Abstract
The fact that most types of sensory stimuli occur naturally over a large range of intensities is a challenge to early sensory processing. Sensory mechanisms appear to be optimized to extract perceptually significant stimulus fluctuations that can be analysed in a manner largely independent of the absolute stimulus intensity. This general principle may not, however, extend to olfaction; many studies have suggested that olfactory stimuli are not perceptually invariant with respect to odour intensity. For many animals, absolute odour intensity may be a feature in itself, such that it forms a part of odour identity and thus plays an important role in discrimination alongside other odour properties such as the molecular identity of the odorant. The experiments with honeybees reported here show a departure from odour-concentration invariance and are consistent with a lower-concentration regime in which odour concentration contributes to overall odour identity and a higher-concentration regime in which it may not. We argue that this could be a natural consequence of odour coding and suggest how an ‘intensity feature’ might be useful to the honeybee in natural odour detection and discrimination.
Keywords: olfaction, invariance, concentration, honeybee, neural coding
1. Introduction
Sensory systems typically encode stimuli in a way that provides subsequent processing mechanisms with several nearly independent stimulus dimensions; for example, visual stimuli have the dimensions of colour, form, speed and orientation. When one considers such a dimensional space in terms of a single perceptual parameter that can be broadly related to stimulus intensity—e.g. brightness or loudness—as opposed to other parameters that appear more qualitative in nature, it becomes apparent that most sensory systems achieve an efficient division of the intensity–quality axes, producing almost complete independence of the intensity axis from the other, qualitative axes over a substantial portion of the operating range. Where such independence is achieved, stimulus intensity does not contribute much to stimulus identity; for example, neither does brightness contribute appreciably to the visual identity of an object (e.g. colour) (Livingstone & Hubel 1988; Lotto & Purves 1999; Purves et al. 2004), nor does loudness contribute to the auditory identity of a sound (e.g. frequency) (Zeng & Shannon 1994; Escabi et al. 2003).
In olfaction, however, it has often been noted anecdotally that increasing the intensity of an odorant affects both its quantitative and qualitative perceptual properties (Wise et al. 2000; Firestein 2001). Thus, a more intense odorant may be perceived to be not merely of greater intensity than the same odorant at lower concentration but also to have different qualitative properties (Gross-Isseroff & Lancet 1988; Marfaing et al. 1989; Laing et al. 2003). This suggests that, whereas visual and auditory stimulus identities are largely intensity-invariant, olfactory stimulus identity may show a substantial departure from concentration invariance, over at least part of the sensory operating range. Such a property would make olfaction different from senses like vision and audition and suggests further that the mechanisms which underlie later cognitive processes such as recognition and categorization must also differ between the senses, as these processes are usually assumed to depend heavily on the computation of invariants (Cavanagh 1978; Gabbiani et al. 2004). Determining how the concentration of an odour affects its perceived identity is thus a vital part of understanding how odours are coded by the entire olfactory system.
Few psychophysical studies have quantified the extent to which intensity modifies the perceptual qualities of odorant molecules in either humans (Gross-Isseroff & Lancet 1988; Laing et al. 2003) or in other animals (Marfaing et al. 1989; Bhagavan & Smith 1996; Pelz et al. 1997), though two recent physiological studies of the insect olfactory system (Sachse & Galizia 2003; Stopfer et al. 2003) and one in humans (Anderson et al. 2002) suggest that odour identity ought to remain invariant in the face of differences in odour concentration. In a previous study with honeybees, we found that odorant intensity affected the ability to differentiate among odorants of the same concentration such that odorants of a low intensity (near the detection threshold) were more difficult to discriminate than odorants of greater intensities (Wright & Smith 2004a). The present work extends this to examine the extent to which odour intensity affects odorant identity.
2. Material and methods
(a) Subjects and conditioning procedure
Worker honeybees (Apis mellifera) were collected from indoor colonies at the Rothenbuhler Honeybee Laboratory, Columbus, OH, USA. Each subject was placed individually in a restraining harness, fed to repletion and held at room temperature overnight. The next day, subjects were conditioned with an odorant as a conditioned stimulus (CS) and a sucrose solution as the unconditioned stimulus (US) in a classical conditioning protocol designed to evaluate olfactory learning (see Smith 1998 for details). After conditioning, subjects were tested with both the CS and other novel stimuli (odorants that differed from the conditioning odorant in either concentration, molecular identity or both).
(b) Odour stimuli
Our odour stimuli were pure monomolecular odorants created at specific molarities using hexane as a solvent (described in Wright & Smith 2004a,b). Each of the odorants, 1-hexanol, geraniol and 2-octanone (Sigma, 99%+ purity), have been used in several previous experiments examining classical conditioning and the odour perception of honeybees (Bhagavan & Smith 1996; Stopfer et al. 1997; Smith 1998; Wright & Smith 2004a,b). To construct each odour stimulus, 5 μl of an odour dilution was placed on a small strip of filter paper; this paper strip was then introduced into a modified, 1 ml tuberculin glass syringe. The syringe was attached to an air supply and controlled by a solenoid valve (see Wright & Smith 2004a,b for details). Each subject received 16 acquisition trials during which a 4 s odour presentation was reinforced with a 0.4 μl droplet of 1.5 M sucrose solution. Each odour stimulus was changed after four trials of use. Ten minutes after the conditioning phase, each subject was tested with four odour presentations; subjects were not given sucrose in association with the test odours. The sequence of test presentations was randomized across subjects.
We ensured that the odour concentrations were within a concentration range that was detectable by the honeybee based on electroantennogram (EAG) data from previous studies (Bhagavan & Smith 1996; Wright & Smith 2004a). The EAG data suggested the following levels of odorant dilution as a reasonable estimate of the mid- and end-points of the honeybee's detection range: 0.0002 M (low), 0.02 M (mid) and 2.0 M (high). Additionally, the low-level (0.0002 M) odorants were detectable above a solvent background (hexane) both as conditioning stimuli and as stimuli in an EAG assay (Wright & Smith 2004a).
(c) Behavioural experiments
We performed two experiments to examine how concentration affected the behavioural response to each odorant. The first experiment compared low-concentration odorants (0.0002 M) and high-concentration odorants (2.0 M). Our subjects were conditioned with either the low (0.0002 M) or the high concentration (2.0 M) of one of the three odorants. After conditioning, they were presented with four test trials: (i) the low concentration of the conditioned odorant; (ii) the high concentration of the conditioned odorant; (iii) the low concentration of another odorant; (iv) the high concentration of another odorant (the same odorant as used in (iii)). For 1-hexanol as the conditioning odorant, we used geraniol as the ‘other’ odorant; for geraniol and 2-octanone, we used 1-hexanol.
The second experiment compared the intermediate (0.02 M) concentration with either the high concentration or the low concentration of the same odorant. We conditioned our subjects with the intermediate concentration and then tested them with the intermediate, low and high concentration of the conditioned odorant. We also tested them with the intermediate concentration of another, novel odorant type; the relationship between the other odorant and the conditioned odorant was the same as in the first experiment.
(d) Statistical analyses
For all of the experiments, the responses of subjects were scored as binary variables; therefore, we used multivariate logistic regression (LR) with least-squares contrasts (LSC) for multiple comparisons to test all hypotheses.
3. Results
We used the probability of responding during the test as an index for the perceptual similarity between the test odorants and the odorant used as the CS. CS refers both to odorant type and concentration.
(a) Odour concentration affects odour quality
In the first experiment, we observed that odorants of the same concentration as the CS elicited higher response rates than did molecularly identical odorants tested at different concentrations. This was true for both the low (figure 1a) and high (figure 1b) concentration variants of the CS (LR: , n=167, p<0.001), and it was true regardless of the molecular identity of odorant used as the CS (LR: , n=167, p=0.297). Because the responses were the same regardless of the molecular identity of the CS, we pooled the data across odorant types and examined the responses separately for each concentration using LSC within the LR.
Figure 1.
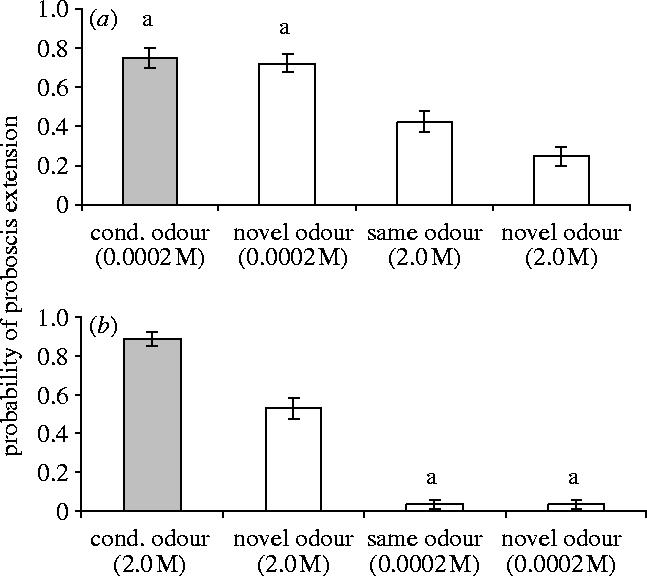
Over a range of concentration of four orders of magnitude, odorants of the same concentration appear more similar than odorants of the same molecular identity. Data were pooled for three conditioning odorants (1-hexanol, 2-octanone and geraniol). (a) At the low conditioning concentration (0.0002 M), honeybees do not appear to differentiate the conditioned odorant (CS) (grey bar) from a novel low-concentration odorant (p=0.704). However, they respond significantly less to the high concentration (2.0 M) of the same conditioning odorant type as the CS; they also respond less to a novel odorant at the high concentration (2.0 M). (b) When the high concentration was used as the CS, honeybees appear to be able differentiate a novel, high-concentration odorant from the CS, but they respond more to the high concentration of a novel odorant than to the low concentration of the same odorant type as the CS or the low-concentration novel odorant. (Bars labelled with the same letter in each graph were not significantly different at α=0.05).
When honeybees were conditioned with a low-concentration CS, the response to the novel, low-concentration odorant was not significantly different from the response to the CS (figure 1a) (LSC: , p=0.704). The response to the high-concentration molecularly identical odorant was lower than both the response to the CS (LSC: , p<0.001) and the response to the low-concentration novel odorant (LSC: , p=0.005). The response to the high-concentration novel odorant was lowest, indicating that it was the most perceptually dissimilar to the CS (LSC: , p<0.001). It was also lower than the response to the novel, low-concentration odorant (LSC: , p<0.001) and lower than the response to the high-concentration molecularly identical odorant (LSC: , p=0.015).
When honeybees were conditioned with a high-concentration CS, the response to the novel, high-concentration odorant was significantly less than the response to the CS (figure 1b) (LSC: , p<0.001). However, the response to the low concentration of a molecularly identical odorant was significantly lower than the response to the CS (LSC: , p<0.001) and lower than the response to the novel high-concentration odorant (LSC: , p<0.001). The test odorant with the least probability of eliciting a response relative to the CS was the low-concentration, novel odorant (LSC: , p<0.001). The responses to the two low-concentration test odorants were not significantly different (LSC: , p=0.976).
(b) Failure of invariance is dependent upon the concentration range
In the second experiment, we examined perceptual differences of the intermediate concentration stimuli (i.e. CS) relative to the low and high concentration of the same odorant type. We observed that a high-concentration molecularly identical odorant was perceptually the most similar to the CS. In contrast with the first experiment, the pattern of response to the test odorants was not the same for each type of CS odorant (LR: , n=109, p=0.050). Specifically, the pattern was the same for the odorants, 1-hexanol and 2-octanone (LSC: , p=0.889), but geraniol was different from both 1-hexanol (LSC: , p=0.041) and from 2-octanone (LSC: , p=0.028). Therefore, we chose to analyse the responses separately for each CS odorant (reported in table 1).
Table 1.
P-values for least-squares multiple comparisons of the intermediate concentration (0.02 M) of each odorant with each test odorant (see figure 2).
| 1-hexanol | 2-octanone | geraniol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | COH | COL | NOI | CS | COH | COL | NOI | CS | COH | COL | NOI | |
| CS | X | 0.023 | <0.001 | <0.001 | X | 0.034 | <0.001 | <0.001 | X | 1.0 | 0.001 | <0.001 |
| COH | 0.023 | X | 0.198 | 0.198 | 0.034 | X | 0.197 | 0.062 | 1.0 | X | <0.001 | <0.001 |
| COL | <0.001 | 0.198 | X | 1.0 | <0.001 | 0.197 | X | 0.551 | 0.001 | <0.001 | X | 0.075 |
| NOI | <0.001 | 0.198 | 1.0 | X | <0.001 | 0.062 | 0.551 | X | <0.001 | <0.001 | 0.075 | X |
Significant differences are in bold. CS, conditioning stimulus; COH, high-concentration conditioning odorant; COL, low-concentration conditioning odorant; NOI, intermediate-concentration novel odorant.
Despite the slight differences observed in the responses to these odorants, it is true of all three that the intermediate concentration (CS) was most similar to the high-concentration molecularly identical odorant than it was to the intermediate concentration of a novel test odorant. For 1-hexanol and 2-octanone, the response to the CS was significantly greater than the response to both the high and low molecularly identical odorants (figure 2a,b). The intermediate concentration of a novel odorant did not elicit a greater response than the molecularly identical low- or high-concentration odorants (figure 2a,b). In the case of these two odours, both concentration and odour type were distinguishable features of the CS. For geraniol, the response to the high concentration of geraniol was not significantly different from the response to the CS (figure 2c). The response to the CS was significantly different from the low concentration of geraniol and from the intermediate concentration of the novel odorant. (See electronic supplementary material for more details with respect to the differences observed among 1-hexanol, 2-octanone and geraniol.)
Figure 2.
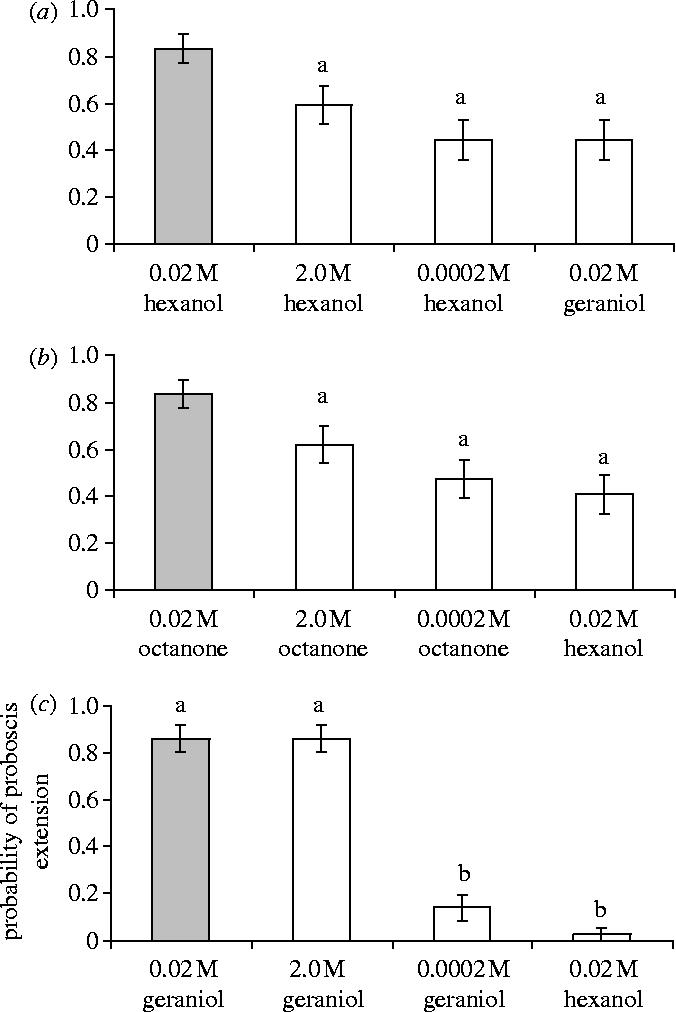
The intermediate concentration was most similar to the high concentration of molecularly identical odorants; the extent of the difference between intermediate and high concentrations was dependent on odorant type (p=0.050). Each graph represents the response to the intermediate concentration of the conditioned odorant (CS) (grey bar) versus the response to the high and low concentration of odorants molecularly identical to the conditioned odorant and versus the response to the intermediate concentration of a molecularly novel odorant. The conditioned odorant was: (a) 1-hexanol; (b) 2-octanone; (c) geraniol. For 1-hexanol and 2-octanone, the response to the intermediate concentration was significantly different from all test odorants. For geraniol, the response to the intermediate concentration was not significantly different from the high concentration (see table 1; bars labelled with the same letter in each graph were not significantly different at α=0.05).
4. Discussion
We show that an odour stimulus with the same molecular identity as the CS, but with a different concentration, was perceived to be less similar to the CS than a molecularly novel odour presented at the same concentration. Since data from previous experiments show that honeybees are sensitive to molecular-identity differences among the same odours at concentrations similar to those tested here (Bhagavan & Smith, 1996; Pelz et al. 1997; Wright & Smith, 2004a,b), our results cannot be explained as a simple failure to discriminate between odorants of different molecular identities. Rather, honeybees extract, after only a modest number of conditioning trials, information about an odour's concentration as well as its molecular identity, and this odour-concentration information may be used as a separate stimulus dimension for odour recognition. Taken together, the two experiments reported here imply additionally that the relative contributions of concentration and molecular identity to overall odour identity—and probably also the extent to which these stimulus attributes can be resolved by the animal as independent stimulus features—depend on the concentration range over which the odours are experienced. Because of the dependence of odour identity on the concentration range, we conclude that odour identity is not invariant as a function of concentration.
(a) Concentration as a salient feature of odours
In an animal's environment, odours are experienced across a wide range of concentrations; it is important to distinguish in our study the difference between the possible role of concentration fluctuations and that of absolute concentration in the identification of odour stimuli. Several previous studies of naturally occurring concentration fluctuations show that odours emitted from sources disperse in such a manner that animals can use changes in concentration to locate odour sources (Murlis et al. 1992; Grasso & Basil 2002; Keller & Weissburg 2004; Wright & Thomson 2005, for a review). This in itself does not mark olfaction out as distinct from other sensory faculties—it is, after all, contrast rather than intensity itself that is critical for visual (Purves et al. 2004; Roe et al. 2005) and auditory processing (Escabi et al. 2003). Moreover, information regarding absolute sound or light levels is not, of course, discarded by the relevant sensory system; it is rather that this information does not contribute to the overall identity of the perceived stimulus. What would make olfaction quite different from the other senses, then, is a generalized failure of concentration invariance such that absolute odour concentrations actually contribute to odour identity.
(b) Ecological relevance of concentration invariance
The fact that honeybees generalize successfully among odorants of the same concentration even though the odorants have different molecular structures may suggest that situations arise when it is more important for a honeybee to learn an odorant's concentration than to learn its molecular identity. Possibly the absolute concentration of a scent is itself an important feature used to identify a salient odour stimulus: when honeybees are foraging, for example, the concentration of the odour stimulus of flowers could be used to identify those flowers as distinct from other previously experienced stimuli at another concentration (Wright & Thomson in press; Wright et al. 2005). Thus, concentration-dependent odour identity could be ecologically appropriate for the honeybee: as it forages for nectar, a high-concentration odorant could have a different meaning when compared with the same odorant at low concentration.
(c) Olfactory perception, coding and odour invariance
An intriguing issue raised by our results is whether the observed departure from odour-concentration invariance is actually to the animal's advantage, possibly even representing an adaptation to its environment as suggested above, or whether it is simply a limitation of olfactory coding. It is certainly tempting to conclude that it might be a natural consequence of neural limits. Human colour vision, for example, works well with just three types of broadband detectors in the retina; olfaction, however, must devote much of its neural capacity to sensing thousands of potential odorant molecules and their combinations in the environment (Firestein 2001). Other sensory systems do not get intensity invariance ‘for free’ (vision, for example, employs a large range of optical, retinal and cortical processes to maintain its high degree of brightness invariance) (Colburn et al. 2003; Escabi et al. 2003; Polyansky et al. 2005; Roe et al. 2005). For olfaction, the cost of adhering strictly to concentration-invariant coding over a wide range of stimulus intensities may simply be too high; this may be supported by the fact that olfactory systems appear to have evolved convergently in diverse organisms (Hildebrand & Shepherd 1997; Eisthen 2002).
There is another reason why the olfactory system might find it difficult to produce concentration invariance: invariance may not be possible in a sensory system that relies on combinatorial coding. Olfactory receptor neurons appear to display highly specific responses to individual odorants, such that the most specific odorant receptors have the highest binding affinities (Araneda et al. 2000; Gaillard et al. 2002; Floriano et al. 2004) for odorant molecules and, therefore, a greater ability to detect odorants at low concentrations. High specificity so early in the olfactory processing stream may result in a trade-off between detectability and discriminability (i.e. olfactory sensitivity versus olfactory acuity); we suggest that at very low odorant concentrations, specificity in the response of receptor neurons to odours confers superb stimulus detectability (Angioy et al. 2003) but at the expense of poor inter-odour discriminability (since no single receptor neuron has shared sensitivity to multiple odours). As odour concentration increases, discrimination will surely improve; less-specific receptor neurons do exist which could detect a wider range of odorant molecules although at higher odour concentrations, and some receptor neurons might show a kind of combined specificity by responding at lower thresholds to their ‘preferred’ odours, and responding to other odour molecules only at high concentrations of odour (Araneda et al. 2000). However, the olfactory system must somehow integrate the responses from the low-threshold, high-selectivity neurons with those of the higher-threshold, less selective neurons (combinatorial coding), and as it does so it may be unable to disconfound ‘concentration’ information from ‘molecular identity’ information. The nature of this integration, usually thought to occur higher up in the olfactory processing stream, such as the antennal lobe (insects) or olfactory bulb (mammals), is crucial to the coding of odour identity and probably takes account not only of the responses of different combinations of subpopulations of receptor neurons (Malnic et al. 1999; Meister & Bonhoeffer 2001) but also of the sequence of activation of these subpopulations (Sachse & Galizia 2003).
Electrophysiological studies conducted at the antennal lobe (Stopfer et al. 2003) or olfactory bulb (Wachowiak & Cohen 2003) are broadly consistent with the idea of a largely odour-concentration-dependent ‘detection regime’ at low odorant concentrations, coupled with a largely odour-concentration-independent ‘discrimination regime’ at higher concentrations of odorant. Such a two-stage model would predict that when the stimulus signal is strong enough, the molecular identity of the odorant may overtake the concentration of the odorant as the key contributor to overall odour identity, such that both concentration invariance and the ability to discriminate on the basis of molecular identity would occur only when odours are experienced at higher concentrations (Cleland & Narla 2003; Wright & Smith 2004b). These results support this hypothesis, since concentration-invariant odour identity was indeed maintained over a range of two orders of magnitude in concentration (within the upper part of the operating range). Support is also provided indirectly by those studies which have concluded that, for odour identity to be encoded in the antennal lobe or olfactory bulb, a ‘critical mass’ of input from the olfactory periphery must exist (Wehr & Laurent 1999; Cleland & Linster 2002; Laurent, 2002). In particular, studies in the locust (Laurent et al. 1996; Stopfer & Laurent 1999; Wehr & Laurent 1999), the slug (Gelperin et al. 1996), the zebrafish (Friedrich & Laurent 2001; Friedrich et al. 2004) and the honeybee (Stopfer et al. 1997) have shown that within the responses of networks of neurons in the antennal lobe there is a higher-order code (e.g. synchronous firing of projection neurons) thought to be critical for producing a representation in which odours can be discriminated according to their molecular identities.
If the hypothesis of a two-stage coding model as outlined above were to be confirmed, it would mean, ironically, that the exquisite specificity of the high-affinity olfactory receptor neuron receptors does not contribute fine-grain information about odorant identity but rather a remarkable detection sensitivity; odour-identity information is more likely to arise instead from the responses of many types of receptor neurons which become active at greater odour concentrations and activate networks of neurons downstream in the olfactory system. Lack of concentration invariance may be the price the olfactory system pays for fine stimulus selectivity at such a low level in the processing stream; by contrast, human colour vision starts out with remarkably unselective receptors (highly broadband cone responses) yet builds from these both excellent wavelength selectivity and near-complete brightness invariance. More research will be necessary in future studies to test these hypotheses.
Acknowledgments
The authors would like to thank Susan Cobey for beekeeping and to thank Michelle Carlton, Brendon Fussnecker, and Amy Lutmerding for help with the honeybee conditioning. This research was funded by a grant to BHS from NIH-NCRR (9 R01 RR1466) and a grant awarded to the Mathematical Biosciences Institute at Ohio State University from NSF (agreement no. 0112050).
Supplementary Material
References
- Anderson A.K, Christoff K, Stappen I, Panitz D, Ghahremani D.G, Glover G, Gabrieli J.D.E, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2002;6:196–202. doi: 10.1038/nn1001. 10.1038/nn1001 [DOI] [PubMed] [Google Scholar]
- Angioy A.M, Desogus A, Barbarossa I.T, Anderson P, Hansson B.S. Extreme sensitivity in an olfactory system. Chem. Sens. 2003;28:279–284. doi: 10.1093/chemse/28.4.279. 10.1093/chemse/28.4.279 [DOI] [PubMed] [Google Scholar]
- Araneda R.C, Kini A.D, Firestein S. The molecular receptive range of an odorant receptor. Nat. Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. 10.1038/81774 [DOI] [PubMed] [Google Scholar]
- Bhagavan S, Smith B.H. Olfactory conditioning in the honeybee, Apis mellifera: the effects of odor intensity. Physiol. Behav. 1996;61:107–117. doi: 10.1016/s0031-9384(96)00357-5. 10.1016/S0031-9384(96)00357-5 [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Size and position invariance in the visual system. Perception. 1978;7:167–177. doi: 10.1068/p070167. [DOI] [PubMed] [Google Scholar]
- Cleland T.A, Linster C. How synchronization properties among second-order sensory neurons can mediate stimulus salience. Behav. Neurosci. 2002;116:212–221. doi: 10.1037//0735-7044.116.2.212. 10.1037//0735-7044.116.2.212 [DOI] [PubMed] [Google Scholar]
- Cleland T.A, Narla V.A. Intensity modulation of olfactory acuity. Behav. Neurosci. 2003;117:1434–1440. doi: 10.1037/0735-7044.117.6.1434. 10.1037/0735-7044.117.6.1434 [DOI] [PubMed] [Google Scholar]
- Colburn H.S, Carney L.H, Heinz M.G. Quantifying the information in auditory-nerve responses for level discrimination. JARO. 2003;4:294–311. doi: 10.1007/s10162-002-1090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisthen H.L. Why are olfactory systems of different animals so similar? Brain Behav. Evol. 2002;59:273–293. doi: 10.1159/000063564. 10.1159/000063564 [DOI] [PubMed] [Google Scholar]
- Escabi M.A, Miller L.M, Read H.L, Schreiner C.E. Naturalistic auditory contrast improves spectrotemporal coding in the cat inferior colliculus. J. Neurosci. 2003;23:11 489–11 504. doi: 10.1523/JNEUROSCI.23-37-11489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. 10.1038/35093026 [DOI] [PubMed] [Google Scholar]
- Floriano W.B, Vaidehi N, Goddard W.A. Making sense of olfaction through predictions of the 3-D structure and function of olfactory receptors. Chem. Sens. 2004;29:269–290. doi: 10.1093/chemse/bjh030. 10.1093/chemse/bjh030 [DOI] [PubMed] [Google Scholar]
- Friedrich R.W, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. 10.1126/science.291.5505.889 [DOI] [PubMed] [Google Scholar]
- Friedrich R.W, Habermann C.J, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat. Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. 10.1038/nn1292 [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp H.G, Hatsopoulos N, Mo C.H, Koch C, Laurent G. Multiplication and stimulus invariance in a looming-sensitive neuron. J. Phys. Paris. 2004;98:19–34. doi: 10.1016/j.jphysparis.2004.03.001. 10.1016/j.jphysparis.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Gaillard I, Rouquier S, Pin J.P, Mollard P, Richard S, Barnabe C, Demaille J, Giorgi D. A single olfactory receptor specifically binds a set of odorant molecules. Eur. J. Neurosci. 2002;15:409–418. doi: 10.1046/j.0953-816x.2001.01871.x. 10.1046/j.0953-816x.2001.01871.x [DOI] [PubMed] [Google Scholar]
- Gelperin A, Kleinfeld D, Denk W, Cooke I.R.C. Oscillations and gaseous oxides in invertebrate olfaction. J. Neurobiol. 1996;30:110–122. doi: 10.1002/(SICI)1097-4695(199605)30:1<110::AID-NEU10>3.0.CO;2-Q. 10.1002/(SICI)1097-4695(199605)30:1%3C110::AID-NEU10%3E3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Grasso F.W, Basil J.A. How lobsters, crayfishes, and crabs find important sources of odor: current perspectives and future directions. Curr. Opin. Neurobiol. 2002;12:721–727. doi: 10.1016/s0959-4388(02)00388-4. 10.1016/S0959-4388(02)00388-4 [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Lancet D. Concentration-dependent changes of perceived odor quality. Chem. Sens. 1988;13:191–204. [Google Scholar]
- Hildebrand J.G, Shepherd G.M. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. 10.1146/annurev.neuro.20.1.595 [DOI] [PubMed] [Google Scholar]
- Keller T.A, Weissburg M.J. Effects of odor flux and pulse rate on chemosensory tracking in turbulent odor plumes by the blue crab, Callinectes sapidus. Biol. Bull. 2004;207:44–55. doi: 10.2307/1543627. [DOI] [PubMed] [Google Scholar]
- Laing D.G, Legha P.K, Jinks A.L, Hutchinson I. Relationship between molecular structure, concentration and odor qualities of oxygenated aliphatic molecules. Chem. Sens. 2003;28:57–69. doi: 10.1093/chemse/28.1.57. 10.1093/chemse/28.1.57 [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat. Rev. Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Laurent G, Wehr M, MacLeod K, Stopfer M, Leitch B, Davidowitz H. Dynamic encoding of odors with oscillating neuronal assemblies in the locust brain. Biol. Bull. 1996;191:70–75. doi: 10.2307/1543064. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth—anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lotto R.B, Purves D. The effects of color on brightness. Nat. Neurosci. 1999;2:1010–1014. doi: 10.1038/14808. 10.1038/14808 [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck L.B. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. 10.1016/S0092-8674(00)80581-4 [DOI] [PubMed] [Google Scholar]
- Marfaing P, Rouault J, Laffort P. Effect of the concentration and nature of olfactory stimuli on the proboscis extension of conditioned honeybees (Apis mellifera ligustica) J. Insect Physiol. 1989;35:949–955. 10.1016/0022-1910(89)90018-8 [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J. Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlis J, Elkinton J.S, Carde R.T. Odor plumes and how insects use them. Annu. Rev. Entomol. 1992;37:505–532. 10.1146/annurev.en.37.010192.002445 [Google Scholar]
- Pelz C, Gerber B, Menzel R. Odorant intensity as a determinant for olfactory conditioning in the honeybee: roles in discrimination, overshadowing, and memory consolidation. J. Exp. Biol. 1997;200:837–847. doi: 10.1242/jeb.200.4.837. [DOI] [PubMed] [Google Scholar]
- Polyansky V.B, Evtikhin D.V, Sokolov E.N. Computation of color and brightness differences by neurons in the rabbit visual cortex. Z. Vyss. Nerv. Deyatel. Imeni I P Pavl. 2005;55:60–70. [PubMed] [Google Scholar]
- Purves D, Williams S.M, Nundy S, Lotto R.B. Perceiving the intensity of light. Psych. Rev. 2004;111:142–158. doi: 10.1037/0033-295X.111.1.142. 10.1037/0033-295X.111.1.142 [DOI] [PubMed] [Google Scholar]
- Roe A.W, Lu H.D.D, Hung C.P. Cortical processing of a brightness illusion. PNAS USA. 2005;102:3869–3874. doi: 10.1073/pnas.0500097102. 10.1073/pnas.0500097102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S, Galizia C.G. The coding of odour-intensity in the honeybee antennal lobe: local computation optimizes odour representation. Eur. J. Neurosci. 2003;18:2119–2132. doi: 10.1046/j.1460-9568.2003.02931.x. 10.1046/j.1460-9568.2003.02931.x [DOI] [PubMed] [Google Scholar]
- Smith B.H. Analysis of interaction in binary odorant mixtures. Physiol. Behav. 1998;65:397–407. doi: 10.1016/s0031-9384(98)00142-5. 10.1016/S0031-9384(98)00142-5 [DOI] [PubMed] [Google Scholar]
- Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. 10.1038/45244 [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith B.H, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. 10.1038/36335 [DOI] [PubMed] [Google Scholar]
- Stopfer M, Jayarman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. 10.1016/j.neuron.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen L.B. Correspondence between odorant-evoked patterns of receptor neuron input and intrinsic optical signals in the mouse olfactory bulb. J. Neurophys. 2003;89:1623–1639. doi: 10.1152/jn.00747.2002. [DOI] [PubMed] [Google Scholar]
- Wehr M, Laurent G. Relationship between afferent and central temporal patterns in the locust olfactory system. J. Neurosci. 1999;19:381–390. doi: 10.1523/JNEUROSCI.19-01-00381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise P.M, Olsson M.J, Cain W.S. Quantification of odor quality. Chem. Sens. 2000;25:429–443. doi: 10.1093/chemse/25.4.429. 10.1093/chemse/25.4.429 [DOI] [PubMed] [Google Scholar]
- Wright G.A, Smith B.H. Variation in complex olfactory stimuli and its influence on odour recognition. Proc. R. Soc. B. 2004;271:147–152. doi: 10.1098/rspb.2003.2590. 10.1098/rspb.2003.2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G.A, Smith B.H. Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera) Chem. Sens. 2004;29:127–135. doi: 10.1093/chemse/bjh016. 10.1093/chemse/bjh016 [DOI] [PubMed] [Google Scholar]
- Wright, G. A. & Thomson, M. G. A. 2005 Odor perception and variability in natural odor scenes. In Recent advances in phytochemistry (ed. J. Romeo). Chemical Ecology and Phytochemistry of Forest Ecosystems, vol.39. Elsevier.
- Wright G.A, Lutmerding A, Dudareva N, Smith B.H. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honey bees (Apis mellifera) J. Comp. Physiol. A. 2005;191:105–114. doi: 10.1007/s00359-004-0576-6. 10.1007/s00359-004-0576-6 [DOI] [PubMed] [Google Scholar]
- Zeng F.G, Shannon R.V. Loudness-coding mechanisms inferred from electric-stimulation of the human auditory-system. Science. 1994;264:564–566. doi: 10.1126/science.8160013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


