Abstract
Exceptionally preserved fossils from the Wenlock Series (Silurian) of Herefordshire, UK, provide unique evidence of metamorphosis from free-swimming cyprid larva to attached juvenile in a Palaeozoic barnacle. The larva had large brush-like anterior limbs. The juvenile shows the head transformed into a stalk and the development of the primordial condition of five mineralized plates within the carapace. The discovery of a cyprid larva indicates that crown group cirripedes had evolved by the Silurian.
Keywords: Thecostraca, Cirripedia, crustacean evolution, Wenlock series, exceptional preservation
1. Introduction
Barnacles have attracted attention since the 1850s when Darwin published his classic monographs (Darwin 1851a,b; 1854a,b) on both living and fossil forms, noting that the present might be styled the ‘age of barnacles’. The barnacles (Cirripedia) fall within the Thecostraca, a group of crustaceans united by a free swimming cypridiform larva (Høeg et al. 2004), which also includes the parasitic ascothoracids and the enigmatic Facetotecta, the latter known only from the so-called y-larva (Grygier 1987). A stem thecostracan, Bredocaris admirabilis, is known from the Upper Cambrian ‘Orsten’ of Sweden (Müller & Walossek 1988; Walossek & Müller 1998). The oldest widely accepted barnacle (Schram 1986; Glenner et al. 1995) is Cyprilepas holmi, a lepadomorph known from juveniles attached to a Silurian eurypterid from Estonia (Wills 1962, 1963), similar in age to the material described here, although a possible lepadomorph has been described from the Cambrian (Collins & Rudkin 1981; but see Briggs 1983). Otherwise there is no record prior to the Carboniferous (Briggs et al. 1993). The Silurian Herefordshire Konservat–Lagerstätte (Briggs et al. 1996) in England (∼425 Myr BP) yields exceptionally preserved three-dimensional fossils that provide unrivalled insights into the palaeobiology of a variety of invertebrates. These include radiolarians (Orr et al. 2000a) and sponges, several arthropods including Haliestes dasos—a pycnogonid (Siveter et al. 2004), Offacolus kingi—a stem-group chelicerate (Orr et al. 2000b; Sutton et al. 2002), Cinerocaris magnifica—a phyllocarid (Briggs et al. 2003), Colymbosathon ecplecticos—a myodocopid ostracode (Siveter et al. 2003), brachiopods (Sutton et al. 2005), Kenostrychus clementsi—a polychaete worm (Sutton et al. 2001c), an aplacophoran-like mollusc Acaenoplax hayae (Sutton et al. 2001a, 2004), orthoconic nautiloids, graptolites, several echinoderms, and a number of organisms whose affinities remain enigmatic. The fossils are preserved as calcitic void in-fills in carbonate concretions within a volcaniclastic horizon (Orr et al. 2000a), and are reconstructed digitally (Sutton et al. 2001b, 2002). Here we report the discovery of a free-living cypridiform larva, a stage in development previously unknown from the fossil record, together with a sessile juvenile from the Herefordshire Lagerstätte. They are interpreted, by association, as two stages in the ontogeny of the same cirripede but the possibility that they represent different species can not be ruled out. Because of the highly derived morphology adopted by the adults following metamorphosis, larval forms are critical to understanding the phylogeny of the group (Høeg et al. 2004).
2. Material and methods
The new arthropod described here is known from two specimens (OUM C.29587, C.29588), the part and counterpart blocks of which were each ground serially and photographed digitally at 20 μm intervals (electronic supplementary material, movies S1–S4). The resulting datasets were used to generate three-dimensional computerized reconstructions (figure 1a–f; electronic supplementary material, movies S5–S6), through the approach detailed in Sutton et al. (2001b). Prior to reconstruction the images were edited carefully to remove extraneous material and to resolve fossil/matrix ambiguities. Images were colour coded manually to identify separate structures such as appendages, head shield, and trunk. This refinement, introduced in Sutton et al. (2002), enables the computer to reconstruct each structure separately and render them in different colours to aid visualization, and to hide structures selectively in order to perform ‘virtual dissections’. It is important to note that the exact point at which one structure meets another (e.g. an appendage and the body) and the colour changes are somewhat arbitrary, and it has not always been possible to maintain consistency. The virtual specimens were studied using pre-rendered video files, printed stereo-pairs, and a custom on-screen visualization system with stereo capabilities.
Datasets from serial grinding are housed in the University Museum of Natural History, Oxford (OUM).
3. Systematic palaeontology
Crustacea, Eucrustacea, Entomostraca
Class: Maxillopoda Dahl, 1856
Subclass: Thecostraca Gruvel, 1905
Infraclass: Cirripedia Burmeister, 1834
Superorder: Thoracica Darwin, 1854
Order: Lepadomorpha Pilsbry, 1916
Genus: Rhamphoverritor gen. nov.
Derivation of name: rhamphos—a curving beak Gr.+converritor—sweeper (masculine), referring to the brush-like antennule.
Diagnosis: cyprid larva with elongate head shield and anterior gape; antennules robust with brush-like terminations. Juvenile with short peduncle and flattened antennules, and thick scutes within the head shield. (Adult unknown.)
Species: Rhamphoverritor reduncus sp. nov.
Derivation of name: reduncus—bent backward, referring to the flexure of the abdomen.
Diagnosis: as for genus.
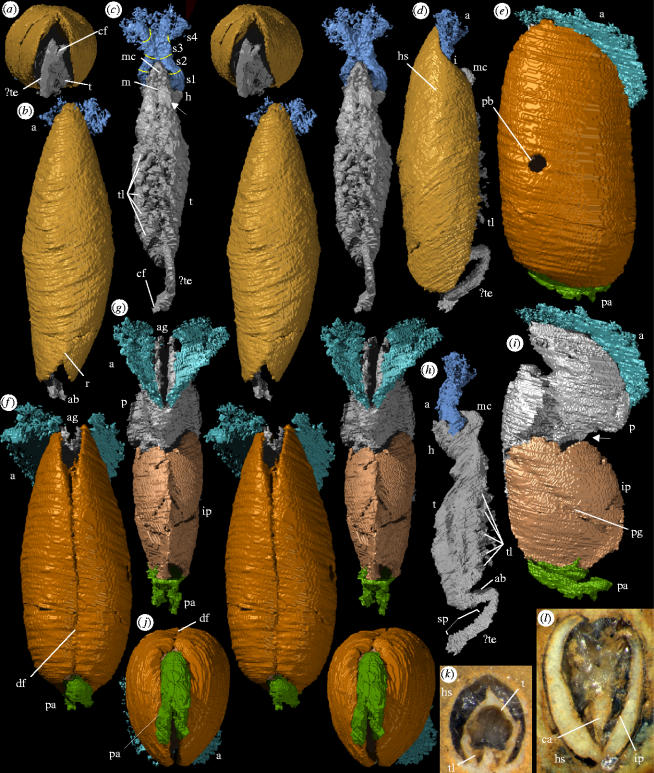
Material: Holotype, a free-swimming cyprid larva OUM C.29587 (figure 1a–d,h,k); other specimen, a settling juvenile, OUM C.29588 (figure 1e–g,i,j,l).
Figure 1.
Rhamphoverritor reduncus. (a–d,h,k) Free swimming stage, holotype (OUM C.29587); (e–g,i,j,l) juvenile (OUM C.29588); Wenlock Series, Herefordshire, England. (a–j) ‘Virtual’ reconstructions; (k,l) specimens in rock. (a) Posterior stereo-pair, magnification×19. (b) Dorsal stereo-pair, magnification×19. (c) Ventral stereo-pair, head shield removed, arrow indicates possible appendage posterior to mouth, magnification×19. (d) Lateral view, magnification×19. (e) Lateral view, magnification×18. (f) Dorsal stereo-pair, magnification×18. (g) Ventral stereo-pair, head shield removed, magnification ×18. (h) Lateral view, head shield removed, magnification×19. (i) Lateral view, head shield removed, arrow indicates constriction between peduncle and inner plates, magnification×18. (j) Posterior stereo-pair, magnification×18. (k,l) Transverse sections prior to serial-grinding, magnification×20. Abbreviations are as follows: a, antennules; ab, abdomen; ag, anterior groove in head; sp, spines; ca, cavity; cf, caudal furca; df, dorsal furrow; h, head; hs, head shield; i, indentation in valves; ip, internal plates; m, mouth; mc, mouth cone; p, peduncle; pa, posterior appendages; pb, predatory boring; pg, (inner) plate groove; r, ridge; s1–4, segments in antennules; t, thorax; te, telson; tl, thoracic limbs.
(a) Cyprid larva
The head shield is elongate oval in lateral outline (figure 1d), ∼4 mm long, the maximum height about 0.3 of the length. There is a pronounced anterior gape created by a shallow indentation in the valves under a tapering rostrum-like projection (figure 1b,d). A ridge at the posterior extremity (figure 1a,b,k) leads to a V-shaped indentation (figure 1b) that marks the division into two valves. The shield is sub-circular in cross-section (figure 1a,k). The valves are about 65 μm thick ventrally but thicken substantially dorsally (figure 1k). The surface appears smooth but structures less than 20 μm are not resolved.
The head is sub-triangular in lateral view with a straight anterior margin (figure 1h). A triangular structure projects beyond the head shield (figure 1d) and may represent a mouth cone, comprising a labrum and appendages. The head tapers anteriorly in ventral view; a small circular depression may represent the mouth (figure 1c). It is flanked posteriorly by a pair of structures that may represent head appendages held against the body (arrow, figure 1c). A pair of large antennules emerges through the anterior gape (figure 1b–d,h); they do not extend far beyond the head shield. Segment boundaries are not well demarcated, but changes in direction indicate four segments in each antennule (figure 1c). Poorly preserved hair-like structures project from both third and fourth segments, giving the appearance of rootlets. Alternatively the two most proximal divisions (s1 and s2 in figure 1c) may represent the two sclerites of the first segment (see Høeg et al. 2004), the second segment consisting of only the short proximal part of s3 in figure 1c.
The thoracic appendages are attached to the trunk ventro-laterally (figure 1c,h); they decrease in size slightly anterior to posterior. Five pairs are clearly evident but details of a possible sixth anterior to these are obscured by merging with the body. The thoracopods are robust proximally and taper abruptly at about half their length before curving posteriorly (figure 1c,h). There is at least one segment in the stout proximal part and three in the slender distal part; the division into rami is not clear. The thorax narrows abruptly just posterior of the last pair of appendages where the slender apodous abdomen projects beyond the head shield (figure 1a,c,d,h). The portion flexed dorsally may represent the telson (figure 1h), which appears to terminate in a pair of caudal rami (i.e. a caudal furca) (figure 1a). The telson bears tens of tiny spines along its dorsal margin (figure 1h).
(b) Juvenile
The juvenile is described in the same orientation as the larva. The head shield is sub-oval in lateral view (figure 1e), ∼4 mm long like the larva, the maximum height about 0.5 of the length. A dorsal furrow (figure 1f) delimits the valves which gape anteriorly; there is no rostrum-like structure. The valves are uniformly thin (about 45 μm) compared to the larva but they show a thickened rim around the ventral margin (figure 1l).
The anterior part of the body is separated from the rest by a pronounced constriction (arrow, figure 1i). It is interpreted as the peduncle that attached the juvenile cirripede to the substrate. A deep median groove along the distal termination (figure 1g) may be equivalent to a short groove on the head of the larva. If this is so, the peduncle in the juvenile is derived largely from the head of the larva. The antennules emerge ventrally and curve parallel to the anterior margin of the valves. The antennules are flattened distally and fringed with short hair-like structures like those in the larva, although these may represent the ragged edge of the appendage (figure 1g,i).
The posterior part of the body is enclosed by an oval structure (figure 1i), subtriangular in section (apex ventral) (figure 1l), that lies inside the head shield. It appears to represent a pair of lateral plates about 300 μm thick, connected dorsally by a wide flat plate (figure 1l) at least in the posterior part. A shallow straight groove traverses the lateral plates antero-dorsally to postero-ventrally (figure 1i). A cavity between the lateral plates (figure 1l) could have accommodated appendages; although none are preserved clearly within this cavity, structures projecting posteriorly beyond the headshield are interpreted as feeding appendages (figure 1e–g,i,j). The juvenile lacks an abdomen.
4. Discussion
The larva of Rhamphoverritor preserves several characters that indicate a thecostracan affinity: head shield shape, presence of probably six swimming thoracopods, highly modified head appendage, and flexed abdomen. The larva is similar to the cypridiform larva of thecostracans (Moyse et al. 1995; Høeg et al. 2004), with its large head shield and modified anterior appendage. This appendage represents the antennule, and is strikingly similar to that in the cirripede cyprid, the distal root-like structure probably representing both third and fourth segments. A seventh trunk segment, which bears the penis in living cirripedes, cannot be distinguished. The abdomen projects beyond the head shield, unlike that in cirripede cyprids. The presence of an abdomen represents the plesiomorphic condition seen in Ascothoracica, where it normally lies within the head shield (Grygier 1987).
The larva is larger than the cypridiform stage in any living thecostracan (the largest, ∼2.5 mm in length, occur in Lepas). An indentation in the valves is unknown in living cypridiforms. The root-like terminations of the antennule differ from the attachment structures in Recent cirripedes (Moyse et al. 1995). There is no evidence of an attachment disc. The villi in Recent cirripedes (Moyse et al. 1995), which produce cement to attach the cyprid to a hard substrate, are smaller (up to 10 per μm2), probably by an order of magnitude, than the preserved terminations in Rhamphoverritor. Such fine structures, however, are beyond the resolution of the fossil. The root-like structures in Rhamphoverritor may be equivalent to setae in living cirripedes (Blomsterberg et al. 2004).
The juvenile is interpreted as an attached thecostracan, just after settling on a substrate the nature of which is unknown. The right valve is perforated by a circular hole about 300 μm in diameter (figure 1e). There is no corresponding mark on the mineralized plate beneath (figure 1i). This hole is interpreted as the result of attack by a tiny predatory gastropod (hatchling Nucella have been reported to make holes 100–200 μm in diameter in plates of Balanus (Palmer 1990), and gastropods are present in the Herefordshire fauna). It is evidence that the juvenile had settled. The antennules are still large structures, but it is not clear how they were attached to the substrate. The featureless anterior part of the body is interpreted as the peduncle at a stage prior to complete unfolding to a vertical orientation. The abdomen has been lost. The lateral structures inside the head shield may represent capitular plates, already detached from the cypridiform valves, which were presumably about to be shed. The oblique groove that traverses each lateral plate may correspond to the division between scutum and tergum (Newman et al. 1969). The flattened median dorsal area may represent the carina. There is no evidence of a rostral plate. We interpret the juvenile as equating to the primordial stage in the ontogeny of living cirripedes that is characterized by five plates: the paired terga and scuta, and the carina, previously reported from a Carboniferous form (Newman 1979; Glenner & Høeg 1993). Thus five plates were apparently present in barnacles by the Silurian.
The Cirripedia comprise three groups, the Acrothoracica (boring forms), Rhizocephala (parasitic forms), and Thoracica (pedunculate and sessile forms, referred to as barnacles). The cypridiform larva is an autapomorphy of the Thecostraca (Høeg et al. 2004; Schram & Høeg 1995), a group including Facetotecta, Ascothoracida and Cirripedia. The morphology of the Herefordshire larva, particularly the nature of the antennule, indicates that it is a true cyprid larva (Høeg et al. 2004), a feature autapomorphic to the Cirripedia. Its discovery in Rhamphoverritor demonstrates the presence of crown group Cirripedia back in the Silurian.
Acknowledgements
We thank the Leverhulme Trust (F/08581/E), the Natural Environment Research Council (GR3/12053), and English Nature for their support; K. Saunders for technical assistance; G. Dietl, J. Høeg and D. Waloszek for discussion; and R. Fenn, T. Hall and J. Sinclair for general assistance.
Supplementary Material
Serial grinding images, slice interval 20 μm. Anterior of OUM C.29587, horizontal field of view 2.90 mm.
Posterior of OUM C.29587, horizontal field of view 2.34 mm.
Anterior of OUM C.29588, horizontal field of view 3.44 mm.
Posterior of OUM C.29588, horizontal field of view 3.44 mm.
Rotating animations of reconstructed specimens, with and without head shield. OUM C.29587.
OUM C.29588.
References
- Blomsterberg M, Høeg J.T, Jeffries W.B, Lagersson N.C. Antennulary sensory organs in cyprids of Octolasmis and Lepas (Crustacea: Thecostraca: Cirripedia: Thoracica): a scanning electron microscope study. J. Morph. 2004;260:141–153. doi: 10.1002/jmor.10131. 10.1002/jmor.10131 [DOI] [PubMed] [Google Scholar]
- Briggs D.E.G. Affinities and early evolution of the Crustacea: the evidence of the Cambrian fossils. In: Schram F.R, editor. Crustacean phylogeny. Balkema; Rotterdam: 1983. pp. 1–22. [Google Scholar]
- Briggs D.E.G, Weedon M.J, Whyte M.A. The fossil record 2. Chapman and Hall; London: 1993. Crustacea, excluding Ostracoda; pp. 321–342. [Google Scholar]
- Briggs D.E.G, Siveter David J, Siveter Derek J. Soft-bodied fossils from a Silurian volcaniclastic deposit. Nature. 1996;382:248–250. 10.1038/382248a0 [Google Scholar]
- Briggs D.E.G, Sutton M.D, Siveter David J, Siveter Derek J. A new phyllocarid (Crustacea: Phyllocarida) from the Silurian Fossil-Lagerstätte of Herefordshire, England. Proc. R. Soc. B. 2003;271:131–138. doi: 10.1098/rspb.2003.2593. 10.1098/rspb.2003.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Rudkin D.M. Priscansermarinus barnetti, a probable lepadomorph barnacle from the Middle Cambrian Burgess Shale of British Columbia. J. Paleont. 1981;55:1006–1015. [Google Scholar]
- Darwin C. The Lepadidae or pedunculated cirripedes. Ray Society; London: 1851a. A monograph on the subclass Cirripedia with figures of all the species. [Google Scholar]
- Darwin C. A monograph on the fossil Lepadidae or pedunculated cirripedes of Great Britain. Palaeont. Soc. Monograph. 1851b;13:88. [Google Scholar]
- Darwin C.R. A monograph on the fossil Balanidae and Verrucidae of Great Britain. Palaeont. Soc. Lond. Monograph. 1854a;30:44. [Google Scholar]
- Darwin C.R. Ray Society; London: 1854b. A monograph on the subclass Cirripedia, with figures of all the species. [Google Scholar]
- Glenner H, Høeg J.T. Scanning electron microscopy of metamorphosis in four species of barnacles (Cirripedia Thoracica Balanomorpha) Mar. Biol. 1993;117:431–439. [Google Scholar]
- Glenner H, Grygier M.J, Høeg J.T, Jensen P.G, Schram F.R. Cladistic analysis of the Cirripedia Thoracica. Zool. J. Linn. Soc. 1995;114:365–404. 10.1006/zjls.1995.0029 [Google Scholar]
- Grygier M.J. New records, external and internal anatomy, and systematic position of Hansen's y-larvae (Crustacea: Maxillopoda: Facetotecta) Sarsia. 1987;72:261–278. [Google Scholar]
- Høeg J.T, Lagersson N.C, Glenner H. The complete cypris larva and its significance in thecostracan phylogeny. In: Scholtz G, editor. Evolutionary developmental biology of Crustacea. Balkema; Rotterdam: 2004. pp. 197–215. [Google Scholar]
- Moyse J, Høeg J.T, Jensen P.G, Al-Yahya H.A.H. Attachment organs in cypris larvae: using scanning electron microscopy. In: Schram F.R, Høeg J.T, editors. New frontiers in barnacle evolution. Balkema; Rotterdam: 1995. pp. 153–177. [Google Scholar]
- Müller K.J, Walossek D. External morphology and larval development of the Upper Cambrian maxillopod Bredocaris admirabilis. Fossils & Strata. 1988;23:1–70. [Google Scholar]
- Newman W.A. A new scalpellid (Cirripedia); a Mesozoic relic living near an abyssal hydrothermal spring. Trans. San Diego Soc. Nat. Hist. 1979;19:153–167. [Google Scholar]
- Newman W.A, Zullo V.A, Withers T.H. Cirripedia. In: Moore R.C, editor. Treatise on invertebrate paleontology, part R. Arthropoda 4. vol. 1. Geological Society of America and University of Kansas Press; Lawrence: 1969. pp. 206–295. [Google Scholar]
- Orr P.J, Briggs D.E.G, Siveter David J, Siveter Derek J. Three-dimensional preservation of a non-biomineralized arthropod in concretions in Silurian volcaniclastic rocks from Herefordshire, England. J. Geol. Soc. 2000a;57:173–186. [Google Scholar]
- Orr P.J, Siveter Derek J, Briggs D.E.G, Siveter David J, Sutton M.D. A new arthropod from the Silurian Konservat-Lagerstätte of Herefordshire, England. Proc. R. Soc. B. 2000b;267:1497–1504. doi: 10.1098/rspb.2000.1170. 10.1098/rspb.2000.1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A.R. Predator size, prey size, and the scaling of vulnerability: hatchling gastropods vs. barnacles. Ecology. 1990;71:759–775. [Google Scholar]
- Schram F.R. Oxford University Press; Oxford: 1986. Crustacea. [Google Scholar]
- Schram F.R, Høeg J.T. New frontiers in barnacle evolution. In: Schram F.R, Høeg J.T, editors. New frontiers in barnacle evolution. Balkema; Rotterdam: 1995. pp. 297–312. [Google Scholar]
- Siveter David J, Sutton M.D, Briggs D.E.G, Siveter, Derek J. An ostracode crustacean with soft parts from the lower Silurian. Science. 2003;302:1749–1751. doi: 10.1126/science.1091376. 10.1126/science.1091376 [DOI] [PubMed] [Google Scholar]
- Siveter Derek J, Sutton M.D, Briggs D.E.G, Siveter David J. A Silurian sea spider. Nature. 2004;431:978–980. doi: 10.1038/nature02928. 10.1038/nature02928 [DOI] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J. An exceptionally preserved vermiform mollusc from the Silurian of England. Nature. 2001a;410:461–463. doi: 10.1038/35068549. 10.1038/35068549 [DOI] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J. Methodologies for the visualization and reconstruction of three-dimensional fossils from the Silurian Herefordshire Lagerstätte. Paleont. Elec. 2001b;4:2. http://palaeo-electronica.org/2001_1/s2/issue1_01.htm [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J. A three-dimensionally preserved fossil polychaete worm from the Silurian of Herefordshire England. Proc. R. Soc. B. 2001c;268:2355–2363. doi: 10.1098/rspb.2001.1788. 10.1098/rspb.2001.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J. The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc. R. Soc. B. 2002;269:1195–1203. doi: 10.1098/rspb.2002.1986. 10.1098/rspb.2002.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J. Computer reconstruction and analysis of a three-dimensionally preserved vermiform mollusc (Acaenoplax hayae) from the Silurian Herefordshire Lagerstätte (Wenlock, England), and implications for molluscan phylogeny. Palaeontology. 2004;47:293–318. 10.1111/j.0031-0239.2004.00374.x [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter, David J, Siveter, Derek J. Silurian brachiopods with soft-tissue preservation. Nature. 2005;436:1013–1015. doi: 10.1038/nature03846. [DOI] [PubMed] [Google Scholar]
- Walossek D, Müller K.J. Early arthropod phylogeny in the light of the Cambrian ‘Orsten’ fossils. In: Edgecombe G.D, editor. Arthropod fossils and phylogeny. Columbia University Press; New York: 1998. pp. 185–231. [Google Scholar]
- Wills L.J. A pedunculate cirripede from the Upper Silurian of Oesel, Esthonia. Nature. 1962;194:567. [Google Scholar]
- Wills L.J. Cyprilepas holmi Wills 1962, a pedunculate cirripede from the Upper Silurian of Oesel, Esthonia. Palaeontology. 1963;6:161–165. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial grinding images, slice interval 20 μm. Anterior of OUM C.29587, horizontal field of view 2.90 mm.
Posterior of OUM C.29587, horizontal field of view 2.34 mm.
Anterior of OUM C.29588, horizontal field of view 3.44 mm.
Posterior of OUM C.29588, horizontal field of view 3.44 mm.
Rotating animations of reconstructed specimens, with and without head shield. OUM C.29587.
OUM C.29588.