Abstract
Social interactions can generate rapid and dramatic changes in behaviour and neuroendocrine activity. We investigated the effects of a changing social environment on aggressive behaviour and brain aromatase activity (bAA) in a sex-changing fish, Lythrypnus dalli. Aromatase is responsible for the conversion of androgen into oestradiol. Male removal from a socially stable group resulted in rapid and dramatic (≥200%) increases in aggression in the dominant female, which will become male usually 7–10 days later. These dominant females and recently sex-changed individuals had lower bAA but similar gonadal aromatase activity (gAA) compared to control females, while established males had lower bAA than all groups and lower gAA than all groups except dominant females. Within hours of male removal, dominant females' aggressive behaviour was inversely related to bAA but not gAA. These results are novel because they are the first to: (i) demonstrate socially induced decreases in bAA levels corresponding with increased aggression, (ii) identify this process as a possible neurochemical mechanism regulating the induction of behavioural, and subsequently gonadal, sex change and (iii) show differential regulation of bAA versus gAA resulting from social manipulations. Combined with other studies, this suggests that aromatase activity may modulate fast changes in vertebrate social behaviour.
Keywords: vertebrate, plasticity, oestrogen, teleost, 11-ketotestosterone, testosterone
1. Introduction
Aromatase, the enzyme that converts testosterone to oestradiol, has been implicated in the early development and later adult expression of sexual behaviour in a wide range of vertebrate species. Early treatments with aromatase inhibitors that generated rats with a bisexual phenotype (Bakker et al. 1993) or reversed gonadal sex in chickens (Elbrecht & Smith 1992) exemplify the important role of aromatase during sexual development. Aromatase in the brain also modulates behaviour in adult birds and mammals (Lephart 1996; Pinckard et al. 2000). Most effects of oestrogens derived from testosterone aromatization are thought to reflect specific changes in the transcription of oestrogen-dependent genes (McEwen & Alves 1999) but oestrogen can have rapid actions through non-genomic mechanisms in a variety of biological systems (Kelly & Ronnekleiv 2002) and its production can be rapidly regulated via changes in brain aromatase activity (AA) in birds (Balthazart et al. 2003). Considering this fast regulation of brain AA and rapid oestrogen effects on brain and behaviour, we investigated a social trigger for rapid changes in sexual phenotype and its effect on AA and behaviour. In a socially stable group of bluebanded gobies, Lythrypnus dalli, removal of the dominant male produces dramatic behavioural and morphological modifications in the dominant female, which then changes sexual phenotype from female to male. Within minutes to hours of male removal, the dominant female increases her aggressive behaviour, an accurate early indicator that the female will morphologically change sex to male (Reavis & Grober 1999). This system therefore exhibits socially mediated transitions between sexual phenotypes and an early and unambiguous behavioural change that robustly predicts the individual that will change sexual phenotype.
In preliminary studies of L. dalli, males had lower AA in both brain and gonad than females, suggesting that a decrease in AA occurs during sex change. Changing social interactions in L. dalli could downregulate AA (Grober 1997) and cause a dramatic change in behaviour (Reavis & Grober 1999). To test this hypothesis, we compared AA and behaviour of females in the early stage of sex change to control females, established males, and recently sex-changed fishes.
2. Material and methods
(a) Subjects and in vivo manipulations
Fishes were collected off the coast of Catalina Island, California (permit no. SC-003083), and then maintained in a fish facility in Atlanta, Georgia. Our experiment ran from February to March of 2003 with 19 social groups. Each group had one large male (standard length (SL)=37.27±0.53 mm, means±s.e.m.), one large female at least 3 mm smaller than the male (SL=30.85±0.61 mm), and two females at least 3 mm smaller than the large female (SL=22.82±0.35 mm). These sizes assured male dominance over all fishes and the largest female's dominance over all females in the group. Each group was placed in a 40 l aquarium with a PVC nesting tube and given 5 days' adjustment to new social conditions. On the fourth day, the largest female's behaviour was observed for 10 min in both the morning and afternoon. Recorded behaviour included approaches, displacements and jerks (Reavis & Grober 1999). Briefly, displacements are ritualized aggression defined as moving within 5 cm of another fish and resulting in that fish moving away, and jerks are male-typical courtship swims. Each behaviour was averaged for the day as baseline frequency.
There were four group types: dominance phase, sex-changed, control females and males. In dominance phase groups (n=8), males were removed in the morning on the fifth day and a female's behaviour was observed for 10 min after male removal, 10 min in the afternoon and 10 min in the morning the next day. The large female in these groups was sacrificed after any of these three observation periods once she met behavioural criteria for dominance, as assessed by exclusive access to the nest tube and/or a doubling of her baseline frequency of aggressive displacement behaviour. All dominance phase fishes had been sacrificed after the third observation period. In each case, we recorded the time from when the male was removed from the social group to when the female's tissue was frozen (see below). These latencies are conservative time estimates because if a male had been in the nest tube, it may have taken some time before the female discovered that the male was missing. The dominance phase fishes are at an early stage in the sex change process.
In sex-changed groups (n=4), the male was also removed on the fifth day and the large female was allowed to fully change sex. Once the sex changer fertilized eggs as a male, it was sacrificed.
In control female groups (n=4), the male remained in the group and the large female was sacrificed at the same time as in the sex changer groups. The sex changer and control groups were paired two by two before experiments began and the large females in these groups were sacrificed in parallel on the same days (8.5±2.53 days) after male removal in the sex-changed groups.
To provide additional reference values, six males that had remained in control groups were also sampled at the same time as control females and their brains and gonads were collected for analysis. Four males came from the same groups as the four control females used in this study. The other two males came from control groups where ‘females’ were excluded because gonad structure in females did not correspond with their genitalia (see below). No difference was detected between these two subgroups of males in brain or gonadal AA or last recorded displacements toward the dominant female (unpaired t-test, t=1.28, p=0.27 and t=1.40, p=0.24, t=0.27, p=0.80, respectively).
All fishes above were rapidly sacrificed via decapitation and the brain and then the gonad were removed and frozen on dry ice. Tissues were kept at −78 °C until shipping, on dry ice, to Belgium for AA assays.
The genital papilla (external genitalia) of each fish was photographed before and after the experiment and length:width ratios were measured (Carlisle et al. 2000). The ratios were used to assess the subjects' sex at the experiment's start. However, the genital papilla of L. dalli is not a perfect predictor of functional sex (St. Mary 1993). Before freezing, gonad inspection verified initial genital-based sex assignment and five fishes coming from three different groups were found to have gonads that were not consistent with the initial sex assignment. These groups were removed from the experiment before we were aware of their brain or gonadal AA, their papillae data were not included, and numbers presented above correspond to the final sample sizes.
(b) Aromatase assay
All frozen brain and gonad samples were weighed, homogenized, and assayed for AA by measuring the tritiated water production from [1β-3H]-androstenedione, as described by Roselli & Resko (1991), with minor modifications (Baillien & Balthazart 1997). Homogenates containing about 1 mg of fresh weight tissue per assay were incubated with 25 nM androstenedione at 37 °C for 1 h for brain and 15 min for gonadal tissue. The incubation durations were selected based on preliminary experiments to limit the amount of substrate metabolized so that the enzymatic reactions could proceed linearly during the entire incubation period (data not shown). Preliminary assays had confirmed that the substrate concentration used here is saturating (at least five times Km) in L. dalli as it is in goldfish (Zhao et al. 2001).
Within each experiment, controls using boiled brain or brain samples with an excess (final concentration 40 μM) of the potent and specific aromatase inhibitor, R76713 (Racemic vorozole, Janssen Pharmaceutica, Beerse, Belgium) never exceeded 300–600 dpm while active control samples had radioactivities ranging between 2000 and 150 000 dpm. Assays were performed so that each run had controls and samples from each of the experimental groups. A recovery of 93±2% was usually obtained from samples of 10 000 dpm tritiated water conducted throughout the entire purification procedure (incubation, centrifugation and Dowex column). Protein content of all homogenates was determined in triplicate by a micromodification of the Bradford method (Bradford 1976). Enzyme activity was expressed in pmol h−1 mg−1 protein after correction of the counts for quenching, recovery, blank values and percentage of tritium in β-position in the substrate.
(c) Data analysis
Statistics were performed using JMP 5.0.1, Statview 5.0 and Sas 8.02 (SAS Institute, Cary, NC). A repeated measures ANOVA was used to compare displacement behaviour before male removal (day 4) and before sacrifice across groups, followed by paired t-tests with Bonferroni correction to compare changes in displacement behaviour within each experimental group. A MANOVA followed by one-way ANOVAs was used to compare differences between groups and was followed when appropriate by Fisher protected least significant difference tests to compare groups two by two. Linear regressions were used to analyse relationships between AA levels, genitalia, behaviour and latency between male removal and sacrifice. All data in the text are presented as means±s.e.m.
3. Results
(a) Behaviour
There was an overall difference in displacement behaviour before male removal (day 4) and displacements before sacrifice, but no overall difference between treatment groups (F1,13=12.09, p=0.004 and F2,13=0.645, p=0.54, respectively; figure 1a; repeated measures ANOVA). There was also a significant interaction between these factors (F2,13=4.21, p=0.039). Further post hoc analysis revealed that dominance females had statistically higher displacement behaviour at sacrifice compared to levels prior to male removal (p=0.003) and sex changers showed a trend in the same direction (p=0.057), while control females did not show a significant change in their levels of displacement behaviour (p=0.767).
Figure 1.
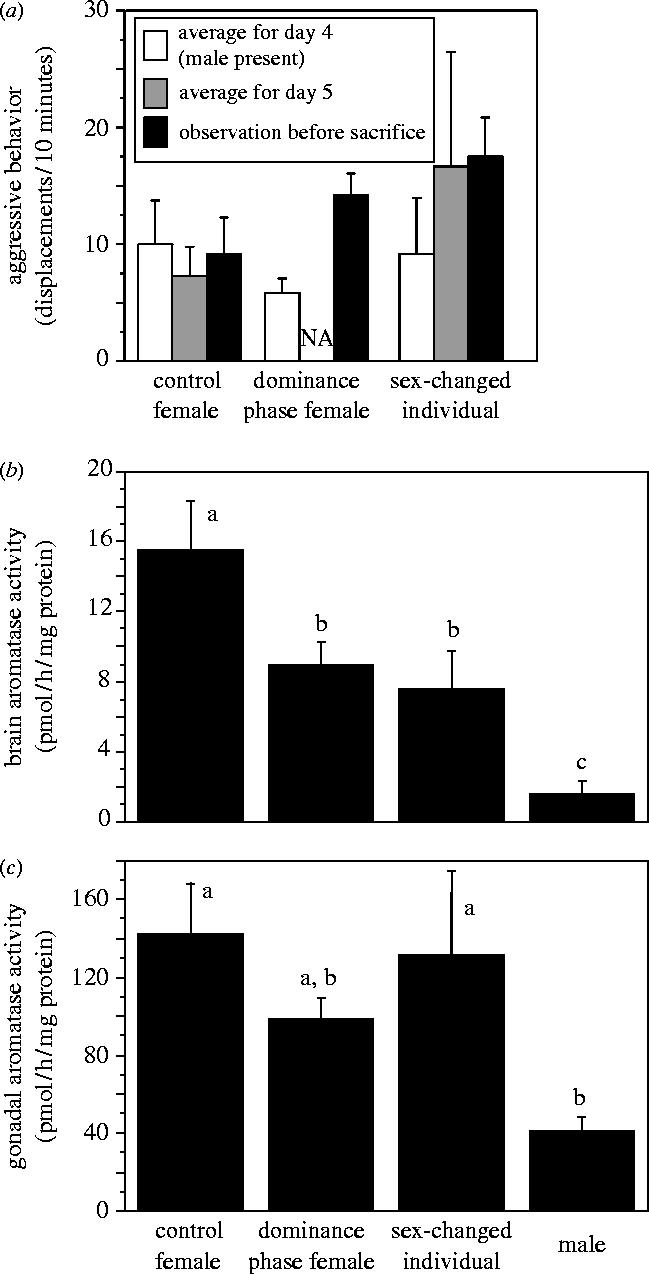
Aggressive displacement behaviour and aromatase activity (AA) in the brain and gonad of Lythrypnus dalli. For behaviour (a), on day 4 (prior to male removal), there was no statistical difference in average daily displacements (baseline frequency) between the largest females. On day 5 the male was removed from dominance phase and sex-changed groups and dominant females increased their aggressive behaviour. Dominance phase fishes have no average (NA) day 5 data because they were sacrificed during day 5 or just after. Brain (b) but not gonadal (c) AA was significantly lower in dominance phase and sex-changed individuals compared to control females, and established males had lower brain AA than all other groups and lower gAA than all groups except dominance phase females (second and third panels). Bars with a different letter are significantly different (p<0.05) based on post hoc Fisher PLSD tests following a significant overall ANOVA.
(b) Brain and gonadal aromatase
Dominance phase females had their brains frozen on dry ice an average of 12.76±4.65 h after male removal. This relatively large average latency is due to the fact that some subjects went through two observation periods without meeting criteria, and so had to be kept overnight for a third observation period the following day. Thus, although most groups reached criterion in the first 10 min observation period (62.5%), our median time to collect brain tissue was about 3.79 h after male removal. All groups that reached criterion in the first observation period were frozen less than 4.25 h after male removal.
Both brain and gonadal AA differed across groups (MANOVA, Wilks' Lambda, F6,34=6.39, p<0.001). Brain AA (bAA) and gonadal AA (gAA) were significantly different between experimental groups (bAA: F3,18=10.71, p<0.001; gAA: F3,18=4.72, p=0.01; figure 1b,c). Post hoc analysis showed that bAA was significantly higher in control females than in the early dominance phase females (p=0.01) and sex changers (p<0.01). The early dominance females were not different from the sex changers (p>0.05). In addition, established males had lower bAA than all other groups (p<0.05). Post hoc analysis showed lower gAA in established males than in all other groups (p<0.05), except dominance phase females (p>0.05). Among all groups other than established males, including those groups that were in the process of changing sex and those that had just changed sex and fertilized eggs as a male, there was no significant difference in gAA (p>0.05; figure 1c).
(c) Regressions
Multiple regression for those dominance phase females that increased their rates of aggression in response to male removal (n=7) showed that there was no significant relationship between bAA and both time after male removal and increases in aggressive behaviour (F2,4=4.193, R2=0.677, p=0.104) and there was no relationship between bAA and time after male removal (p-value for individual regression coefficient=0.455), but there might be for bAA and increases in aggression (p-value for individual regression coefficient=0.044). Simple linear regression on the individual variables demonstrated that bAA levels were not associated with the amount of time after male removal (F1,5<0.001, R2<0.001, p=0.99), but rather with the increased aggressive behaviour of these dominance phase fishes (figure 2; F1,5=8.22, R2=0.62, p=0.04). Moreover, the level of aggression in these females, either prior to removal of the male or just before sacrifice, was not significantly associated with bAA (F1,5=0.006, R2=0.001, p=0.94 and F1,5=0.881, R2=0.15, p=0.39, respectively). Thus, it is the increase in aggressive behaviour following male removal that appears to linked to lower bAA.
Figure 2.
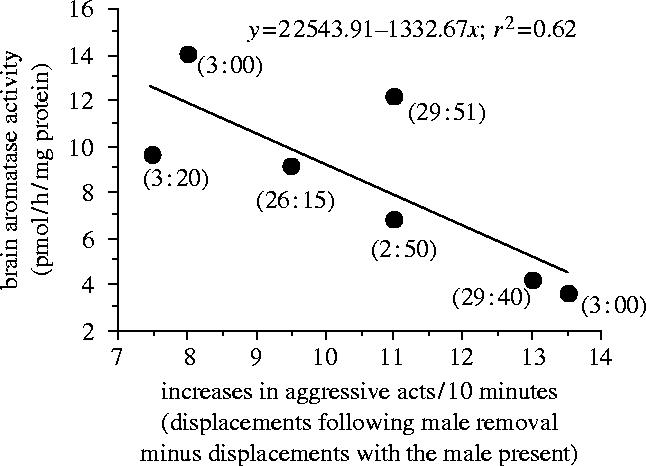
Regression of brain aromatase activity against socially induced increases in aggression in Lythrypnus dalli during the dominance phase of sex change. Increased aggression is scored as the number of aggressive acts (displacements) performed after male removal during the last test period before sacrifice minus the number of acts performed in the presence of the male (prior to male removal). The number next to each data point represents the time from male removal (the social cue) to when the brain was frozen on dry ice (e.g. 3 : 20 is 3 h and 20 min).
Regression showed no relationship between final genitalia length : width ratio and bAA or gAA of all fishes sacrificed (R2<0.03, p>0.05). Further, there was no relationship between gAA and bAA or the increase in aggression of dominance phase fishes (R2<0.03, p>0.05).
4. Discussion
We show here higher bAA in females than in male L. dalli, contrary to what is observed in birds and mammals (Schumacher & Balthazart 1986; Roselli 1991), but consistent with some fishes (e.g. Callard et al. 1978; Contractor et al. 2004). More importantly, removal of the male from stable social groups results in a rapid (within hours) increase in aggression in the largest female, correlated with lower bAA but not gAA. The female that establishes dominance through this increased aggression will fertilize eggs as a male, but the sex-changed individual resulting from this process still has similar bAA and gAA levels as dominance phase females. In contrast, in established males, bAA and gAA are significantly lower than in individuals that have recently changed sex from female to male. These results are novel in that they are the first to: (i) demonstrate socially induced decreases in bAA levels that correspond with increases in aggressive behaviour, (ii) identify this process as a possible neurochemical mechanism regulating the induction of behavioural, and subsequently gonadal, sex change and (iii) show differential regulation of bAA versus gAA resulting from social manipulations.
As noted above, established males showed much lower bAA and gAA relative to recently sex-changed fishes. Because testicular tissue is built up faster than ovarian tissue is broken down, ovarian tissue remains in recently sex-changed L. dalli (Black et al. 2005). Visual inspection of gonads confirmed that sex-changed individuals in our study still had ovarian tissue while males did not. The ovarian tissue remaining in sex-changed fishes may both generate high levels of gAA and regulate bAA through gonadal oestrogen production. Since oestrogen upregulates bAA in other fishes (Pasmanik et al. 1988; Kishida & Callard 2001), ovarian oestrogen may prevent a drop in bAA to levels observed in established males that have completely degraded their ovarian tissue.
We demonstrated dramatic differences in AA between the brain and gonad, but the differential regulation of bAA and gAA is not unexpected. In goldfish and zebrafish, the aromatase CYP19B gene is expressed more in the brain, while CYP19A predominates in the gonad (Callard & Tchoudakova 1997; Tchoudakova & Callard 1998). Moreover, tissue-specific promoters can differentially regulate aromatase expression in mice and humans (Simpson et al. 2000). On a shorter-term basis, it has also been shown in quail that brain and ovarian aromatase react differentially to calcium and various phosphorylating conditions (M. Baillien & J. Balthazart, unpublished data). Several mechanisms are therefore available to differentially regulate AA in the brain and gonad.
One important question that arises from the rapid change in both behaviour and bAA, but not gAA is, does the decrease in bAA cause behavioural sex change? Our data suggest that early in the process of brain reorganization from female to male, bAA drops dramatically. Decreased bAA in L. dalli should limit oestrogen synthesis, leave more testosterone available for conversion into 11-ketotestosterone (11-KT), a potent fish androgen (Borg 1994; figure 3), and thus increase the brain androgen : oestrogen (A : E) ratio. This increased A : E ratio (or an increase in 11-KT production alone) may be responsible for the increased aggression that negatively correlates with bAA and is the earliest predictor of sex change (Reavis & Grober 1999). In fishes, behaviour can change rapidly in response to 11-KT (Remage-Healey & Bass 2004), and several studies support the idea that increased production of androgen, such as 11-KT, increases aggression (e.g. Brantley et al. 1993; Borg 1994; Oliveira et al. 2001). Moreover, similar negative correlations between bAA and aggression have been observed in mammals. For example, in Peromyscus mice, increases in aggressive behaviour correlate with reduced bAA in the bed nucleus of the stria terminalis, and experimentally reduced aromatase levels resulted in shorter attack latencies (Trainor et al. 2004).
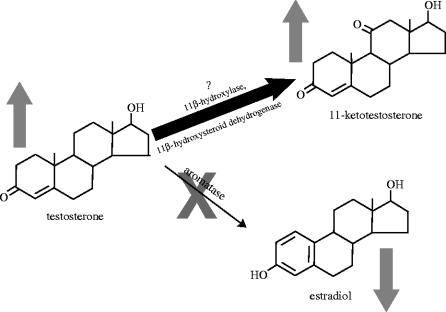
Figure 3.
Model for the potential neurosteroidal consequences of decreased aromatase activity (note grey X). First, estradiol production decreases, while testosterone levels increase (indicated by grey arrows). Higher levels of testosterone (T) substrate could (see question mark) then increase conversion to 11-ketotestosterone (11-KT; grey arrow). The increased T and/or greater conversion to 11-KT, reduced oestrogen, or the greater androgen : oestrogen ratio could be affecting the brain, behaviour and morphology of sex changing individuals.
A second important question that arises from our data is, does the decrease in bAA cause morphological sex change? Androgens promote a variety of male-typical traits in L. dalli, including testicular growth, secondary sex characters like the accessory gonadal structure, and increases in genitalia length : width ratios (Carlisle 2001). As changes in bAA can affect peripheral levels of steroids in the zebra finch (male oestrogen levels; Schlinger & Arnold 1991), the lower bAA in dominance phase and sex-changed fishes may have been sufficient to affect peripheral levels of oestrogen and androgens. The results of the present study suggest the intriguing possibility that a change in the social environment causes early downregulation of bAA, which can act in two possible ways to affect morphological sex: (i) decreased bAA triggers a cascade of events resulting in altered serum androgen levels and morphological sex change, or (ii) decreased bAA changes morphological sex via direct affects on serum hormone levels. In either case, the brain leads the gonad in this process of sexual redifferentiation (e.g. Grober & Bass 1991; Francis 1992). This mechanism is consistent with studies showing that bluehead wrasse behaviourally change sex in the absence of their gonads and gonadally derived steroids (Godwin et al. 1996). This is also consistent with our model of downregulation of AA (Grober 1997) driving behavioural changes that independently precede gonadal changes.
Finally, changes in aromatase function may significantly alter brain and serum steroid levels and steroids are known to have potent effects on sex-changing fishes (Devlin & Nagahama 2002). Variation in AA among different sexual phenotypes has been found in several fishes (e.g. Schlinger et al. 1999) including sex changers (e.g. Kincl et al. 1987; Lee et al. 2002). Consistent with our results, treatment with aromatase inhibitor induces female to male sex change in blackeye and coral gobies (Kroon & Liley 2000; Kroon et al. 2005), but blocks protandrous (male to female) sex reversal in the black porgies (Lee et al. 2002). These studies implicate the role of aromatase in the sex change process, but do not identify the exact nature or timing of that role. Future studies in L. dalli will focus on how quickly bAA changes following male removal, whether a causal relationship between bAA, aggression and sex change exists, and what mechanisms decrease bAA.
Acknowledgments
We thank C. Mizell, E. Stokes, E. Rodgers, J. Netherton and K. Felton for help with behavioural observations, J. Pylkkanen and C. Drilling for the help in catching fishes, E. Broadwater for papilla measurements, R. Earley and C. Derby for statistical consulting, and R. Earley and E. Rodgers for helpful comments on the manuscript. This material is based upon work supported in part by the STC Program of the National Science Foundation under Agreement no. IBN-9876754, the Georgia Research Alliance and GSU-RPE program, NSF-IBN 9723817 to M.S.G. and NIMH (MH50388) and the Belgian FRFC (2.4562.05) to J.B.
References
- Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J. Steroid Biochem. Mol. Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. 10.1016/S0960-0760(97)00080-0 [DOI] [PubMed] [Google Scholar]
- Bakker J, Brand T, van Ophemert J, Slob A.K. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav. Neurosci. 1993;107:480–487. doi: 10.1037//0735-7044.107.3.480. 10.1037//0735-7044.107.3.480 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier T.D, Ball G.F. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur. J. Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. 10.1046/j.1460-9568.2003.02598.x [DOI] [PubMed] [Google Scholar]
- Black M.P, Moore B, Canario A.V.M, Ford D, Reavis R.H, Grober M.S. Reproduction in context: field testing a laboratory model of socially controlled sex change in Lythrypnus dalli (Gilbert) J. Exp. Mar. Biol. Ecol. 2005;318:127–143. [Google Scholar]
- Borg B. Androgens in teleost fishes. Comp. Biochem. Physiol. C. 1994;109:219–245. 10.1016/0305-0491(94)90005-1 [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brantley R.K, Wingfield J.C, Bass A.H. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm. Behav. 1993;27:332–347. doi: 10.1006/hbeh.1993.1025. 10.1006/hbeh.1993.1025 [DOI] [PubMed] [Google Scholar]
- Callard G.V, Tchoudakova A.V. Evolutionary and functional significance of two CYP19 genes differentially expressed in brain and ovary of goldfish. J. Steroid Biochem. Mol. Biol. 1997;61:387–392. doi: 10.1016/s0960-0760(97)80037-4. 10.1016/S0960-0760(97)80037-4 [DOI] [PubMed] [Google Scholar]
- Callard G.V, Petro Z, Ryan K. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- Carlisle, S. L. 2001 Androgens mediate changes in sexually dimorphic structures in the bluebanded goby. Master Thesis, Arizona State University, Tempe, AZ, USA.
- Carlisle S.L, Marxer-Miller S.K, Canario A.V.M, Oliveira R.F, Carneiro L, Grober M.S. Effects of 11-ketotestosterone on genital papilla morphology in the sex changing fish Lythrypnus dalli. J. Fish Biol. 2000;57:445–456. 10.1006/jfbi.2000.1320 [Google Scholar]
- Contractor R.G, Foran C.M, Shuanfang L, Willett K.L. Evidence of gender- and tissue-specific promoter methylation and the potential for ethinylestradiol-induced changes in Japanese medaka (Oryzias latipes) estrogen receptor and aromatase genes. J. Toxicol. Environ. Health A. 2004;67:1–22. doi: 10.1080/15287390490253633. [DOI] [PubMed] [Google Scholar]
- Devlin R.H, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. 10.1016/S0044-8486(02)00057-1 [Google Scholar]
- Elbrecht A, Smith R.G. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255:467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Francis R. Sexual lability in teleosts—developmental factors. Q. Rev. Biol. 1992;67:1–18. 10.1086/417445 [Google Scholar]
- Godwin J, Crews D, Warner R.R. Behavioral sex change in the absence of gonads in a coral reef fish. Proc. R. Soc. B. 1996;263:1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- Grober M.S. Conserved neuroendocrine foundations give rise to diverse sexual phenotypes in fish. In: Ellis L, Ebertz L, editors. Sexual orientation: toward biological understanding. Praeger; Westport, CT: 1997. pp. 2–20. [Google Scholar]
- Grober M.S, Bass A. Neuronal correlates of sex/role change in labrid fishes: LHRH-like immunoreactivity. Brain Behav. Evol. 1991;38:302–312. doi: 10.1159/000114396. [DOI] [PubMed] [Google Scholar]
- Kelly M.J, Ronnekleiv O.K. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. Academic Press; San Diego: 2002. pp. 361–380. [Google Scholar]
- Kincl F.A, Kramer C.H, Koulish S. Sex reversal in wrasses. I. Uptake of testosterone by the gonads and central nervous system and its aromatization in the CNS of Thalassoma duperrey (Teleostei: Labridae) Endocrinol. Exp. 1987;21:115–123. [PubMed] [Google Scholar]
- Kishida M, Callard G.V. Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology. 2001;142:740–750. doi: 10.1210/endo.142.2.7928. 10.1210/en.142.2.740 [DOI] [PubMed] [Google Scholar]
- Kroon F.J, Liley N.R. The role of steroid hormones in protogynous sex change in the blackeye goby, Coryphopterous nicholsii. Gen. Comp. Endocrinol. 2000;118:273–283. doi: 10.1006/gcen.2000.7459. 10.1006/gcen.2000.7459 [DOI] [PubMed] [Google Scholar]
- Kroon F.J, Munday P.L, Westcott D.A, Hobbs J.-P.A, Liley N.R. Aromatase pathway mediates sex change in each direction. Proc. R. Soc. B. 2005;272:1399–1405. doi: 10.1098/rspb.2005.3097. 10.1098/rspb.2005.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-H, Yueh W.-S, Du J.-L, Sun L.-T, Chang C.-F. Aromatase inhibitors block natural sex change and induce male function in the protandrous black porgy, Acanthopagarus schlegeli Bleeker: possible mechanism of natural sex change. Biol. Reprod. 2002;66:1749–1754. doi: 10.1095/biolreprod66.6.1749. [DOI] [PubMed] [Google Scholar]
- Lephart E.D. A review of brain aromatase cytochrome P450. Brain Res. Brain Res. Rev. 1996;22:1–26. 10.1016/0165-0173(96)00002-1 [PubMed] [Google Scholar]
- McEwen B.S, Alves S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. 10.1210/er.20.3.279 [DOI] [PubMed] [Google Scholar]
- Oliveira R.F, Canario A.V, Grober M.S. Male sexual polymorphism, alternative reproductive tactics, and androgens in combtooth blennies (Pisces: Blenniidae) Horm. Behav. 2001;40:266–275. doi: 10.1006/hbeh.2001.1683. 10.1006/hbeh.2001.1683 [DOI] [PubMed] [Google Scholar]
- Pasmanik M, Schlinger B.A, Callard G.V. In vivo steroid regulation of aromatase and 5 alpha-reductase in goldfish brain and pituitary. Gen. Comp. Endocrinol. 1988;71:175–182. doi: 10.1016/0016-6480(88)90308-5. 10.1016/0016-6480(88)90308-5 [DOI] [PubMed] [Google Scholar]
- Pinckard K.L, Stellflug J, Resko J.A, Roselli C.E, Stormshak F. Review: brain aromatization and other factors affecting male reproductive behavior with emphasis on the sexual orientation of rams. Domest. Anim. Endocrinol. 2000;18:83–96. doi: 10.1016/s0739-7240(99)00065-x. 10.1016/S0739-7240(99)00065-X [DOI] [PubMed] [Google Scholar]
- Reavis R.H, Grober M.S. An integrative model of sex change: social, behavioral and neurochemical changes in Lythrypnus dalli (Pisces) Acta Ethol. 1999;2:51–60. [Google Scholar]
- Remage-Healey L, Bass A.H. Rapid hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 2004;24:5982–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. 10.1523/JNEUROSCI.1220-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C.E. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- Roselli C.E, Resko J.A. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine research methods. vol. 2. Harwood Academic Publishers; Switzerland: 1991. pp. 937–951. [Google Scholar]
- Schlinger B.A, Arnold A.P. Brain is the major site of estrogen synthesis in a male songbird. Proc. Natl Acad. Sci. USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B.A, Greco C, Bass A.H. Aromatase activity in the hindbrain vocal control region of a teleost fish: divergence among males with alternative reproductive tactics. Proc. R. Soc. B. 1999;266:131–136. 10.1098/rspb.1999.0612 [Google Scholar]
- Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. 10.1016/0006-8993(86)90483-X [DOI] [PubMed] [Google Scholar]
- Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Jones M, Davis S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol. Metab. 2000;11:184–188. doi: 10.1016/s1043-2760(00)00254-x. 10.1016/S1043-2760(00)00254-X [DOI] [PubMed] [Google Scholar]
- Mary St.C.M. Novel sexual patterns in two simultaneous hermaphroditic gobies, Lythrypnus dalli and Lythrypnus zebra. Copeia. 1993;4:1062–1072. [Google Scholar]
- Tchoudakova A, Callard G.V. Identification of multiple CYP19 genes encoding different cytochrome P450 aromatase isozymes in brain and ovary. Endocrinology. 1998;139:2179–2189. doi: 10.1210/endo.139.4.5899. 10.1210/en.139.4.2179 [DOI] [PubMed] [Google Scholar]
- Trainor B.C, Bird I.M, Marler C.M. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 2004;43:113–121. doi: 10.1016/j.yhbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Zhao J, Mak P, Tchoudakova A, Callard G, Chen S. Different catalytic properties and inhibitor responses of the goldfish brain and ovary aromatase isozymes. Gen. Comp. Endocrinol. 2001;123:180–191. doi: 10.1006/gcen.2001.7661. 10.1006/gcen.2001.7661 [DOI] [PubMed] [Google Scholar]