of day, take considerably longer to migrate, can nest in one location for a number of months, and seem to lack any cyclical activity schedule (Rettenmeyer 1963; Powell personal observation). Members of the remaining genera share some or all of these characteristics with Labidus.
In addition to all Eciton species living primarily above ground, which is unusual for army ants, they all prey on other ants. However, some are strict ant specialists, whereas others also take non-ant prey (Rettenmeyer et al. 1983; Powell & Franks submitted). Moreover, mapping Eciton prey records onto the army ant phylogeny (Brady 2003) suggests that a non-ant dietary component is derived within the genus. Crucially, however, the diet of E. burchellii is unique because although approximately 50% of their diet is ant prey, the remaining proportion comes from large arthropods (katydids, spiders, etc.) that they capture and dismember (Franks 1983; Powell & Franks submitted). No other Eciton species preys upon arthropods that are not social insects.
We hypothesise that there are significant challenges associated with the transport of the derived, non-ant component of the diet of E. burchellii and that the exaggerated submajor caste of this species has evolved to overcome them. These hypotheses provide numerous testable predictions. First, we predict that the mixed prey of E. burchellii will constrain loading efficiency (i.e. the relationship between ant size and load size) and transport speed below that of other Eciton, which have a strict diet of more uniformly shaped social insects. Second, we predict that the mixed prey of E. burchellii will constrain the loading efficiency for prey-transport below that for the transport of their own larvae, and that submajors will specialise in transporting non-ant prey. Third, we predict that if non-ant prey is indeed more awkward to transport, thus having selected for the exaggerated submajor in E. burchellii, it will be possible to replicate the constraints it places on loading efficiency and overall transport efficiency (incorporating loading efficiency and transport speed). Here we address these predictions with data from observational studies and controlled experiments.
2. Material and Methods
(a) Study site, species and sampling
Studies were conducted on Barro Colorado Island, Panama with Eciton burchellii foreli, Eciton hamatum, and Eciton dulcium crassinode. Each transport sample consisted of a load (a prey item or a larva) and the ant(s) that transported it. Unbiased sampling was achieved by collecting the next sample of the appropriate category to pass a predetermined point, after processing the previous sample. Each sample was taken without disturbing the surrounding ants, placed into a vial, and put on ice. The fresh wet mass of each load was taken within three hours of collection, and the right back-leg of each ant was measured. To allow comparison among different samples (see later), back-leg length (G) was transformed to dry mass (D) using the following relationships: E. burchellii, D=0.01978G2.28313 (r2=99.5%, p<0.0001); E. hamatum, D=0.03975G2.02608 (r2=99.3%, p<0.0001); E. dulcium, D=0.01412G2.53360 (r2=99.3%, p<0.0001). The submajor caste in E. burchellii was defined from 5.6 to 9.5 mg, dry mass (Franks 1985).
(b) Comparative prey-transport in Eciton
Prey-transport samples were collected for E. burchellii, E. hamatum, and E. dulcium, to compare loading efficiency with their natural prey. For each species, a hundred individual prey-transport (IPT) samples were taken from each of four colonies. From an additional three colonies of E. burchellii and E. hamatum, fifty prey-laden individuals were timed on a controlled substrate, to compare the speed of prey-transport among species. A section of the principle foraging column was redirected over the same horizontal piece of wood (0.05×0.1×1.5 m3), controlling for substrate rugosity within and between colonies, and individuals were timed over 1 m and collected. For these timed samples, prey was not weighed. E. dulcium foragers could not be redirected over the controlled substrate.
(c) Comparative load-transport in E. burchellii
In addition to the IPT samples for E. burchellii (see earlier), other samples were collected to compare loading efficiency for different load-types. Fifty team prey-transport (TPT) samples were collected from each of four colonies, and the relative size of each team's front-runner was noted. A hundred larva-transport samples were also collected from each of four colonies during the last emigration of a nomadic phase, when larvae are fully developed. These were taken downstream of a ‘larvae cache’, to control for the conditions under which larvae and prey were collected (prey is collected from caches during foraging). These data were also used to compare the relative involvement of E. burchellii submajors in the transport of larvae and prey.
(d) E. burchellii submajors and different prey types
A hundred IPT samples from each of three colonies were categorised as either ant prey or non-ant prey to compare the type of prey transported by submajors and standard workers. Prey was also categorised in the TPT samples (see earlier) and in fifty IPT samples from the same colonies, allowing comparison of the type of prey transported by individuals and teams.
(e) Prey type experiment
To determine if non-ant prey of E. burchellii constrains loading and overall transport efficiency compared to ant prey, prey-type was manipulated experimentally. All experiments were conducted on the same controlled substrate (see earlier). Experimental prey was initially killed by foragers held in a closed container, ensuring it would be accepted for transport. Two types of prey were used: katydids (Orthoptera: Tettigoniidae), typifying non-ant prey; and mealworms (Coleoptera: Tenebrionidae), characterising the cylindrical shape and smooth exterior of ant prey. Katydids and mealworms were of various sizes, approximating the diversity of prey naturally captured by E. burchellii. Experimental prey was placed at the beginning of the controlled substrate, where workers dismembered it, and a mixture of individuals and teams transported it to the nest. Each transport sample was timed over 0.5 m and collected. Twenty-five samples were colleted for each type of prey, from each of three colonies.
(f) Substrate type experiment
To determine if overall transport efficiency in E. burchellii is constrained by interactions between prey and substrate, we manipulated substrate rugosity experimentally. For each of four colonies, a section of the principle column was redirected over the same horizontal piece of wood (see earlier). Thirty team samples were collected directly off the wood, constituting a smooth substrate, while another thirty were collected from a rough substrate, consisting of leaf-litter placed onto the wood (leaf-litter was slowly relocated from the surrounding area without alarming the ants). These experimental substrates mimicked the smoothest and roughest substrates over which E. burchellii runs naturally. Teams were timed over 1 m and collected. The relative size of any ant(s) that joined a focal team was noted, and any ant(s) that dropped out from a team were collected. Samples were collected from the smooth substrate first for two colonies and second for the other two.
(g) Statistical analyses
Loading efficiency was defined by the bivariate relationship of natural-log of the wet mass of a load plotted against the natural-log of a size-based measure of the transport capability of the ant(s). For comparisons among species, relative back-leg length (back-leg/dry mass1/3) was used as the measure of transport capability because it adjusts for differences in relative morphology. For different load-types transported by E. burchellii, dry mass was used because by using the combined dry mass of each team, individuals and teams can be compared. For the experimental data, both prey wet mass and transport rate (prey wet mass×speed) were plotted against the dry mass of the ant(s), defining ‘loading efficiency’ and ‘overall transport efficiency’ (incorporating loading efficiency and transport speed), respectively. Differences in loading efficiency and overall transport efficiency among data sets were tested with Analysis of Covariance (ANCOVA). An interaction term was used to account for differences in the slopes of the bivariate relationships. A simpler main factor model was used whenever an interaction term was not significant. A colony effect was included whenever degrees of freedom permitted; otherwise differences among colonies were addressed in separate ANCOVAs before running the main ANCOVA. The natural-log transformed data sets did not deviate significantly from the assumptions of ANCOVA.
Differences in the involvement of submajors in the transport of different types of load were assessed with Log-likelihood ratio tests (G-tests).
3. Results
(a) Comparative prey-transport in Eciton
These data address the prediction that the mixed prey of E. burchellii causes lower loading efficiency and transport speed than the social insect prey of other Eciton. Figure 1 shows that loading efficiency in E. burchellii is significantly lower than that of E. hamatum, which has a less exaggerated submajor caste, and E. dulcium, which has no submajor. It is important to note that loading efficiency among species is inversely correlated with submajor development: The presence of a submajor was associated with lower loading efficiency, and the species with the most exaggerated submajor, E. burchellii, achieved the lowest loading efficiency of the three. Figure 2 shows that, in addition to lower loading efficiency, E. burchellii also transports prey at a slower speed than E. hamatum.
Figure 1.
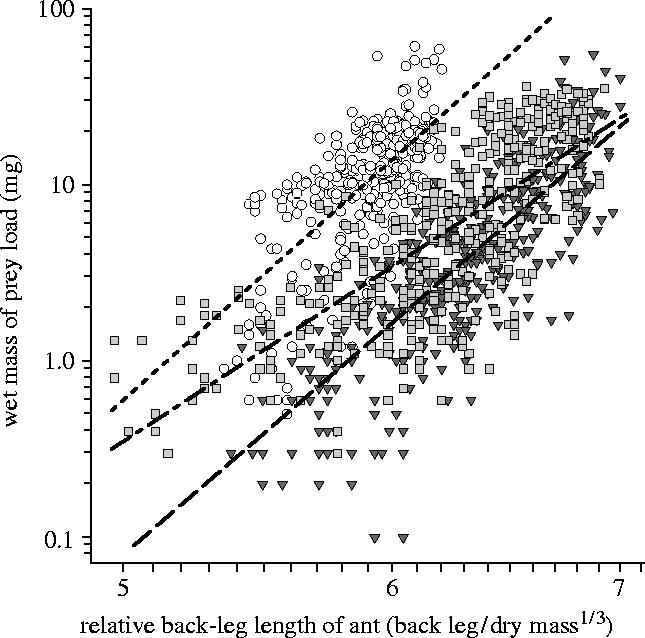
Loading efficiency (relationship between ant size and load size) for natural prey-transport in E. burchellii (grey triangles, dashed regression line), E. hamatum (light grey squares, dashed/dotted regression line), and E. dulcium (white circles, dotted regression line). The difference in the loading efficiency achieved by the three species was highly significant (species effect, F2,1192=536.65, p<0.0001, η2=47%; interaction term, F2,1192=16.51, p<0.0001, η2=3%). Pairwise comparisons showed E. burchellii's loading efficiency was significantly lower than that of E. hamatum (species effect, F1,796=115.65, p<0.0001, η2=13%; interaction term, F1,796=23.42, p<0.0001, η2=3%) and E. dulcium (species effect, F1,795=1493.93, p<0.0001, η2=65%). Loading efficiency was also significantly different between E. hamatum and E. dulcium (species effect, F1,794=669.91, p<0.0001, η2=46%; Interaction term, F1,794=14.54, p=0.0001, η2=2%). Loading efficiency was not significantly different among E. burchellii colonies. Significant differences were found among both E. hamatum colonies (colony effect, F3,392=35.02, p<0.0001, η2=20%; interaction term, F3,392=5.29, p=0.0014, η2=4%) and E. dulcium colonies (colony effect, F3,393=17.74, p<0.0001, η2=12%). However, for all species, the data from all four colonies were used in the main ANCOVA, so that the data set for each species retained the full natural variation in loading efficiency.
Figure 2.
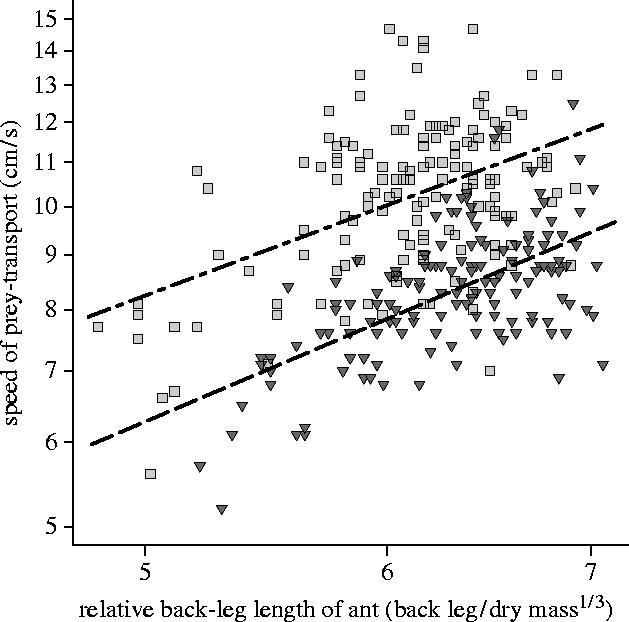
Transport speed achieved by prey-laden foragers in E. burchellii (grey triangles, dashed regression line) and E. hamatum (light grey squares, dashed/dotted regression line). E. burchellii workers transported prey at a significantly slower speed than E. hamatum workers (species effect, F1,295=234.23, p<0.0001, η2=44%). Initial analysis showed significant differences among E. burchellii colonies (Colony effect, F2,146=32.0991, p>0.0001, η2=25%) and E. hamatum colonies (colony effect, F2,144=12.54, p>0.0001, η2=14%). However, for both species, the data from all three colonies were used in the main ANCOVA, so that the data set for each species retained the full natural variation in transport speed.
(b) Comparative load-transport in E. burchellii
(i) Loading efficiency with different load types
These data address the prediction that E. burchellii achieves lower loading efficiency during prey-transport than during larva-transport. Figure 3 shows that when E. burchellii transport their own larvae, they achieve significantly higher loading efficiency than when they transport prey, regardless of whether prey is transported by individuals or teams.
Figure 3.
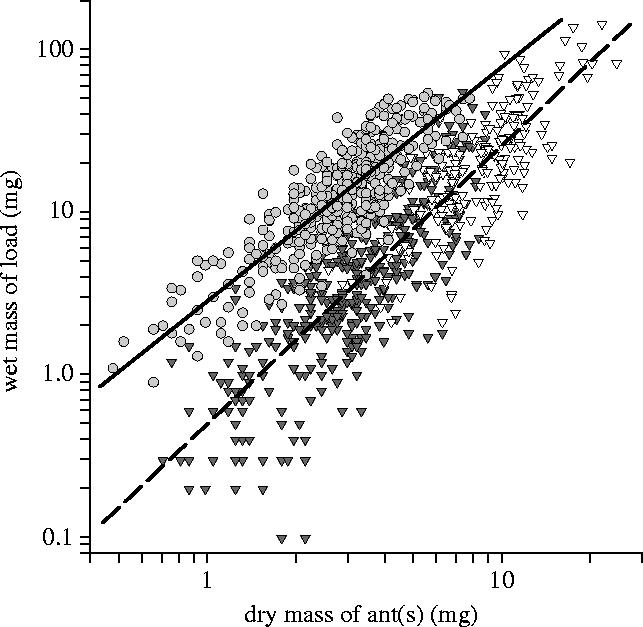
Loading efficiency (relationship between ant size and load size) for the transport of larvae (light grey circles), prey by individuals (grey triangles), and prey by teams (white triangles) in E. burchellii. Loading efficiency for individual and team prey-transport was not significantly different (transport-method effect, F1,597=0.23, p=0.63), and for the overall loading efficiency for prey-transport (combining individual and team data), P=0.50477A1.70714 (dashed regression line, n=600, r2=0.75, p<0.0001); P=wet mass of prey, and A=dry mass of ant(s)). However, loading efficiency for larva-transport was significantly higher than that for prey-transport (load-type effect, F1,996=1156.7, p<0.0001, η2=54%; interaction term, F1,996=15.53, p<0.0001, η2=2%). Initial analysis showed no significant differences among colonies for individual prey-transport. Differences were found among colonies for both team prey-transport (colony effect, F3,195=5.89, p=0.0007, η2=8%) and larva-transport (colony effect, not significant; interaction term, F3,392=3.86, p=0.0097, η2=6%). However, for all types of load-transport, the data from all four colonies were used in the main ANCOVA, so that the data set for each load-type retained the full natural variation in loading efficiency. For the combined data for larva-transport, L=2.88420D1.43171 (solid regression line, L=wet mass of larva, D=dry mass of ant, n=400, r2=0.73, p<0.0001).
(ii) Submajors and different load types
These data address the prediction that E. burchellii submajors should specialize in prey-transport, with a particular specialization for non-ant prey. An upper-range was defined for individual prey-transport between the heaviest individual prey item and the mean mass of prey transported by submajors (14.4–54.1 mg; see figure 3). Of all prey transported by individuals, 11.8% were within this range, compared to 43.5% of all larvae. Submajors transported 68.1% of prey in this range, but only 8.6% of larvae. The difference between the proportional employment of submajors in transporting these different load-types of equivalent mass was highly significant (G1=67.66, p<0.0001). Figure 4a shows typical transport of larvae by E. burchellii.
Figure 4.
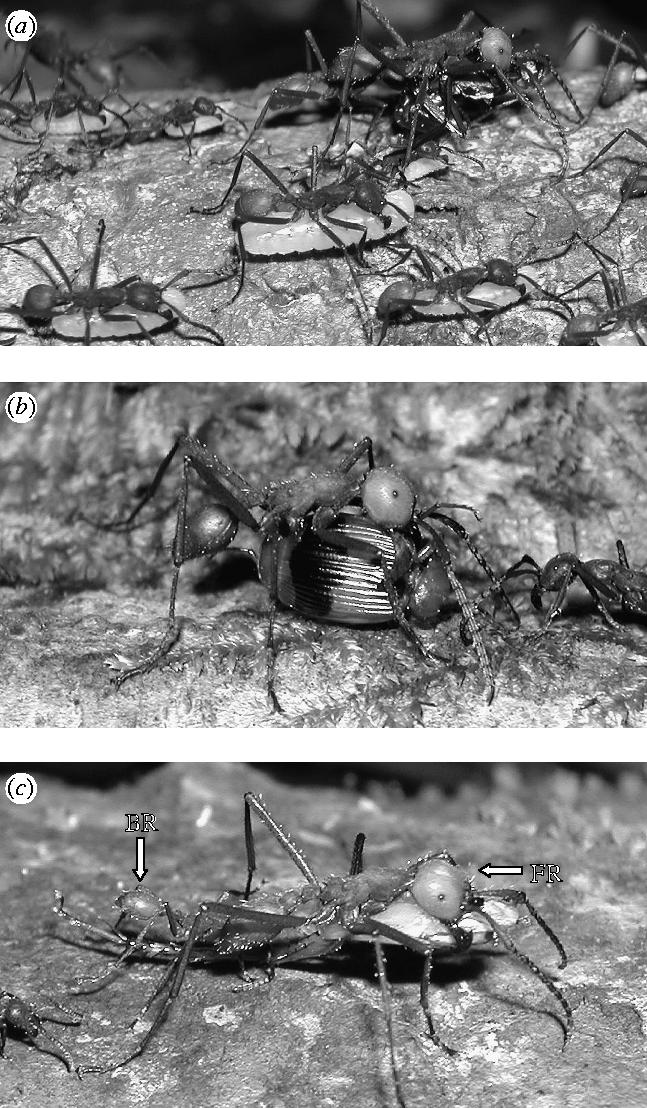
Typical load-transport in the army ant E. burchellii. (a) Workers transporting mature larvae of various sizes during a colony migration, while a submajor-led team transports prey leftover from the day's raid. Teams never transported larvae, despite the fact that most larvae (96.7%) were within the range of prey transported by teams (2.1–141 mg). Submajors specialized in the transport of irregularly shaped, but not necessarily heavy, non-ant prey, both alone (b) transporting dorso-ventrally flattened beetle) and as front-runners (FR) in teams (c) transporting cockroach leg). Team back-runners (BR) were often partially obscured beneath larger front-runners (c) gaster of back-runner visible at the rear).
Most individual prey (73.3%) and team prey (89%) was within a common range (2.1–54.1 mg). Within this range, the difference between the proportion of prey transported by submajors alone (21.2%) and the proportion of prey transported by submajor-led teams (54.0%) was highly significant (G1=37.36, p<0.0001). Thus, in addition to specializing in individual prey-transport, submajors are a fundamental component of teams, and teams carry prey that is usually no heavier than that carried by individuals.
The difference between the type of prey transported by submajors and standard workers was highly significant in all sampled colonies (G1=24.29, p<0.0001; G1=11.94, p=0.0005; G1=9.87, p=0.0017). Submajors specialized in the transport of non-ant prey (100; 100; 79.2%) and workers transported a higher proportion of ant prey (42.5; 32.5; 56.6%). Thus, submajors specialize specifically in the transport of non-ant prey. Figure 4b show typical submajor prey-transport.
The difference between the type of prey carried by individuals and teams was highly significant in all sampled colonies (G1=6.87, p=0.0088; G1=31.42, p<0.0001; G1=34.25, p<0.0001). Individuals transported a high percentage of ant prey (60; 46; 78%), and teams specialized in transporting non-ant prey (66; 98; 78%). Thus, in addition to individual submajors, teams, which are usually led by submajors, also specialize in the transport of non-ant prey. Figure 4c shows a typical submajor-led team.
(c) Load type experiment
Here we addressed experimentally the prediction that non-ant prey imposes greater constraints on loading and overall transport efficiency than ant prey. E. burchellii workers achieved significantly higher loading efficiency with ant-like prey than with non-ant prey (Figure 5). Similarly, overall transport efficiency (incorporating loading efficiency and transport speed) was significantly higher for ant-like prey than non-ant prey (prey-type effect, F1,145=124.98, p<0.0001, η2=46%; colony effect, not significant).
Figure 5.
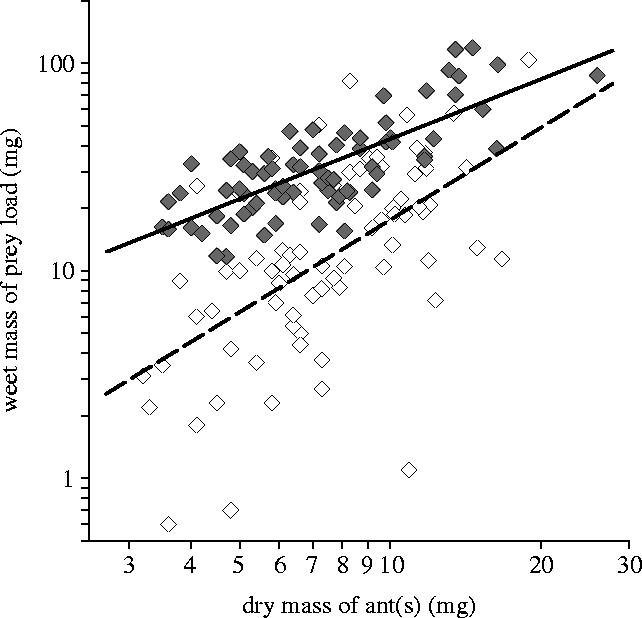
Loading efficiency for the transport of two experimentally manipulated types of prey by E. burchellii. Loading efficiency was significantly lower for non-ant prey (white diamonds) than for ant-like prey (grey diamond; prey-type effect, F1,145=94.82, p<0.0001, η2=40%; colony effect, not significant). For the loading efficiency for ant-like prey P=4.69227A0.95938 (solid regression line, n=75, r2=0.58, p<0.0001), and for the loading efficiency for non-ant prey P=1.69963A1.47265 (dashed regression line, n=75, r2=0.34, p<0.0001), where P is the wet mass of a prey item, and A is the dry mass of the ant(s).
(d) Substrate type experiment
These experiments addressed the prediction that interactions between prey and substrate constrain the overall transport efficiency achieved by those that specialize in the transport of non-ant prey, such as teams (see above). Substrate rugosity did not have a significant effect on the loading efficiency of teams, as would be expected because teams were chosen at random and all transported natural prey. However, substrate rugosity did have a significant effect on overall transport efficiency (F1,234=115.47, p<0.0001, η2=33%), meaning that transport speed was significantly reduced over the rough substrate. Colony also had a significant effect on overall transport efficiency, but accounted for a fraction of the variance (F3,234=6.09, p=0.0005, η2=7%). Moreover, 13.3% of teams lost back-runners over the smooth substrate, with 6.7% breaking-down to leave individual transporters. Over the rough substrate, 15% of teams gained a member, and one team lost a member. The difference between the changes in team composition over the two substrates was highly significant (no significant colony differences, combined analysis: G2=40.96, p<0.0001). Thus, changes in the constraining interactions between prey and substrate prompted behavioural changes in the ants.
4. Discussion
Here we provide compelling support for the hypothesis that an important novel task, associated with the invasion of a derived niche, can select for a special caste. E. burchellii achieves significantly lower loading efficiency than other Eciton species, and they transport prey at a slower speed. This indicates that their mixed diet of polymorphic ants and large arthropods imposes greater constraints on transport efficiency than does the strict social insect diet of its congeners. E. burchellii also achieves significantly lower loading efficiency during the transport of prey than during the transport of its own larvae. Thus, the prey of E. burchellii imposes greater constraints on load-transport than their own brood; the other load-type they transport routinely. Moreover, within the substantial region of overlap between the masses of prey and larvae, both submajors and teams, which are often submajor-led, specialize in the transport of non-ant prey. This establishes an explicit link between the derived, non-ant component of the diet of E. burchellii and its morphologically exaggerated submajor caste. Crucially, we have shown experimentally that the irregular shape of non-ant prey constrains loading and overall transport efficiency (incorporating loading efficiency and transport speed), and that overall transport efficiency is further constrained by problematic interactions between prey and substrate. This demonstrates the significant constraints associated with transporting non-ant prey, and this explains the lower transport efficiency and specialized involvement of submajors with this type of load. Thus broadly, our findings strongly suggest that in the army ant E. burchellii, the challenges associated with the novel task of transporting awkwardly shaped prey drove the evolution of an exaggerated transport caste.
Previous work has hypothesized that E. burchellii submajor morphology, and the distinct composition of teams, effectively counterbalances rotational forces that might constrain transport efficiency (Franks 1986; Franks et al. 2001). Here, we have demonstrated experimentally that inefficient loading and frictional forces impose the key constraints on the efficient transport of the non-ant prey usually transported by submajors and teams. However, our findings also support a contributing role of rotational forces, in some situations. Well packaged loads, like E. burchellii larvae, are lifted clear of the substrate (figure 4a), but irregularly shaped non-ant prey are usually dragged. Although loading difficulties and friction seem inevitable and independent of any lifting force for most non-ant prey, rotational forces probably contribute to the trailing end of long, rigid loads touching the substrate. When this happens, the large head and mandibles of submajors probably help overcome rotational forces (Franks 1986; Franks et al. 2001). While submajors seem adapted to increase the transport efficiency of non-ant prey, it is important to consider them in a broader perspective. If E. burchellii had retained a strict ant diet, transport efficiency would be significantly higher and submajors are unlikely to have evolved. E. burchellii submajors are large and therefore expensive to produce, so despite their importance in transporting non-ant prey, they can be seen ultimately as a cost of expanding out of the ancestral feeding niche.
The exaggerated submajor of E. burchellii seems to have evolved to transport non-ant prey, but less exaggerated submajors are found in other Eciton. Do constraints associated with prey type explain these submajors and the lack of the caste in other Eciton? All existing data support this interpretation. E. dulcium has a strict diet of monomorphic ponerine ants (Rettenmeyer et al. 1983; Powell & Franks submitted), which are elongate and cylindrical like Eciton larvae. Consequently, the challenges associated with transporting prey are likely to be comparable to those for transporting larvae, explaining why E. dulcium has no submajor. Moreover, the shape similarities between E. dulcium prey and Eciton larvae suggest this species should achieve higher loading efficiency than E. burchellii, with its mixed diet, and we have demonstrated this. The key comparison, however, is with E. hamatum. If constraints associated with a novel prey-type have selected for the less exaggerated submajor of this species, their largest prey should differ significantly from the monomorphic ant prey of E. dulcium, but be less irregularly shaped than the non-ant prey of E. burchellii. Concordantly, E. hamatum takes the brood of large polymorphic ants and specializes more than other Eciton on the brood of large social wasps (Rettenmeyer et al. 1983; Powell & Franks submitted). Moreover, with this type of prey, E. hamatum should achieve intermediate loading efficiency between E. dulcium and E. burchellii, and indeed, we have shown this to be the case. The diet of other Eciton is poorly known, due to their more cryptic and nocturnal foraging, but one other species that lacks a submajor, E. mexicanum, also seems to take monomorphic poneroid ant prey (Rettenmeyer et al. 1983; Powell & Franks submitted). For other species, we can make clear predications: if novel prey has driven the independent evolution, or elaboration of a submajor caste, the submajors should show a significant specialization in transporting the novel prey type, and their degree of morphological exaggeration should reflect the extent to which the shape of the novel prey diverges from the ant prey of species without a submajor. Indeed, if a new species is found with submajors that are more exaggerated than those of E. burchellii and they specialize in transporting a novel prey type, we predict that the novel prey will constrain prey-transport even more than the non-ant prey of E. burchellii. Conversely, if others species are shown to lack a submajor, we predict that their prey will closely approximate the shape of Eciton larvae.
The evolution of a distinct caste for transporting food items that diverge strongly in shape from the species own brood is unusual among ants, but a diet including irregularly shaped food items is not (Hölldobler & Wilson 1990). So, why has item shape been so important in caste evolution in Eciton, and particularly in E. burchellii, and not in countless others? The evidence suggests that selection for a special caste to transport novel prey is likely to have been intensified by unique phylogenetic constraints associated with the Eciton lifestyle.
Eciton's uniquely time-limited lifestyle makes rapid load-transport essential (Franks et al. 1999). Accordingly, Eciton hold all loads below the body, permitting mechanically efficient and rapid transport (Bartholomew et al. 1988). However, we have shown that below-body transport constrains loading efficiency and invites friction between load and substrate. These constraints are not significant for long, cylindrical ant prey because it fits neatly below the body and can be held above the substrate. However, more irregularly shaped loads, from a shift in diet, make these limitations pronounced, as demonstrated here experimentally. Other ants avoid these constraints by carrying relatively larger, heavier loads in front or over their bodies (Traniello 1989), often resulting in a relatively weak or non-existent relationship between ant and load size (e.g. Wetterer 1994; Kaspari 1996; Morehead & Feener 1998), but this reduces transport speed (Traniello 1989; Roschard & Roces 2002). In contrast, E. burchellii, is bound to the fast below-body transport method fundamental to the Eciton lifestyle, and this seems to have necessitated the evolution of a special caste adapted to transport awkward loads: the disproportionately longer legs of a submajor increase the loading space and ground clearance, while their disproportionately large head and mandibles allow for a more powerful grip (Paul 2001) to combat the friction between load and substrate.
The lack of control over the size and shape of non-ant prey is also likely to have intensified selection on worker morphology in E. burchellii. Like other New World army ants, E. burchellii workers kill prey with their sting (Powell & Clark 2004; Powell unpublished data), and while ant prey is transported whole, large arthropods are pulled apart at the intersegmental membranes. The unusually high level of proteases in their venom (Schmidt et al. 1986) assists this process (Powell personal observation), but the shape of the resulting loads are dictated by the shape of the prey's sclerites because workers cannot cut through cuticle (Rettenmeyer 1963). Exemplifying how this method of prey capture constrains prey shape, whole wings are frequently retrieved by E. burchellii workers because they cannot cut the consumable biomass from the base. Other ants do not face such problems. For instance, Atta leaf-cutting ants have fine control over load-shape (Rudolph & Loudon 1986; Roces & Hölldobler 1994), and it is likely that an evolutionary trade-off between cutting and transporting underlies load size and shape, and the overall pattern of load-transport in these ants (Roschard & Roces 2002). In E. burchellii, loads must be retrieved at high speed, and the irregular and uncontrollable shape of non-ant prey ultimately seems to have intensified selection for a special transport caste.
It is widely accepted that special morphological characteristics often evolve in animals in relation to the exploitation of new ecological niches, but the relationship between ecology and the evolution of specialized morphological castes within animal societies remains poorly understood. Our results strongly suggest that significant challenges associated with a novel task selected for a special caste in E. burchellii. Yet such castes should be more common if novel tasks alone were enough to select for them, and given the multitude of remarkable behaviours in ants (see Hölldobler & Wilson 1990), selection seems to produce behavioural solutions most often. So why do special castes still evolve? Our analysis also suggests that selection for the exaggerated submajor caste in E. burchellii was intensified by phylogenetic constraints associated with the Eciton lifestyle. Thus, we suggest that significant challenges associated with a novel task may only select for a special caste when phylogenetic constraints preclude the evolution of alternative behavioural solutions. This provides a new and potentially general scenario for caste evolution that can be tested in other groups. Have important novel tasks selected for special castes in other groups because unique constraints prevent behavioural solutions? What underlying organizational principles explain why in social evolution, novel tasks usually select for behavioural solutions in the existing workforce, and why special castes seem to be an evolutionary last-option? These are key questions for future studies, and an assessment of the evolutionary balance between behavioural and morphological solutions to novel problems will be fundamental to deepening our understanding of the evolution of social organization in animal societies.
Acknowledgements
S.P. thanks the residents of Barro Colorado Island and Egbert Giles Leigh Jr. for helpful discussion, the BCI staff for logistical support, and Beatriz Baker and Sally Powell for help in the field. We thank Ana Sendova-Franks for statistical advice, and three anonymous referees for their useful comments on earlier versions of this manuscript. This work was funded by a CASE Studentship from the Natural Environment Research Council, U.K., with additional support from the CASE partner, the Smithsonian Tropical Research Institute, Panama. N.R.F. also thanks the BBSRC (UK) for their support through research grant E19832.
References
- Baroni Urbani C. The number of castes in ants, where major is smaller than minor and queens wear the shield of the soldiers. Insect. Soc. 1998;45:315–333. 10.1007/s000400050091 [Google Scholar]
- Bartholomew G.A, Lighton J.R.B, Feener D.H., Jr. Energetics of trail-running, load carriage, and emigration in the column-raiding army ant Eciton hamatum. Physiol. Zool. 1988;61:57–68. [Google Scholar]
- Bolton B. Harvard University Press; Cambridge, MA: 1995. A new general catalogue of the ants of the world. [Google Scholar]
- Bourke A.F.G, Franks N.R. Princeton University Press; 1995. Social evolution in ants. [Google Scholar]
- Brady S.G. Evolution of the army ant syndrome: The origin and long-term evolutionary stasis of a complex of behavioral and reproductive adaptations. Proc. Natl Acad. Sci. USA. 2003;100:6575–6579. doi: 10.1073/pnas.1137809100. 10.1073/pnas.1137809100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feener D.H, Jr., Lighton J.R.B, Bartholomew G.A. Curvilinear allometry, energetics and foraging ecology: a comparison of leaf-cutting ants and army ants. Funct. Ecol. 1988;2:509–520. [Google Scholar]
- Franks N.R. Ecology and population regulation in the army ant Eciton burchelli. In: Leigh E.G Jr., Rand A.S, Windsor D.M, editors. The ecology of a tropical forest: seasonal rhythms and long-term changes. Oxford University Press; 1983. pp. 389–395. [Google Scholar]
- Franks N.R. Reproduction, foraging efficiency and worker polymorphism in army ants. In: Holldobler B, Lindauer M, editors. Experimental behavioral ecology and sociobiology: in memoriam Karl von Frisch,1886-1982. vol. 31. Sinauer Associates; Sunderland, MA: 1985. pp. 91–107. [Google Scholar]
- Franks N.R. Teams in social insects: group retrieval of prey by army ants (Eciton burchelli Hymenoptera: Formicidae) Behav. Ecol. Sociobiol. 1986;18:425–429. 10.1007/BF00300517 [Google Scholar]
- Franks N.R, Fletcher C.R. Spatial patterns in army ant foraging and migration: Eciton burchelli on Barro Colorado, Panama. Behav. Ecol. Sociobiol. 1983;12:261–270. 10.1007/BF00302894 [Google Scholar]
- Franks N.R, Sendova-Franks A.B, Simmons J, Mogie M. Convergent evolution, superefficient teams and tempo in Old and New World army ants. Proc. R. Soc. B. 1999;266:1697–1701. 10.1098/rspb.1999.0834 [Google Scholar]
- Franks N.R, Sendova-Franks A.B, Anderson C. Division of labour within teams of New World and Old World army ants. Anim. Behav. 2001;62:635–642. 10.1006/anbe.2001.1794 [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Kaspari M. Worker size and seed size selection by harvester ants in a Neotropical forest. Oecologia. 1996;105:397–404. doi: 10.1007/BF00328743. 10.1007/BF00328743 [DOI] [PubMed] [Google Scholar]
- Morehead S.A, Feener D.H. Foraging behavior and morphology: seed selection in the harvester ant genus. Pogonomyrmex. Oecologia. 1998;114:548–555. doi: 10.1007/s004420050479. 10.1007/s004420050479 [DOI] [PubMed] [Google Scholar]
- Oster G.F, Wilson E.O. Princeton University Press; 1978. Caste and ecology in the social insects. [PubMed] [Google Scholar]
- Paul J. Mandible movements in ants. Comp. Biochem. Physiol. A—Mol. Integr. Physiol. 2001;131:7–20. doi: 10.1016/s1095-6433(01)00458-5. 10.1016/S1095-6433(01)00458-5 [DOI] [PubMed] [Google Scholar]
- Powell S, Clark E. Combat between large derived societies: A subterranean army ant established as a predator of mature leaf-cutting ant colonies. Insect. Soc. 2004;51:342–351. 10.1007/s00040-004-0752-2 [Google Scholar]
- Powell, S. & Franks, N. R. Submitted. Ecology and evolution of worker polymorphism: a new comparative approach exemplified with Eciton army ants
- Rettenmeyer C.W. Behavioral studies of army ants. Univ. Kans. Sci. Bull. 1963;44:281–465. [Google Scholar]
- Rettenmeyer C.W, Chadab-Crepet R, Naumann M.G, Morales L. Comparative foraging by neotropical army ants. In: Jaisson P, editor. Social insects in the tropics. vol. 2. Université Paris-Nord; Paris: 1983. pp. 59–73. (see also p. 252) [Google Scholar]
- Roces F, Hölldobler B. Leaf density and a trade-off between load-size selection and reruitment behavior in the ant Atta cephalotes. Oecologia. 1994;97:1–8. doi: 10.1007/BF00317902. 10.1007/BF00317902 [DOI] [PubMed] [Google Scholar]
- Roschard J, Roces F. The effect of load length, width and mass on transport rate in the grass-cutting ant Atta vollenweideri. Oecologia. 2002;131:319–324. doi: 10.1007/s00442-002-0882-z. 10.1007/s00442-002-0882-z [DOI] [PubMed] [Google Scholar]
- Rudolph S.G, Loudon C. Load size selection by foraging leaf-cutter ants (Atta cephalotes) Ecol. Entomol. 1986;11:401–410. [Google Scholar]
- Schmidt J.O, Blum M.S, Overal W.L. Comparative enzymology of venoms from stinging Hymenoptera. Toxicon. 1986;24:907–921. doi: 10.1016/0041-0101(86)90091-7. 10.1016/0041-0101(86)90091-7 [DOI] [PubMed] [Google Scholar]
- Schneirla, T. C. 1971 Army ants A study in social organization (ed. H. R. Topoff). San Francisco: W. H. Freeman & Co.
- Traniello J.F.A. Foraging strategies of ants. Annu. Rev. Entomol. 1989;34:191–210. 10.1146/annurev.en.34.010189.001203 [Google Scholar]
- Wetterer J.K. Forager polymorphism, size-matching, and load delivery in the leaf-cutting ant, Atta Cephalotes. Ecol. Entomol. 1994;19:57–64. [Google Scholar]


