Abstract
Variation partitioning and hierarchical partitioning are novel statistical approaches that provide deeper understanding of the importance of different explanatory variables for biodiversity patterns than traditional regression methods. Using these methods, the variation in occupancy and abundance of the clouded apollo butterfly (Parnassius mnemosyne L.) was decomposed into independent and joint effects of larval and adult food resources, microclimate and habitat quantity. The independent effect of habitat quantity variables (habitat area and connectivity) captured the largest fraction of the variation in the clouded apollo patterns, but habitat connectivity had a major contribution only for occupancy data. The independent effects of resources and microclimate were higher on butterfly abundance than on occupancy. However, a considerable amount of variation in the butterfly patterns was accounted for by the joint effects of predictors and may thus be causally related to two or all three groups of variables. Abundance of the butterfly in the surroundings of the focal grid cell had a significant effect in all analyses, independently of the effects of other predictors. Our results encourage wider applications of partitioning methods in biodiversity studies.
Keywords: autocovariate, collinearity, connectivity, habitat quality, Parnassius mnemosyne, semi-natural grasslands
1. Introduction
Conclusions about the determinants of biodiversity patterns in ecological studies are often derived from correlative multiple regression settings. However, identification of the predictor variables most probably affecting the variation in the response variable by commonly used regression methods can be problematic, particularly if predictor variables are significantly intercorrelated (Chevan & Sutherland 1991; Mac Nally 2000; Graham 2003).
Multicollinearity among predictors may result in the exclusion of ecologically more causal variables from multiple regression models if other intercorrelated variables explain the variation in response variable better in statistical terms (Mac Nally 2000). There are currently many approaches available for tackling collinearity problems. In studies aiming at predictive regression analysis, valuable insights can be developed by methods such as sequential regression and structural equation modelling (Graham 2003). Collinearity can also be addressed by variation partitioning (Borcard et al. 1992) and hierarchical partitioning methods (Chevan & Sutherland 1991; Mac Nally 2000). These techniques aim to provide understanding of the probable causalities and explanatory powers of predictors in multivariate datasets, not at generating a predictive equation (Watson & Peterson 1999). In this study, we focus on variation partitioning and hierarchical partitioning methods. These methods can provide new insights into species–environment relationships by decomposing the variation in response variable(s) into independent components which reflect the relative importances of individual predictors or groups of predictors and their joint effects (Liu 1997; Anderson & Gribble 1998; Watson & Peterson 1999; Mac Nally 2000; Lichstein et al. 2002; Cushman & McGarigal 2004; Gibson et al. 2004; Heikkinen et al. 2004).
Butterfly–environment studies have often relied on traditional regression methods: relationships between species richness or abundance of butterflies and the environment have commonly been examined with (stepwise) regression techniques (e.g. Clausen et al. 2001; Krauss et al. 2003; Maes et al. 2003), and single species occupancy patterns using (stepwise) logistic-regression (e.g. Hill et al. 1996; Dennis & Eales 1997, 1999; Cowley et al. 2000; Thomas et al. 2001; Fleishman et al. 2002; WallisDeVries 2004). Both variation and hierarchical partitioning methods have rarely been applied in assessing the contributions of different environmental variables to studied butterfly patterns (but see Mac Nally et al. 2003; Sawchik et al. 2003; Cleary & Gennert 2004). This is surprising, because butterflies have been used as model taxa in ecological and population biological, e.g. metapopulation studies which have compared the explanatory power of two or more groups of predictor variables to explain the variation in species abundance or occupancy, e.g. landscape factors versus local factors (Krauss et al. 2003; Bergman et al. 2004), or habitat patch size and isolation versus habitat quality factors (Thomas et al. 2001; Fleishman et al. 2002; Anthes et al. 2003; WallisDeVries 2004). Such study designs may provide insufficient information concerning the relative importance of predictors when traditional regression methods are employed to model species–environment data affected by multicollinearity (cf. Mac Nally 2000; Heikkinen et al. 2004).
Heikkinen et al. (2004) showed that both variation partitioning and hierarchical partitioning approaches can provide important insights into bird abundance–environment relationships. Here, we present the first simultaneous application of two partitioning methods to explain the occupancy and abundance patterns of a single butterfly species. We explored the relative importance of predictors representing three ecological groups of variables (larval and adult food resources, microclimate, habitat quantity) in explaining the spatial variation of the threatened clouded apollo butterfly (Parnassius mnemosyne L.) in southwestern Finland. Using data on P. mnemosyne recorded in a 50×50 m grid system we determined the independent and joint effects of predictor variables on the variation in occupancy and abundance of the butterfly. Specifically, we asked the following questions: (i) How great are the independent contributions of the three groups of predictors in explaining the occupancy and abundance of the clouded apollo? (ii) Do the relative importances of the predictor groups differ between the variation partitioning results for the butterfly occupancy and abundance? (iii) How great are the joint contributions of the explanatory variables? (iv) Which variables have the highest independent correlations with the occupancy and abundance of the butterfly in hierarchical partitioning? Furthermore, we explored whether the spatial autocorrelation in the clouded apollo abundance (‘autocovariate’, i.e. the abundance of butterfly individuals in the surroundings of the focal grid square; see Augustin et al. 1996; Ferrier et al. 2002) exerts an independent, significant effect on the butterfly patterns even if the effects of other factors are controlled.
2. Material and Methods
The study area and material are described in detail in Luoto et al. (2001) and here we provide only a brief description.
(a) Study area
The study area is situated in an agriculturally dominated landscape in southwestern Finland (60°N 32′–60°N 36′, 23°19′–23°30′E). The landscape is generally characterized by flat agricultural plains, scattered forest-covered hills and steep-sided river valleys along the river Rekijoki. Coniferous forest (Norway spruce Picea abies and Scots pine Pinus sylvestris) is the main forest type in the area. Deciduous forests (birches Betula pendula and Betula pubescens, grey alder Alnus incana and trembling aspen Populus tremula) and semi-natural grasslands are found on the slopes of river valleys.
(b) Study species
The clouded apollo (P. mnemosyne) is a palearctic butterfly which is classified as threatened in Finland (Rassi et al. 2001) and elsewhere in Europe (Konvicka & Kuras 1999; van Swaay & Warren 1999). In Finland, the clouded apollo is a rare inhabitant of traditionally managed flower-rich semi-natural meadows and pastures accompanied by deciduous forest patches abundant with Corydalis solida, the larval host plant of the butterfly (Luoto et al. 2001).
(c) Butterfly survey
In 1999 the distribution of P. mnemosyne was surveyed in a total of 2408 grid squares, 50×50 m in size, within a 6 km2 study area. All parts of the study area were visited at least five times during the adult flight season. In each grid square the potentially suitable habitat was thoroughly sampled using mark–release–recapture method (Luoto et al. 2001). The sampling effort varied between different parts of the study area. However, the results of Luoto et al. (2001) showed that the impact of study effort on modelling results was small, particularly when spatial autocorrelation (autocovariate) was considered simultaneously, as was also done here. From the survey results we produced two variables measuring the distribution and abundance of the butterfly. Distribution was measured for each of the 2408 grid squares as a binary variable, presence (>0 observations) or absence (0 observations) of butterflies during the whole flight season. For each of the 349 grid cells (‘core’ set of grid cells), where the butterfly was present its abundance was measured as the total number of butterflies observed during all visits divided by the number of visits in each grid square.
(d) Environmental variables
Eight environmental predictor variables were recorded for each of the 2408 0.25 ha grid squares. Data for the ninth environmental variable, the abundance of nectar plants, was available only for the core set of 349 grid squares. These variables were assigned into three groups: (i) habitat quantity (habitat area and connectivity), (ii) resource and (iii) microclimate variables. An additional predictor was the ‘autocovariate’, which incorporated the effect of the butterfly abundance in the surroundings into the analysis.
The four habitat area variables included were the amount of agricultural land, semi-natural grassland, deciduous and mixed forest and coniferous forest in each of the 0.25 ha grid squares. The distribution of these habitats was defined from aerial low altitude black and white photographs (1 : 7500 scale) and from topographic maps, and verified in the field (Luoto et al. 2001).
Habitat connectivity was measured only for semi-natural grasslands, the habitat for P. mnemosyne (Luoto et al. 2001). Connectivity (S) to semi-natural grasslands in the surroundings of grid square i was measured as follows (Hanski 1994):
| 2.1 |
where Aj is the area of semi-natural grassland (in ha) in the square, and dij is the distance between squares i and j. The dispersal kernel α was set as 1 based on mark–recapture data on the movements of the butterfly in the study area (Kuussaari & Luoto, unpublished work). Connectivity of the focal cell to semi-natural grasslands in adjacent grid cells was measured up to 330 m, which matches closely with the observed average dispersal distance of P. mnemosyne in boreal areas (Välimäki & Itämies 2003).
The two resource variables were the abundances of nectar plants and larval host plants. The average density of host plant (C. solida) shoots per square metre was estimated from each grid square in April–May. The abundance of the nectar plants (Anthriscus sylvestris, Geranium sylvaticum, Trifolium spp., Vicia cracca, Vicia sepium, Ranunculus spp.) was recorded only from the core set of 349 grid squares using a scale from 0 to 10 (0=no nectar plants, 10=nectar plants extremely abundant) in June.
The two variables reflecting microclimate were radiation and average wind speed. Following Luoto et al. (2001), an estimate of maximum theoretical solar radiation for each grid square was produced using a computer model of clear sky insolation and the exposure of different slopes, based on the equation given by Griffiths (1985). The average wind speed for each grid square was produced by the Finnish Meteorological Institute on the basis of a field survey and topographical and habitat maps using a four-class scale: (i) <0.5; (ii) 0.5–1.5; (iii) 1.5–2.3 and (iv) >2.3 m s−1. Wind speed and the abundance of nectar plants were considered as continuous predictors in the analysis.
The tenth predictor variable, autocovariate, incorporated the effect of the spatial autocorrelation structure of butterfly abundance in the neighbourhood of the focal cell into the analysis (cf. Ferrier et al. 2002). The autocovariate for each focal cell i was measured using the formula of Hanski (1994):
| 2.2 |
where Nj was the number of butterfly individuals in cell j and dij the distance (measured up to 330 m) between squares i and j in metres, and α was set at 1. The exponential function assigns more weight to those squares which are close to the focal square.
(e) Statistics
Variation partitioning was used to decompose the variation in the occupancy and abundance of the clouded apollo among the three groups of predictors: habitat quantity (H), resource (R) and microclimate (M) variables. Variation in the occupancy data was partitioned using a series of (partial) logistic regression analysis with binomial generalized linear models as implemented in the statistical package R (R Development Core Team 2004). As a first step, within each of the three groups of predictors forward selection of variables was performed to exclude variables that did not contribute significantly (p>0.01) to the explained variation (Borcard et al. 1992; Heikkinen et al. 2004). Here, we also entered the quadratic terms of predictors in order to take potential curvilinear relationships between butterfly occupancy and predictor variables into account. The goodness-of-fit for each added variable was measured by the deviance statistics and the change in deviance was tested by an F-ratio test (Venables & Ripley 2002).
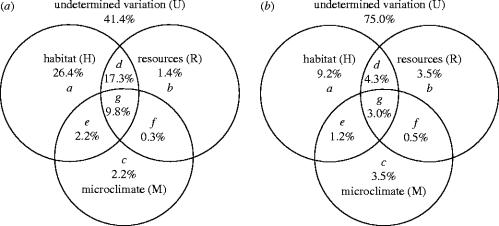
Variation partitioning with three explanatory matrices has been described in detail by Liu (1997), Anderson & Gribble (1998) and Heikkinen et al. (2004). Here, it led to eight fractions: (i) pure effect of habitat quantity; (ii) pure effect of resources; (iii) pure effect of microclimate; and combined variation due to the joint effects of (iv) habitat quantity and resources; (v) habitat quantity and microclimate; (vi) resources and microclimate; (vii) the three groups of explanatory variables and finally (viii) unexplained variation (figure 1). Several fractions, or groups of fractions, can be obtained directly by a (partial) logistic regression run: a+d+e+g: butterfly occupancy by habitat quantity (H); b+d+f+g: butterfly occupancy by resources (R); c+e+f+g: butterfly occupancy by microclimate (M); a: butterfly occupancy by habitat quantity, controlling for resources and microclimate (i.e. fitting resource and microclimate variables first as ‘covariables’ and habitat quantity variables subsequently to explain the residual variation); a+b+d; butterfly occupancy by habitat quantity and resources, controlling for microclimate and so on. Fractions d, e, f and g were calculated by the following equations:
and
Figure 1.
Results of variation partitioning for (a) the occupancy and (b) abundance of P. mnemosyne in terms of the fractions of variation explained. Variation of the species data matrix is explained by three groups of explanatory variables: H (habitat quantity), R (resources) and M (microclimate), and U is the undetermined variation; a, b and c are unique effects of habitat quantity and resources and microclimate, respectively; while d, e, f and g are fractions indicating their joint effects.
The total explained variation in the butterfly data (a+b+c+d+e+f+g in figure 1) was obtained by regressing the occupancy of P. mnemosyne with the selected statistically significant variables of the three groups of explanatory variables together (‘final model’). The importance of the autocovariate in variation partitioning was measured, both for the occupancy and abundance data, by fitting it as an additional predictor to the final model.
The variation in the abundance data of P. mnemosyne was decomposed using a similar process, but using only the 349 grid squares, where P. nmemosyne was present. However, here we performed a series of (partial) regression analysis with redundancy analysis (RDA) using the program CANOCO (ter Braak & Smilauer 2002). Statistical testing for each added variable was conducted with the Monte Carlo permutation tests (9999 permutations).
In hierarchical partitioning, all possible models for the occupancy and abundance of the clouded apollo were considered in a hierarchical multivariate regression setting. This process involved computation of the increase in the fit of all models with a particular predictor compared to the equivalent model without that variable, and averaging the improvement in the fit across all possible models with that predictor. As a result, hierarchical partitioning provides, for each explanatory variable separately, an estimate of the independent and conjoint contribution with all other variables (for more details see Chevan & Sutherland 1991; Mac Nally 2000; Quinn & Keough 2002). Hierarchical partitioning does not result in the development of a predictive model because this is not its purpose (Mac Nally 2002). Instead it aims at generating a detailed basis for inferring causality in multivariate regression settings (Watson & Peterson 1999).
Hierarchical partitioning was conducted using the ‘hier.part package’ version 0.5–1 (Mac Nally & Walsh 2004) which was run as a part of R statistical package (R Development Core Team 2004). Hierarchical partitioning for the butterfly occupancy data was conducted using logistic regression and log-likelihood as the goodness-of-fit measure, and for the abundance data using linear regression and R2 as the goodness-of-fit measure, respectively. Hierarchical partitioning, as currently implemented in the ‘hier.part package’, depends on monotonic relationships between the response and predictor variables. To improve the linearity of relationships between predictors and butterfly variables, the areas of semi-natural grassland and deciduous-mixed forest were square root-transformed and the abundance of larval host plant was log-transformed. The abundance of butterflies was also log-transformed, as suggested by the results of the Box–Cox maximum-likelihood procedure for choosing power transformations of the response (Venables & Ripley 2002). Following Mac Nally (2002), statistical significances of the independent contributions of variables were tested by a randomization routine which yielded Z-scores for the generated distribution of randomized independent contributions and a measure of statistical significance (*) based on an upper 0.95 confidence limit.
3. Results
The majority (34 out of 36) of the bivariate correlations between our predictor variables in the 2408 grid squares were statistically significant (p<0.01; results not shown, but see Luoto et al. 2001), which indicates potential collinearity problems. The highest correlations occurred between habitat connectivity and the autocovariate (Spearman's rs=0.973), windiness and cover of agricultural field (rs=0.816), and larval host plant abundance and cover of grassland (rs=0.634).
(a) Butterfly occupancy
The results of forward selection of explanatory variables for the occupancy of the clouded apollo from the three variable groups are presented in table 1. The selected habitat quantity variables, i.e. linear and quadratic terms of habitat connectivity and cover of semi-natural grassland, together accounted for 54.8% of the variation in the butterfly distribution data (explained deviance 1092.5 out of the total deviance 1993.0), resource variables for 20.1% (401.7 out of 1993.0) and microclimate variables for 14.5% (298.2 out of 1993.0). The importance of quadratic terms of predictors pointed towards curvilinear relationships between predictors and the butterfly occupancy. Closer examination of the bivariate plots and fitted response curves (results not shown) indicated that these relationships were largely asymptotically saturating. For example, in the case of cover of semi-natural grassland and connectivity, the incidence of species occupancy first increased when moving towards grid squares with higher amounts of grassland or higher connectivity and then levelled off (cf. Heikkinen et al. 2004).
Table 1.
The linear and quadratic terms of the variables selected from the three groups of explanatory variables and used in the variation partitioning procedures.
| clouded apollo occupancy | clouded apollo abundance | |
|---|---|---|
| habitat quantity | ||
| agricultural field | NS | NS |
| semi-natural grassland | L***+Q*** | L***+Q*** |
| coniferous forest | NS | NS |
| deciduous–mixed forest | L*** | L***+Q*** |
| habitat connectivity | L***+Q*** | NS |
| resources | ||
| host plant | L***+Q*** | L*** |
| nectar plant | — | L*** |
| microclimate | ||
| radiation | L***+Q*** | L*** |
| windiness | L***+Q*** | L*** |
The forward selection of statistically significant (p<0.01) variables was conducted separately for each of the variable groups, and using multiple logistic regressions for the clouded apollo occupancy data and redundancy analysis (RDA) for the clouded apollo abundance data. NS=not selected; L=linear term; Q=quadratic term; **p<0.01, ***p<0.001. Note: one grid cell that appeared as an outlier in the residual plots of the regression models was excluded from the partitioning procedures for the abundance data.
In variation partitioning, the largest fractions in the occupancy of the butterfly were accounted for by the pure effect of habitat quantity variables (fraction a in figure 1a; 26.4%) and the joint effect of habitat quantity and resources (fraction d; 17.3%), or all three groups of predictors (fraction g; 9.8%). The pure effects of resource and microclimate variables were small, but statistically significant. Fitting the autocovariate as an additional variable to the final model resulted in a statistically significant (p<0.001) deviance change, capturing 3.0% of the deviance in the butterfly data.
In hierarchical partitioning, the independent effects of all included variables were statistically significant (table 2), although some of the contributions were small (figure 2a). As in the case of the variation partitioning results, cover of semi-natural grassland and habitat connectivity showed also here the highest independent contributions to the occupancy of P. mnemosyne. The independent contributions of autocovariate and larval host plant were also noteworthy. The negative joint contribution of radiation indicates that the majority of the relationships between radiation and the other predictors are suppressive and not additive (see Chevan & Sutherland 1991).
Table 2.
Results of the randomization tests for the independent contributions of separate predictor variables in hierarchical partitioning to explaining variation in the occupancy and abundance of P. mnemosyne.
| occupancy | abundance | |
|---|---|---|
| agricultural field | 87.17* | 10.19* |
| semi-natural grasslanda | 241.19* | 15.52* |
| coniferous forest | 14.50* | 3.37* |
| deciduous–mixed foresta | 21.98* | 2.80* |
| habitat connectivity | 184.98* | 1.88* |
| host plantb | 87.18* | 7.41* |
| nectar plant | — | 11.86* |
| radiation | 35.84* | 4.87* |
| windiness | 20.36* | 7.87* |
| autocovariate | 233.08* | 6.27* |
Results are expressed as Z-scores. *p<0.05.
Square-root transformed variable.
log-transformed variable.
Figure 2.
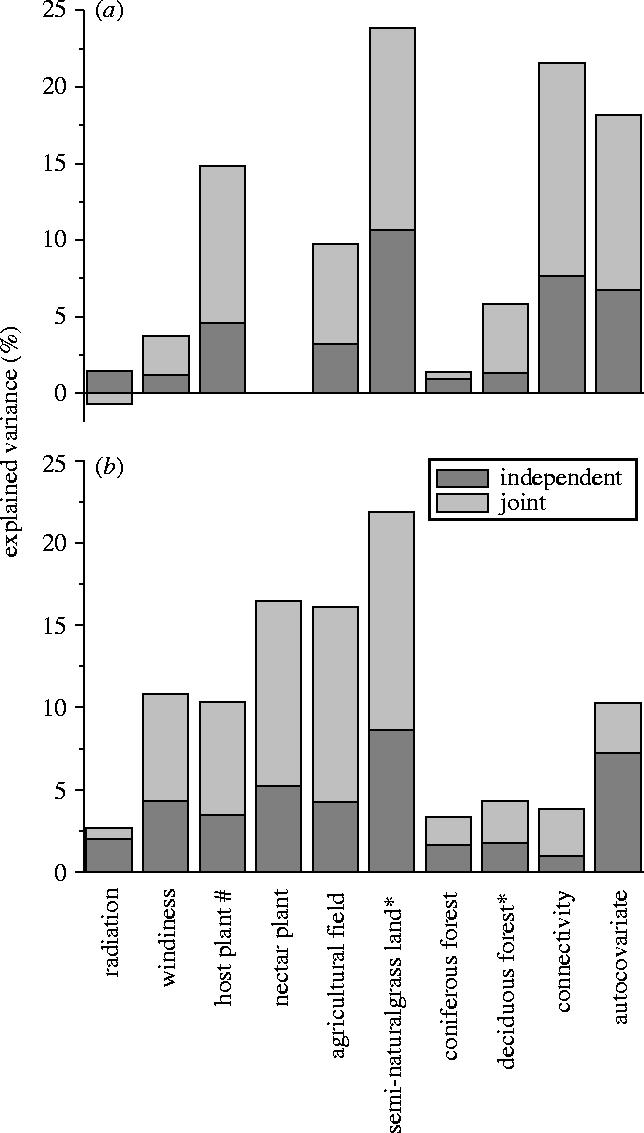
The independent and joint contributions (given as the percentage of the total explained variance) of the predictor variables for (a) the occupancy and (b) the abundance of P. mnemosyne, as estimated from hierarchical partitioning. Data on the nectar plant abundance were only available for (b); #=log-transformed variable, *=square root transformed variable.
(b) Butterfly abundance
Habitat quantity variables selected in the forward selection runs with RDA (see table 1) accounted for 17.7% (the proportion of the sum of all canonical eigenvalues (0.177) of the sum of all eigenvalues (1.00 for RDA)) of the variation in the abundance of the butterfly, whereas resource variables captured 11.3% and microclimate variables 9%, respectively. The quadratic relationship between butterfly abundance and semi-natural grasslands was asymptotically saturating. In contrast, the relationship between the cover of deciduous forest and butterfly abundance was hump-shaped (unimodal), suggesting that the highest density of butterflies occurred in grid squares with intermediate cover of deciduous forest.
Fitting the autocovariate to the final model explained an additional 3.6% (p=0.0002) of the variation in the clouded apollo abundance. In the variation partitioning results, the pure effect of habitat cover variables and the joint effect between them and the resource variables were the largest fractions (figure 1b). However, the pure effects of resource and microclimate variables were higher than the corresponding results for occupancy data.
In hierarchical partitioning, cover of semi-natural grassland had the highest independent contribution, followed by autocovariate and abundance of nectar plants (figure 2b). Independent effects of all predictors were statistically significant (table 2) and a considerable part of the explained variation was related to the joint effects of predictors (figure 2b). Deciduous forest made a smaller contribution than in variation partitioning results, very probably because of the humped response. Habitat connectivity had here the second lowest independent contribution, whereas in the butterfly occupancy data it had the second highest independent contribution.
4. Discussion
Ecological studies on butterflies that have assigned predictor variables to ‘competing’ variable groups have yielded contrasting inferences about the importance of various environmental and spatial factors to butterflies. The importance of habitat quality on butterfly patterns has been emphasized by, e.g. Dennis & Eales (1997, 1999), Thomas et al. (2001), Fleishman et al. (2002), Anthes et al. (2003) and WallisDeVries (2004), and the effects of habitat patch area and/or isolation by, e.g. Hanski & Thomas (1994), Hanski et al. (1996), Moilanen & Hanski (1998), Cowley et al. (2000) and Krauss et al. (2003).
Several methodological issues may contribute to the differences in the reported contributions of habitat quality versus habitat quantity (area and connectivity) between different studies, two of which are discussed here. First, the discrepancies between studies may stem from the varying efficiency of the connectivity or isolation measures employed in different modelling exercises. The connectivity index Si used in this study is currently considered superior to more simple isolation measures such as distance to the nearest patch (Moilanen & Nieminen 2002). Thus, the role of connectivity may have been underestimated in studies based on simple isolation indices (e.g. Thomas et al. 2001).
Second, a large number of butterfly studies have employed traditional regression techniques, which may have distorted inferences about the relative importance of explanatory variables. This is because traditional regression approaches do not provide separate measures of the amounts of variation explained independently and jointly by two or more variables or groups of variables. Due to collinearity among explanatory variables, the variables selected for the model will inevitably pick up some of the explanatory power of, and thereby downplay the importance of the other intercorrelated variables.
Variation and hierarchical partitioning methods used in this study provide measures of the independent and joint explanatory capacities of single predictors or groups of predictors (Mac Nally 2000; Cushman & McGarigal 2004). Overall, the results of the two methods were in our case similar, and the variables highlighted as significant by the two approaches were the same. Our results for the occupancy of P. mnemosyne indicate a dominant independent contribution of habitat quantity and only a very small independent contribution of resources and microclimate. This supports the conclusions of Hanski et al. (1996), Cowley et al. (2000), Krauss et al. (2003) and Bergman et al. (2004) on the importance of habitat area and connectivity. Moilanen & Hanski (1998) reported that adding information on habitat quality did not greatly improve the goodness-of-fit of the incidence function models (based on patch area and isolation) for the patch occupancy and density of Melitaea cinxia.
However, almost half (27.1 of 59.6%) of the total explained variation of the clouded apollo occupancy in our variation partitioning results was related to the joint variation between habitat quantity and resources, or to the joint variation between all three groups of variables. The amount of joint contributions of predictors was also notable in hierarchical partitioning. The shared variation in variation partitioning results may be related to the variation in resources, as well as to microclimate or habitat area and connectivity. If we consider larval host plant abundance as the most potential causal explanatory variable for the joint variation, the total relative importance of resources increases more than tenfold from the pure effect of resources. It is noteworthy that modelling exercises where resource variables are entered only as additional predictors after all the statistically significant habitat quantity variables will give only an estimation of the pure effect of resources, and vice versa.
Habitat quantity (area of semi-natural grassland and deciduous-mixed forest) also had the highest independent fraction in the variation partitioning of the clouded apollo abundance data (see also Luoto et al. 2001, 2002). However, connectivity of semi-natural grasslands made a minor contribution to the abundance of the butterfly both in variation and hierarchical partitioning, possibly because in our study area semi-natural grasslands often occur in a riverside corridor system and not as clearly separated distinct patches.
Resource variables (host-plant and nectar-plant abundance) and microclimate had a relatively higher independent importance for the abundance than for the occupancy patterns of P. mnemosyne. The proportions of joint contributions of predictors on butterfly abundance were also notable. The importance of the amounts of larval host plant and adult nectar sources for butterfly abundance (or occupancy) has been demonstrated by numerous studies (e.g. Dennis 1992; Loertscher et al. 1995; Clausen et al. 2001; Thomas et al. 2001; Fleishman et al. 2002; Anthes et al. 2003; Auckland et al. 2004; WallisDeVries 2004).
The contribution of microclimate reflects the fact that in our study area the clouded apollo occurs most abundantly in sheltered river valleys with warm microclimate and low wind speed. This is not surprising because our study area is at the northern margin of the European distribution of P. mnemosyne. The importance of microclimate for butterfly abundance has been emphasized by many other butterfly studies (e.g. Thomas et al. 1999; Clausen et al. 2001).
The abundance of the butterfly in the surroundings (autocovariate) appeared as a significant predictor in all our partitioning analyses. Thus, the number of butterfly individuals in the surroundings of the focal square had an explanatory power, which was independent of the effects of other variables, including connectivity of semi-natural grasslands, and this effect should be taken into account in modelling. The importance of the autocovariate may reflect occurrences of sites with favourable amounts of resources or sheltered patches in the surroundings, or the behaviour of P. mnemosyne. The latter assumption is supported by the results of Välimäki & Itämies (2003), who reported that the most important single factor explaining the number of immigrating P. mnemosyne individuals was the butterfly density in the target patch.
To reiterate, our results show that habitat quantity, i.e. habitat connectivity and particularly habitat area, have a major effect on the clouded apollo occupancy. Resource and microclimate variables made substantial contributions to the abundance of P. mnemosyne, but their independent impact on the occupancy patterns was small. This suggests that the components of habitat quality become increasingly important for butterflies when examining the finer-scale variation of abundance, whereas habitat quantity and spatial arrangement of habitat patches may largely determine the species distribution patterns in a wider landscape. Nevertheless, in our results a considerable amount of variation in the butterfly patterns was related to joint effects of explanatory variables. Whether this variation is ultimately more causally related to habitat quantity, resources or microclimate can only by assessed by experimental work or by accumulating information from new similar study settings. Overall, the considerable amount of conjoined contributions suggests that the relative importance of different competing predictors may have been insufficiently scrutinized in several earlier butterfly studies.
In conclusion, our study shows that variation and hierarchical partitioning methods provide deeper insights into biodiversity–environment relationships than traditional regression methods, particularly if used in a complementary manner. A potential shortcoming of hierarchical partitioning is that the importance of polynomial variables cannot be assessed by this method. Asymptotic relationships between explanatory and response variables can be improved by taking transformation of the variables, as was done in our study. However, in the case of humped relationships (here P. mnemosyne abundance versus deciduous forest), improving the linearity between response variables and predictors is difficult, if not impossible. Under such circumstances hierarchical partitioning is a less powerful method than variation partitioning, in which the model fit can be improved by including polynomial terms (Heikkinen et al. 2004). A possible weak point in variation partitioning is the potential of collinearity problems during the forward selection within the groups of predictor variables. In such cases, hierarchical partitioning can be used in confirming whether the predictors selected in variation partitioning are among the most likely causal variables (cf. Gibson et al. 2004). Hitherto both partitioning methods have been applied only sporadically in butterfly studies (but see Mac Nally et al. 2003; Sawchik et al. 2003; Cleary & Gennert 2004). We conclude that when applied with caution, butterfly ecology and biodiversity–environment studies in general would benefit from a wider application of the partitioning methods.
Acknowledgments
We thank B. Schröder and an anonymous referee for valuable comments. Different parts of this research were funded by the EC FP6 Integrated Project ALARM (GOCE-CT-2003-506675).
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Anderson M.J, Gribble N.A. Partitioning the variation among spatial, temporal and environmental components in a multivariate data set. Aust. J. Ecol. 1998;23:158–167. [Google Scholar]
- Anthes N, Fartmann T, Hermann G, Kaule G. Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J. Insect Conserv. 2003;7:175–185. 10.1023/A:1027330422958 [Google Scholar]
- Auckland J.N, Debinski D.M, Clarke W.R. Survival, movement, and resource use of the butterfly Parnassius clodius. Ecol. Entomol. 2004;29:139–149. 10.1111/j.0307-6946.2004.00581.x [Google Scholar]
- Augustin N, Mugglestone M.A, Buckland S.T. An autologistic model for spatial distribution of wildlife. J. Appl. Ecol. 1996;33:339–347. [Google Scholar]
- Bergman K.-O, Askling J, Ekberg O, Ignell H, Wahlman H, Milberg P. Landscape effects on butterfly assemblages in an agricultural region. Ecography. 2004;27:619–628. 10.1111/j.0906-7590.2004.03906.x [Google Scholar]
- Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. [Google Scholar]
- Chevan A, Sutherland M. Hierarchical partitioning. Am. Stat. 1991;45:90–96. [Google Scholar]
- Clausen H.D, Holbeck H.B, Reddersen J. Factors influencing abundance of butterflies and burnet moths in the uncultivated habitats of an organic farm in Denmark. Biol. Conserv. 2001;98:167–178. 10.1016/S0006-3207(00)00151-8 [Google Scholar]
- Cleary D.F.R, Gennert M.J. Changes in rain forest butterfly diversity following major ENSO-induced fires in Borneo. Global Ecol. Biogeogr. 2004;13:129–140. 10.1111/j.1466-882X.2004.00074.x [Google Scholar]
- Cowley M.J.R, Wilson R.J, León-Cortes J.L, Gutiérrez D, Bulman C.R, Thomas C.D. Habitat-based statistical models for predicting the spatial distribution of butterflies and day-flying moths in fragmented landscape. J. Appl. Ecol. 2000;37:60–72. 10.1046/j.1365-2664.2000.00526.x [Google Scholar]
- Cushman S.A, McGarigal K. Hierarchical analysis of forest bird species–environment relationships in the Oregon coast range. Ecol. Appl. 2004;14:1090–1105. [Google Scholar]
- Dennis R.L.H, editor. The ecology of butterflies in Britain. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- Dennis R.L.H, Eales H.T. Patch occupancy in Coenonympha tullia (Müller, 1764) (Lepidoptera: Satyrinae): habitat quality matters as much as patch size and isolation. J. Insect Conserv. 1997;1:167–176. 10.1023/A:1018455714879 [Google Scholar]
- Dennis R.L.H, Eales H.T. Probability of site occupancy in the large heath butterfly Coenonympha tullia determined from geographical and ecological data. Biol. Conserv. 1999;87:295–301. 10.1016/S0006-3207(98)00080-9 [Google Scholar]
- Ferrier S, Watson G, Pearce J, Drielsma M. Extended statistical approaches to modeling spatial pattern in biodiversity: the north-east New South Wales experience. I. Species-level modeling. Biodivers. Conserv. 2002;11:2275–2307. 10.1023/A:1021302930424 [Google Scholar]
- Fleishman E, Ray C, Sjögren-Gulve P, Boggs C.L, Murphy D.D. Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv. Biol. 2002;16:706–716. 10.1046/j.1523-1739.2002.00539.x [Google Scholar]
- Gibson L.A, Wilson B.A, Cahill D.M, Hill J. Spatial prediction of rufous bristlebird habitat in a coastal heathland: a GIS-based approach. J. Appl. Ecol. 2004;41:213–223. 10.1111/j.0021-8901.2004.00896.x [Google Scholar]
- Graham M. Confronting multicollinearity in ecological multiple regression. Ecology. 2003;84:2809–2815. [Google Scholar]
- Griffiths J.F. Climatology. In: Houghton D.D, editor. Handbook of applied meteorology. Wiley; New York: 1985. pp. 62–132. [Google Scholar]
- Hanski I. A practical model of metapopulation dynamics. J. Anim. Ecol. 1994;63:151–162. [Google Scholar]
- Hanski I, Thomas C.D. Metapopulation dynamics and conservation: a spatially explicit model applied to butterflies. Biol. Conserv. 1994;68:167–180. 10.1016/0006-3207(94)90348-4 [Google Scholar]
- Hanski I, Moilanen A, Pakkala T, Kuussaari M. The quantitative incidence function model and persistence of an endangered butterfly metapopulation. Conserv. Biol. 1996;10:578–590. 10.1046/j.1523-1739.1996.10020578.x [Google Scholar]
- Heikkinen R.K, Luoto M, Virkkala R, Rainio K. Effects of habitat cover, landscape structure and spatial variables on the abundance of birds in an agricultural-forest mosaic. J. Appl. Ecol. 2004;41:824–835. 10.1111/j.0021-8901.2004.00938.x [Google Scholar]
- Hill J.K, Thomas C.D, Lewis O.T. Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. J. Anim. Ecol. 1996;65:725–735. [Google Scholar]
- Konvicka M, Kuras T. Population structure, behaviour and selection of oviposition sites of an endangered butterfly, Parnassius mnemosyne, in Litovelske Pomoravi, Czech Republic. J. Insect Conserv. 1999;3:211–223. 10.1023/A:1009641618795 [Google Scholar]
- Krauss J, Steffan-Dewenter I, Tscharntke T. How does landscape context contribute to effects of habitat fragmentation on diversity and population density of butterflies? J. Biogeogr. 2003;30:889–900. 10.1046/j.1365-2699.2003.00878.x [Google Scholar]
- Lichstein J.W, Simons T.R, Shriner S.A, Franzreb K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002;72:445–463. [Google Scholar]
- Liu Q. Variation partitioning by partial redundancy analysis (RDA) Environmetrics. 1997;8:75–85. 10.1002/(SICI)1099-095X(199703)8:2%3C75::AID-ENV250%3E3.0.CO;2-N [Google Scholar]
- Loertscher M, Erhardt A, Zettel J. Microdistribution of butterflies in a mosaic-like habitat: the role of nectar sources. Ecography. 1995;18:15–26. [Google Scholar]
- Luoto M, Kuussaari M, Rita H, Salminen J, von Bonsdorff T. Determinants of distribution and abundance in the clouded apollo butterfly: a landscape ecological approach. Ecography. 2001;24:601–617. 10.1034/j.1600-0587.2001.d01-215.x [Google Scholar]
- Luoto M, Kuussaari M, Toivonen T. Modelling butterfly distribution based on remote sensing data. J. Biogeogr. 2002;29:1027–1037. 10.1046/j.1365-2699.2002.00728.x [Google Scholar]
- Mac Nally R. Regression and model-building in conservation biology, biogeography and ecology: The distinction between—and reconciliation of—‘predictive’ and explanatory models. Biodivers. Conserv. 2000;9:655–671. 10.1023/A:1008985925162 [Google Scholar]
- Mac Nally R. Multiple regression and inference in ecology and conservation biology: further comments on identifying important predictor variables. Biodivers. Conserv. 2002;11:1397–1401. 10.1023/A:1016250716679 [Google Scholar]
- Mac Nally R, Walsh C.J. Hierarchical partitioning public-domain software. Biodivers. Conserv. 2004;13:659–660. 10.1023/B:BIOC.0000009515.11717.0b [Google Scholar]
- Mac Nally R, Fleishman E, Fay J.P, Murphy D.D. Modelling butterfly species richness using mesoscale environmental variables: model construction and validation for mountain ranges in the Great Basin of western North America. Biol. Conserv. 2003;110:21–31. 10.1016/S0006-3207(02)00172-6 [Google Scholar]
- Maes D, Gilbert M, Titeux N, Goffart P, Dennis R.L.H. Prediction of butterfly diversity hotspots in Belgium: a comparison of statistically focused and land use-focused models. J. Biogeogr. 2003;30:1907–1920. 10.1046/j.0305-0270.2003.00976.x [Google Scholar]
- Moilanen A, Hanski I. Metapopulation dynamics: effects of habitat quality and landscape structure. Ecology. 1998;79:2503–2515. [Google Scholar]
- Moilanen A, Nieminen M. Simple connectivity measures in spatial ecology. Ecology. 2002;83:1131–1145. [Google Scholar]
- Quinn G.P, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2004. R: a language and environment for statistical computing. See http://www.R-project.org. [Google Scholar]
- Rassi P, Alanen A, Kanerva T, Mannerkoski I, editors. The 2000 Red List of Finnish species. The II Committee for the monitoring of threatened species in Finland (In Finnish with English Summary) Ministry of the Environment & Finnish Environment Institute; Helsinki, Finland: 2001. [Google Scholar]
- Sawchik J, Dufrêne M, Lebrun P. Estimation of habitat quality based on plant community, and effects of isolation in a network of butterfly habitat patches. Acta Oecol. 2003;24:25–33. 10.1016/S1146-609X(02)00005-X [Google Scholar]
- ter Braak C.J.F, Smilauer P. Microcomputer Power; Ithaca, NY, USA: 2002. CANOCO reference manual and canodraw for windows user's guide: software for canonical community ordination (version 4.5) [Google Scholar]
- Thomas J.A, Rose R.J, Clarke R.T, Thomas C.D, Webb N.R. Intraspecific variation in habitat availability among ectothermic animals near their climatic limits and their centres of range. Funct. Ecol. 1999;13(Suppl. 1):55–64. 10.1046/j.1365-2435.1999.00008.x [Google Scholar]
- Thomas J.A, Bourn N.A.D, Clarke R.T, Stewart K.E, Simcox D.J, Pearman G.S, Curtis R, Goodger B. The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc. R. Soc. B. 2001;268:1791–1796. doi: 10.1098/rspb.2001.1693. 10.1098/rspb.2001.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swaay C.A.M, Warren M.S. Nature and environment 99. Council of Europe Publishing; Strasbourg: 1999. Red Data Book of European Butterflies (Rhopalocera) [Google Scholar]
- Välimäki P, Itämies J. Migration of the clouded Apollo butterfly Parnassius mnemosyne in a network of suitable habitats—effects of patch characteristics. Ecography. 2003;26:679–691. 10.1034/j.1600-0587.2003.03551.x [Google Scholar]
- Venables W.N, Ripley B.D. Springer; Berlin: 2002. Modern applied statistics with S. [Google Scholar]
- WallisDeVries M.F. A quantitative conservation approach for the endangered butterfly Maculinea alcon. Conserv. Biol. 2004;18:489–499. [Google Scholar]
- Watson D.M, Peterson A.T. Determinants of diversity in a naturally fragmented landscape: humid montane forest avifaunas of Mesoamerica. Ecography. 1999;22:582–589. [Google Scholar]