Abstract
The role of the extracellular matrix in cutaneous morphogenesis is poorly understood. Here, we describe the essential role of laminin-10 (α5β1γ1) in hair follicle development. Laminin-10 was present in the basement membrane of elongating hair germs, when other laminins were downregulated, suggesting a role for laminin-10 in hair development. Treatment of human scalp xenografts with antibodies to laminin-10, or its receptor β1 integrin, produced alopecia. E16.5 Lama5 –/– mouse skin, lacking laminin-10, contained fewer hair germs compared with controls, and after transplantation, Lama5 –/– skin showed a failure of hair germ elongation followed by complete hair follicle regression. Lama5 –/– skin showed defective basement membrane assembly, without measurable increases in anoikis. Instead, Lama5 –/– skin showed decreased expression of early hair markers including sonic hedgehog and Gli1, implicating laminin-10 in developmental signaling. Intriguingly, treatment of Lama5 –/– skin with purified laminin-10 corrected basement membrane defects and restored hair follicle development. We conclude that laminin-10 is required for hair follicle development and report the first use of exogenous protein to correct a cutaneous developmental defect.
Keywords: basement membrane/extracellular matrix/hair follicle/laminin
Introduction
Hair is a small organ but provides many important functions to mammals. Hair helps to maintain body temperature, provides sensation and promotes social interaction. In humans, loss of hair affects millions of men and women of all ethnic backgrounds and can be psychologically devastating. Hair follicle development is critical not only to hair growth, but also to a number of other biologically important processes. Hair follicles are a reservoir of pluripotent stem cells (Fuchs and Raghavan, 2002), which can regenerate epidermis and are thought to play an important role during wound healing. Aberrations of hair follicle development can produce a number of neoplastic conditions, including pilomatricoma and basal cell carcinoma (Callahan and Oro, 2001).
Epithelial development during hair follicle morphogenesis starts with the induction of epithelial placodes by underlying mesenchyme, and progresses through the development of hair germs and elongation of hair germs downward into the underlying dermis, to form hair follicles. These developmental process take place through a complex and incompletely understood series of mesenchymal–epithelial tissue interactions (Oro and Scott, 1998; Millar, 2002). Secreted proteins including members of the WNT (a family of highly conserved secreted signaling molecules that regulate cell–cell interactions during embryogenesis), fibroblast growth factor (FGF), transforming growth factor (TGF) and bone morphogenetic protein (BMP) families have been shown to be important in the early stages of hair placode and hair germ formation. This group of proteins also contributes to expression of sonic hedgehog (Shh) and activation of the Shh signaling pathway, which is essential to the elongation of hair germs during hair follicle development. Despite these advances, it is clear that many of the events of hair follicle morphogenesis remain to be elucidated, including the role of the extracellular matrix.
Extracellular matrix molecules are known to play important roles in tissue morphogenesis, for example, elastin has an important regulatory function in arterial mophogenesis (Li et al., 1998), and expression of extra cellular matrix molecules has been shown to play a key role in epithelial morphogenesis of the hydra (Shimizu et al., 2002). A highly organized and complex collection of extracellular matrix molecules, the basement membrane zone (BMZ), lies at the epithelial–mesenchymal interface in skin (Burgeson and Christiano, 1997). Once thought to be purely a structural entity, the BMZ has recently been shown to have important developmental roles. In particular, BMZ components termed laminins have been implicated in the development of many different types of epithelia (Ekblom et al., 1998; Klinowska et al., 1999; Martin et al., 1999; Miner and Li, 2000; Willem et al., 2002). Laminins are a family of large extracellular BMZ glycoproteins (Engvall and Wewer, 1996; McGowan and Marinkovich, 2000) which link the BMZ to epithelial receptors such as integrins (Hynes, 1992). Laminins contain α, β and γ chains and, to date, five α chains, three β chains and three γ chains have been identified (Burgeson et al., 1994; McGowan and Marinkovich, 2000). Differential expression of these chains leads to the assembly of functionally diverse laminin isoforms in distinct tissue distributions. The laminin α5 chain (Miner et al., 1995) is expressed in the BMZ of many epithelial tissues, where it is assembled into laminin-10 (α5β1γ1). Laminin-10 (Maatta et al., 2001), laminin-5 (α3β3γ2) (Rousselle et al., 1991), laminin-6 (α3β1γ1) (Marinkovich et al., 1992a) and laminin-1 (α1β1γ1) each localize to the dermal–epidermal BMZ. Interestingly, laminin-5 (Nanba et al., 2000) and laminin-1 (Hayashi et al., 2002) each show reduced expression during hair germ elongation; however, the expression of laminin-10 in developing hair follicles has not been studied.
Lama3 –/– mice (Ryan et al., 1999) and patients with Herlitz’s junctional epidermolysis bullosa (HJEB) (Marinkovich et al., 1999), who lack laminin-5/6 expression, display significant epidermal adhesive defects but show no significant hair or other developmental abnormalities. On the other hand, Lama5 –/– mice, which lack laminin-10, show multiple developmental defects (Miner et al., 1998) including exencephaly, syndactyly and placentopathy. However, these mice die at ∼E16.5, before hair follicle morphogenesis is completed. As the laminin α5 chain is important in the development of many tissues, we hypothesized that laminin-10 could play an important role in hair follicle development in the skin. To test this hypothesis, we studied laminin-10 in both human and mouse models, and in this report demonstrate that laminin-10 is essential for hair follicle development. Furthermore, we show that exogenous laminin-10 restores hair formation in a laminin-10-deficient mouse model, providing the first example of protein-mediated correction of a developmental defect in the skin.
Results
Laminin-10 is present in the specialized BMZ of elongating hair germs
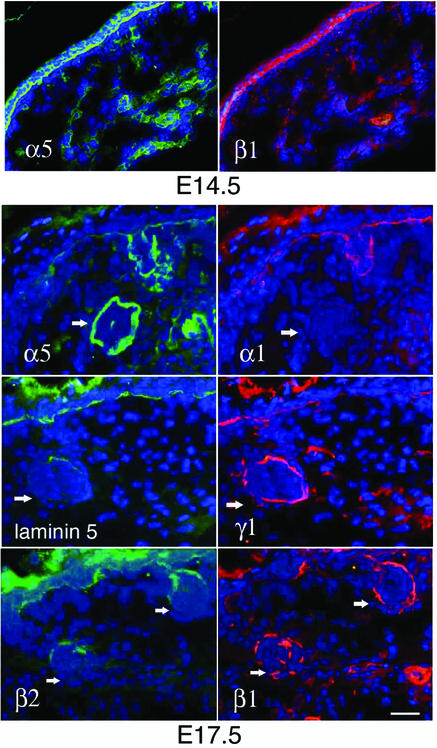
As a part of ongoing studies on the cutaneous functions of laminins, we examined the role of laminin-10 during skin development. As a first step, we assessed the expression of the known laminin isoforms in developing embryonic mouse skin by dual-label indirect immunofluorescence microscopy (IDIF) using a panel of laminin antibodies. Through staining with a combination of laminin α5 and β1 chain antibodies, we found that laminin-10 was prominently expressed in the dermal epidermal BMZ at E14.5 (Figure 1, upper panels). We found specific changes in laminin chain deposition taking place in E17.5 mid-back skin (Figure 1, lower panels). While laminins 1, 5 and 10 each showed linear expression at the dermal–epidermal junction, significant differences in the expression of laminins were noted in developing hair follicle BMZ during the hair germ elongation stage. Laminin α5 chain was a prominent component of elongating hair germ BMZ (Figure 1), while laminin α1 chain and laminin-5 each showed reduced expression compared with adjacent epidermis. Polyclonal antiserum to laminin-5 is also known to cross-react with laminin-6 (Marinkovich et al., 1992a), therefore these results indicate that laminin-6 is also not significantly expressed in elongating hair follicle BMZ. Laminin γ1 and β1 chains showed significant expression, while laminin β2 chain showed only a low level of expression in elongating hair germ BMZ. In total, these results point to laminin-10 (α5β1γ1) as the primary laminin present in the BMZ underlying elongating hair germ epithelium and suggested that it may play a role in hair development.
Fig. 1. Laminin-10 is the major laminin of elongating hair germ BMZ. E14.5 or E17.5 wild-type murine skin was analyzed using a panel of antibodies against the indicated laminin chains or trimer, and analyzed by dual-label IDIF using fluorescein-conjugated anti-rabbit IgG and Texas Red-conjugated anti-rat IgG secondary antibodies. Cell nuclei were visualized with Hoechst stain. Note expression of laminin-10 component chains (α5β1γ1) in elongating hair germs (arrows). Bar = 30 µm.
Laminin-10 inhibition induces hair loss in human scalp xenografts
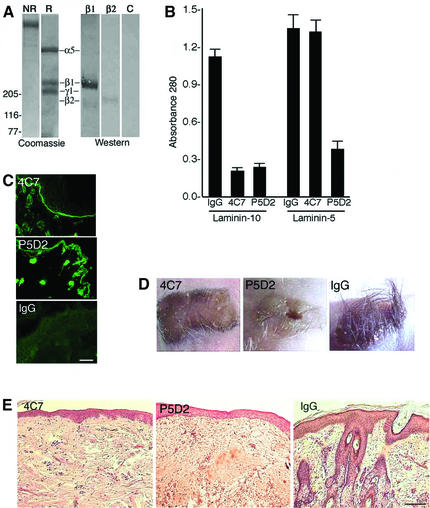
To assess the role of laminin-10 in hair development, we injected laminin-10 blocking antibodies into nude mice bearing full-thickness embryonic human scalp skin xenografts. Monoclonal antibody (mAb) 4C7 has been shown to recognize the globular domain of the laminin α5 chain (Engvall et al., 1986), which is the principal cell-binding domain (Timpl et al., 2000). The mAb P5D2 (Wayner et al., 1993) is a blocking antibody to the β1 integrin subunit, contained in laminin-binding integrins α3β1 and α2β1. Non-reduced (NR) SDS–PAGE analysis of purified laminin-10 revealed a single high-molecular-weight species of the predicted size (Figure 2A). Upon reduction, three bands were noted corresponding to the laminin α5, β1 and γ1 chains of laminin-10. Western blot analysis of the β chains showed a predominance of the β1 chain; however, a small amount of β2 chain was noted in the sample by western blotting, which was not appreciated in the total protein stain. Purified laminin-10 or purified laminin-5 (Marinkovich et al., 1992b) was coated onto tissue culture plates for cell attachment studies. As expected, mAb 4C7 significantly blocked the attachment of keratinocyte to laminin-10 but not laminin-5 (Figure 2B). In contrast, P5D2 inhibited attachment to laminin-10 and, to a lesser extent, laminin-5.
Fig. 2. Inhibition of laminin-10 produces alopecia in human scalp xenografts. (A) Affinity-purified laminin-10 was separated on a 3–5% gradient acrylamide gel. Total protein staining (Coomassie Blue) was assessed under non-reducing (NR) and reducing conditions (R). Western blotting was performed using primary antibodies to laminin β1 chain (β1), laminin β2 chain (β2) or no primary antibody (C). Positions of molecular weight markers in kilodaltons are shown on the left. Positions of laminin chains are shown as indicated. (B) Primary human keratinocytes were seeded onto laminin-10- or laminin-5-coated wells for 30 min, in the presence of either 10 mg/ml mAb 4C7, P5D2 or mouse IgG. Attached cells were stained with crystal violet, solubilized and cell attachment was quantified by assessing the absorbance at 570 λ. Note that 4C7 mediated inhibition of cell attachment to laminin-10 but not laminin-5. (C) Mice bearing human scalp xenografts were injected with mAb 4C7, P5D2 or mouse IgG for 3 weeks, as described in Materials and methods, then a 1:1000 dilution of treated mouse sera was applied to frozen neonatal foreskin sections and analyzed by IDIF microscopy. Note the linear BMZ staining in 4C7 and P5D2 conditions, indicating maintenance of a high titer of circulating antibody. Bar = 40 µm. (D) Clinical appearance of human scalp xenografts after 3 weeks of treatment with mAb 4C7, P5D2 or mouse IgG. Note the loss of hair in the 4C7- and P5D2-treated xenografts. (E) Histological appearance of human scalp xenograft after 3 weeks of treatment with mAb 4C7, P5D2 or mouse IgG. Note the loss of hair and associated appendages in 4C7- and P5D2-treated grafts. Bar = 60 µm.
As a next step, we injected nude mice bearing human scalp skin xenografts intraperitoneally with mAbs 4C7 or P5D2. mAbs 4C7 and P5D2 are known to react with human but not mouse tissues, ensuring that the effects of the antibodies would be localized to the human graft rather than the surrounding skin. The dose of antibody was assessed by measurement of serum from treated mice applied to human foreskin sections analyzed by IDIF. One milligram of injected antibody per week was found to consistently maintain a titer >1:1000 in treated mouse sera (Figure 2C). After 3 weeks, significant lack of hair growth was present in all of the 4C7- and P5D2-treated xenografts (Figure 2D). Biopsy of alopecic regions of the 4C7- and P5D2-treated xenografts showed a lack of hair follicles or related appendageal structures (Figure 2E). No lack of hair growth was noted in any of the xenografts treated with control antibody. Blistering was not clinically apparent in the 4C7- or P5D2-treated grafts, consistent with abundant previous data showing that the binding of laminin-5 with α6β4 integrin is the major interaction that promotes dermal–epidermal cohesion in the skin (Burgeson and Christiano, 1997). These results implicated laminin-10, an extracellular matrix ligand of β1-containing integrins, in the process of hair follicle development, and prompted further studies in a transgenic mouse model system.
Lama5 –/– skin fails to support hair development
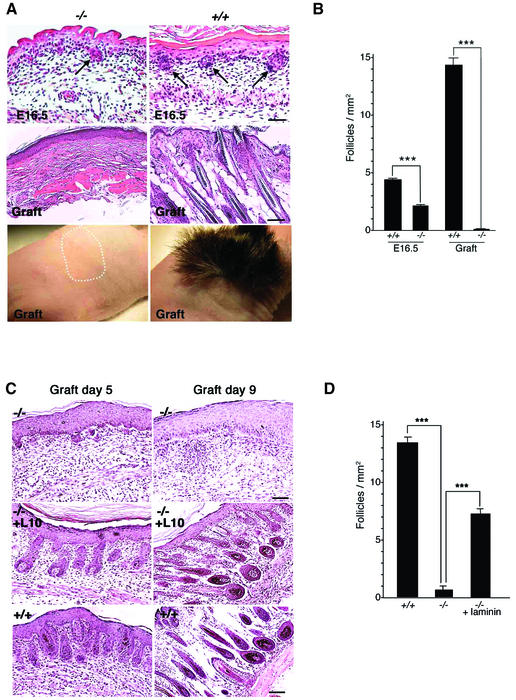
As a disease model affecting laminin-10 has not been described, we therefore chose to further elucidate the role of laminin-10 in hair follicle development by studying Lama5 –/– mice, which lack laminin-10. At E16.5, hair germs were evident in Lama5 –/– mouse skin, and they were morphologically similar to those in wild-type control skin (Figure 3A). However, germs were decreased in number in the mutant to ∼50% of those observed in wild-type sibling controls (Figure 3B).
Fig. 3. Lama5 –/– skin fails to support hair development. (A) Morphology of Lama5 –/– and Lama5 +/+ E16.5 skin was assessed before (E16.5) or 28 days after grafting to nude mice (Graft). Note the decreased numbers of hair germs (arrows) in Lama5 –/– E16.5 skin, and a lack of hair development in Lama5 –/– skin after grafting. The dotted line indicates the area of non-hair-bearing Lama5 –/– skin graft. E16.5 panels: bar = 40 µm; graft panels: bar = 60 µm. (B) Hair germs per millimeter of epidermal length in mid-back skin of E16.5 Lama5 –/– and Lama5 +/+ skin (E16.5), and the number of hair follicles per millimeter of epidermal length of Lama5 –/– and Lama5 +/+ skin 28 days after grafting (Graft). Vertical bar = mean ± SEM. A significant decrease in the number of hair germs was found in the Lama5 –/– skin compared with wild-type sibling control. A dramatic decline of hair follicles was also found in the Lama5 –/– skin grafts, compared with the wild-type siblings ***P < 0.001. (C) E16.5 Lama5 –/– skin was incubated overnight at 4°C in PBS (–/–) or PBS containing 40 µg/ml purified human laminin-10 (–/–, +L10). E16.5 Lama5 +/+ skin was incubated overnight at 4°C in PBS (+/+). After the incubation step, skin was grafted to nude mice, then analyzed after 5 and 9 days by hematoxlyin and eosin staining of tissue sections. (D) Hair follicles per millimeter of epidermal length of Lama5 –/– skin, Lama5 –/– skin treated with laminin-10, and Lama5 +/+ skin, 9 days after grafting. The dramatic decrease in the number of hair follicles found in the Lama5 –/– skin, compared with wild-type sibling control, was significantly reversed after application of laminin-10. ***P < 0.001. Graft day 5 panels and graft day 9 –/– panel: bar = 40 µm; graft day 9 –/–, +L10 and +/+ panels: bar = 60 µm.
As Lama5 –/– embryos typically fail to survive past E16.5 (Miner et al., 1998), further study of hair follicle development in the absence of laminin-10 was not possible. Therefore, we harvested full-thickness skin from E16.5 mutant or wild-type sibling control embryos and grafted the skin to nude mice. After 28 days, significant hair growth was evident in the wild-type control grafts, but a complete lack of hair was observed in the mutant grafts, as shown in Figure 3A. Skin samples were biopsied at 28 days and histological evaluation showed a complete lack of hair appendages in mutant grafts (Figure 3A). The decrease in hair germ number noted in E16.5 Lama5 –/– skin could not account for the total lack of hair follicles noted after grafting, quantified in Figure 3B. These results suggested that hair germs in Lama5 –/– skin regressed upon further development in the absence of laminin-10.
Exogenous laminin-10 restores hair follicle development in Lama5 –/– skin
To evaluate further the cause of hair follicle regression in mutant skin, we examined grafts at earlier developmental time points, at both 5 and 9 days after grafting (Figure 3). At 5 days, hair germs were still visible, however they were reduced in size. By 9 days, hair germs were not visible; only small invaginations of the epidermis were occasionally seen. These changes suggested that hair follicles underwent nearly complete regression during the 9 day period following skin grafting.
We next sought to determine whether exogenous laminin-10 could restore defective hair follicle development in Lama5 –/– grafts. We incubated E16.5 mutant skin in purified human laminin-10 prior to grafting, then analyzed the effects of this treatment at 5 and 9 days post-grafting (Figure 3C, middle panels). Intriguingly, this treatment had a dramatic effect on rescuing hair follicle formation in mutant grafts. Laminin-10-treated mutant grafts produced hair follicles that were similar in morphology to wild-type controls (Figure 3C, lower panels) at both 5 and 9 days. The numbers of hair follicles in laminin-10-treated mutant grafts were ∼50% of wild-type controls at 9 days post-grafting (Figure 3D).
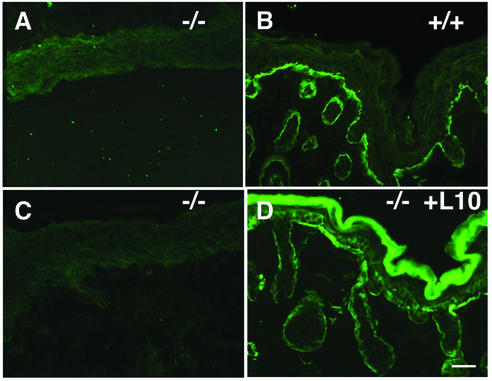
As untreated mutant skin failed to show any laminin-10 staining even at 9 days after grafting, as assessed by IDIF using an anti-mouse laminin α5 chain antibody (Figure 4A), we attributed the hair follicle regression observed in mutant grafts to a lack of laminin-10. As an additional control, we wished to verify the localization and persistence of exogenous laminin-10 in human laminin-10-treated mutant skin grafts coincident with hair follicle development. We analyzed 9 day grafts using mAb 4C7 specific to human laminin α5 chain. As shown in Figure 4D, exogenous laminin-10 was found to localize in a linear fashion in areas surrounding hair follicles as well as the dermal–epidermal junction. These results suggest that exogenous laminin-10, applied to mutant skin prior to grafting, localized correctly and persisted long enough to mediate hair follicle development up to 9 days.
Fig. 4. Lack of mouse laminin-10 and persistence of exogenous human laminin-10 in 9 day Lama5 –/– skin grafts. (A and B) Lama5 –/– skin (A) and Lama5 +/+ skin (B) 9 days after grafting, analyzed by IDIF with anti-mouse laminin α5 pAb. Note the absence of mouse laminin-10 diffusion into Lama5 –/– graft. (C and D) Lama5 –/– skin was treated with PBS (C) or exogenous laminin-10 (D) grafted to nude mice, and analyzed after 9 days by IDIF using 4C7–fluorescein mAb. Note the persistence of laminin-10 in BMZ of treated grafts. Bar = 30 µm.
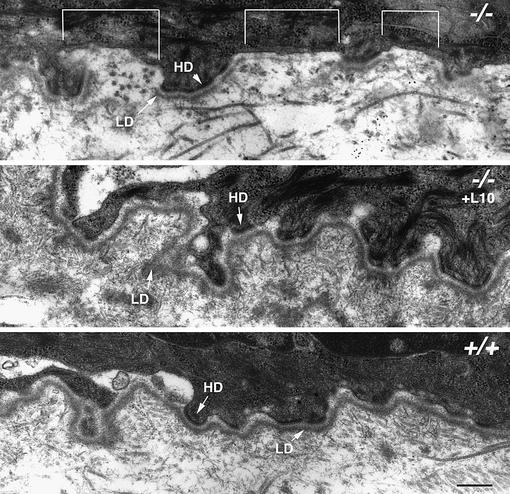
To further assess the effects of exogenous laminin-10 on mutant skin, we examined the BMZ of 5 day skin grafts by transmission electron microscopy, as shown in Figure 5. Untreated Lama5 –/– skin grafts showed discontinuous lamina densa formation (shown by brackets) in areas between hemidesmosomes. Exogenous laminin-10 treatment restored a continuous lamina densa over the entire BMZ in Lama5 –/– skin grafts, which was comparable to wild-type controls. These results suggest that laminin-10 is necessary for correct lamina densa assembly in the BMZ underlying hair follicle epithelium.
Fig. 5. Aberrant BMZ assembly in developing Lama5 –/– skin is corrected with exogenous laminin-10. Follicular BMZ in Lama5 –/– skin (–/–), Lama5 –/– skin treated with laminin-10 (–/–, +L10) and Lama5 +/+ skin was analyzed 5 days after grafting, using transmission electron microscopy. Note the discontinuity of the lamina densa (LD) in areas between hemidesmosomes (HD) in Lama5 –/– skin (indicated by brackets), and its correction after treatment of Lama5 –/– skin with exogenous laminin-10. Bar = 250 nm.
Lama5 –/– mouse skin shows a failure of hair germ elongation
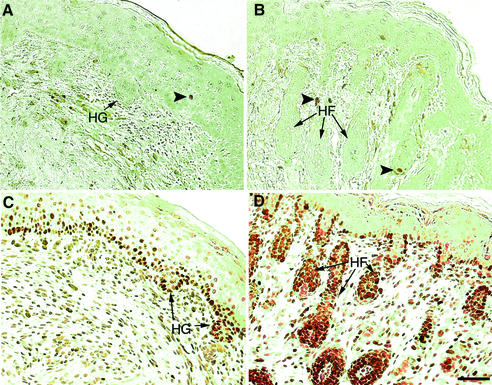
As it is known that adhesion to laminin can influence cell survival during early embryogenesis (Li et al., 2002), one possible explanation for regression of hair follicles in mutant skin was that a lack of adhesion to laminin-10 could trigger apoptosis in hair germ epithelium. However, examination of mutant skin 5 days after grafting showed no increase in apoptosis compared with wild-type skin grafts (Figure 6A and B). These results suggest that apoptosis cannot explain the lack of hair follicle development in mutant grafts.
Fig. 6. Impairment of hair follicle-associated epithelial proliferation in Lama5 –/– skin. Lama5 –/– (A and C) or Lama5 +/+ (B and D) skin was assessed 5 days after grafting to nude mice by TUNEL assay (A and B) or Ki67 antibody expression (C and D). Note the lack of hair follicle-associated Ki67 staining in Lama5 –/– skin. HG, hair germ; HF, hair follicle. Arrowheads indicate apoptotic nuclei. Bar = 60 µm.
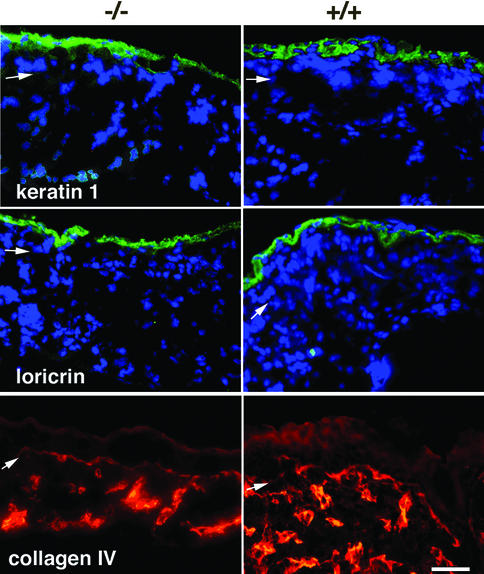
We next compared cellular proliferation in Lama5 –/– and Lama5 +/+ grafts, by examining the proliferation marker Ki67 (Figure 6C and D). While mutant grafts showed a lack of the hair follicle-associated Ki67 staining that was observed in wild-type grafts, mutant and wild-type grafts showed similar Ki67 staining at the dermal–epidermal junction. These results suggested that a selective loss of cellular proliferation associated with hair follicle development was present in mutant grafts. The hair follicle developmental defect in mutant skin appeared to be selective, as epidermal differentiation, assessed by keratin 1 and loricrin expression, was similar in both mutant and wild-type grafts, and blood vessel development, assessed by type IV collagen expression, was also evident in mutant grafts, as shown in Figure 7.
Fig. 7. Selective impairment of epithelial development in Lama5 –/– skin. Lama5 –/– or Lama5 +/+ skin was analyzed 5 days after grafting to nude mice by IDIF microscopy using antibodies against the indicated proteins. Note the normal expression of epidermal differentiation markers in Lama5 –/– skin. Bar = 30 µm.
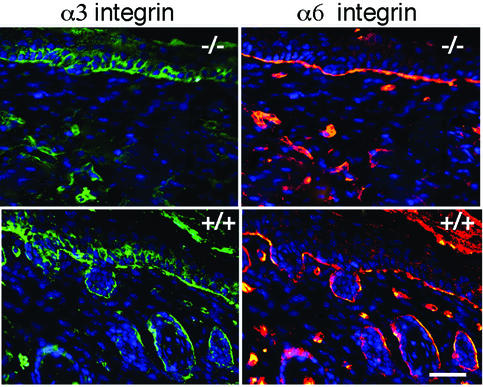
Alterations of integrin expression can influence cellular proliferation and differentiation (Giancotti and Ruoslahti, 1999). To determine whether the absence of laminin-10 affected integrin distribution in the skin, we examined the two major integrin receptors at the dermal–epidermal junction, α3β1 and α6β4 integrin in day 5 Lama5 –/– and Lama5 +/+ skin grafts. As seen by dual-label immunofluorescence microscopy in Figure 8, α3 integrin localized in a basal–lateral distribution, and α6 integrin localized in a basal distribution in both Lama5 –/– and Lama5 +/+ skin. Similar findings were observed in ungrafted E16.5 skin (not shown). Thus, we were unable to demonstrate differences in integrin expression or distribution that could account for the lack of hair follicle development in Lama5 –/– skin.
Fig. 8. Absence of laminin-10 does not affect integrin distribution in the skin. Lama5 –/– or Lama5 +/+ skin samples 5 days after grafting were analyzed by dual-label indirect immunofluorescent microscopy using antibodies against the indicated integrin subunits. Bar = 30 µm.
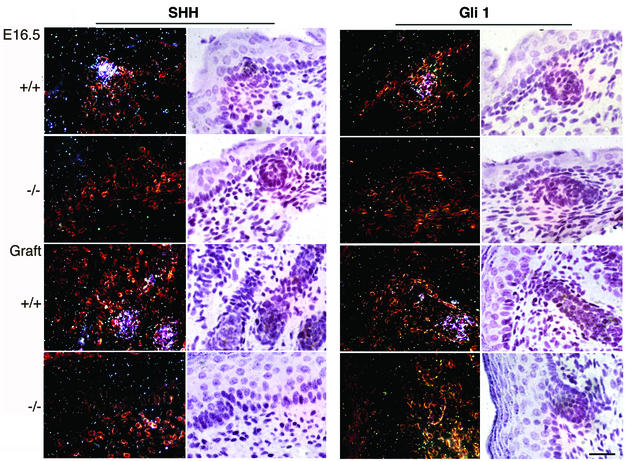
Increased cellular proliferation in elongating hair germs is known to be driven by the Shh signaling pathway, and Shh null mice fail to show hair germ elongation (St-Jacques et al., 1998; Chiang et al., 1999). Thus, we wished to determine whether absence of laminin-10 affected Shh expression. To test this hypothesis, we examined Lama5 –/– skin and wild-type skin at E16.5 and 5 days after grafting by in situ hybridization using probes for Shh and its downstream target, Gli1. While hair follicle-associated Shh and Gli1 expression were observed in E16.5 wild-type skin and in 5 day wild-type grafts, no Shh or Gli1 expression was noted in mutant skin or graft samples (Figure 9).
Fig. 9. Lama5 –/– and Lama5 +/+ skin was analyzed at E16.5 days or 5 days after grafting by in situ hybridization using 35S-labeled probes for Shh or Gli1. Dark-field visualization is shown in the left panels, while bright-field images are shown in the right panels. Note the lack of significant Shh or Gli1 expression in Lama5 –/– hair germs in either E16.5 or graft conditions. Bar = 20 µm.
Discussion
This study, for the first time, describes the critical role of the extracellular matrix/BMZ in skin morphogenesis. We have shown that laminin-10 is the primary laminin of elongating hair germs, and that absence of laminin-10 results in arrest of hair follicle development at the hair germ elongation phase. Interestingly, the application of exogenous laminin-10 promoted restoration of hair follicle development in Lama5 –/– skin. To our knowledge, this is the first instance of protein-mediated therapy in the correction of a cutaneous developmental defect. The method we employed, incubation of full-thickness embryonic skin in a laminin-10-containing solution, effectively resulted in the diffusion of laminin-10 into the BMZ of developing hair follicles. While exogenous laminin-10 persisted in grafts for up to 9 days following application, it is likely that additional methods of application would need to be employed, such as intradermal injection, to facilitate further persistence of exogenous laminin-10 in skin. Studies examining the effects of laminin-10 delivery to later stages of skin development are currently under way.
As small amounts of laminin-11 were present in our purified laminin-10 samples, we cannot rule out the possibility that laminin-11 might also facilitate hair follicle development. However, as laminin β2-deficient mice have no hair defects (Noakes et al., 1995), and a downregulation of the laminin β2 chain was seen during mouse hair germ elongation in our studies, it is not likely that laminin-11 plays a major role in hair follicle development in the skin.
Our results, as well as the results of a number previous studies, suggest that laminin-10 supports hair follicle development through a mechanism other than the maintenance of dermal–epithelial cohesion. A comparison between inhibition of laminin-5 and laminin-10 in the skin illustrates this point. In human skin xenografts, laminin-5 antibodies induced extensive epidermal detachment (Lazarova et al., 2000; M.P.Marinkovich, unpublished data) within 24 h of application, while in our studies, skin treated with laminin-10 antibodies did not show blisters or epidermal detachment even after 3 weeks. Similarly, extensive blistering is seen in patients with HJEB, who lack laminin-5. Absence of laminin-5 in Lama3 –/– mice produced extensive epidermal detachment as well as detachment-associated cell death (anoikis) (Ryan et al., 1999). In contrast, in our studies, we were unable to demonstrate significant epidermal detachment or anoikis in Lama5 –/– mouse skin. On the other hand, while inhibition of laminin-10 in both human and murine skin produced marked effects on hair follicle development, no hair follicle defects were demonstrated in laminin-5/6 null mouse skin (Ryan et al., 1999), and hair follicle development is typically normal in HJEB patients (Skoven and Drzewiecki, 1979). In conclusion, laminin-5 and laminin-10 appear to have non-overlapping functions in the skin, with laminin-5 promoting epidermal adhesion and laminin-10 promoting epithelial development.
In our studies of BMZ structure in Lama5 –/– skin, lamina densa assembly was markedly abnormal, while exogenous laminin-10 corrected these defects. These results highlight the role that laminin-10 plays in lamina densa assembly in epithelial BMZs. However, the question arises as to whether BMZ assembly is necessary for hair follicle formation. For example, a number of alterations of BMZ assembly have been described in skin that do not affect hair follicle development. The absence of β4 integrin in humans produces junctional epidermolysis bullosa with pyloric atresia. In this disease, hemidesmosome formation is markedly abnormal and dermal– epidermal cohesion is severely impaired, but hair follicle abnormalities have never been reported in conjunction with this syndrome (Fine et al., 2000). Similarly, β4 integrin null mice show a similar lack of epidermal cohesion and hemidesmosome formation, but normal hair follicle development (Dowling et al., 1996). Even the combined absence of α6β4 integrin and α3 integrin in Intα3/Intα6 –/– mice, which produced extensive impairment of lamina densa and hemidesmosome formation as well as extensive disruption of epidermal adhesion, did not significantly affect hair follicle formation (DiPersio et al., 2000). From these observations, it appears likely that neither an epithelial attachment defect nor a BMZ assembly defect can by themselves account for the lack of hair follicle development in Lama5 –/– skin.
In contrast to β4 or α3 integrin deficiency, ablation of β1 integrin markedly inhibited hair follicle development (Brakebusch et al., 2000; Raghavan et al., 2000). Findings in β1 integrin conditionally null skin were remarkably similar to our findings with Lama5 –/– skin, and include formation of an interrupted lamina densa and arrest of hair follicle development at the hair germ elongation stage. These previous findings correlate well with our studies of antibody-induced β1 integrin inhibition in the developing human scalp xenografts, in which β1 inhibition inhibited hair follicle development. As α3β1 integrin is a major laminin-10 receptor (Kikkawa et al., 2000), these results support a model in which laminin-10 promotes hair follicle development through β1 integrin signaling. This remains to be further studied.
It appears likely that the lack of epithelial proliferative downgrowths in mutant skin grafts are the direct result of impaired Shh production and signaling evidenced by a decrease in expression of Shh and its downstream effector Gli1 in Lama5 –/– skin. Thus, it is possible that interruption of a specific signaling pathway arrested hair follicle development in Lama5 –/– mice. Shh expression and signaling are known to depend on the correct signaling of regulators such as isoforms of the BMP, WNT and TGF families of proteins, and it is possible that laminin-10 may in turn influence the localization, expression or activity of one or more of these proteins. Of note, it is interesting that the alterations of epithelial development in Lama5 –/– skin are selective, and do not affect other aspects of skin development such as epidermal differentiation or blood vessel formation.
As the effects of laminin-10 appear specifically directed at the elongating hair germ, it is tempting to speculate that laminin-10 required for this process would be located in the follicular epithelial BMZ and be of keratinocyte origin. Alternatively, it has previously been suggested that a dermal signal is required for elongation of hair germs (Hardy, 1992). In particular, laminin-10 is a component of dermal blood vessels (Sorokin et al., 1997), and has been shown to act as a potent substrate for β1 integrin-mediated endothelial cell signaling and migration (Doi et al., 2002). Thus, it is possible that interactions mediated by laminin-10 in blood vessels could account for dermal contributions necessary for hair germ elongation. Additional experiments are currently under way to compare the contributions of keratinocyte and blood vessel laminin-10 towards hair follicle development.
It is also possible that lack of laminin-10 could impair blood vessel function. This could produce hypoxic conditions which impact on hair follicle development either in the hair cycle or embryonic skin morphogenesis. However, blood vessels were shown to form in mutant skin, and viability in both Lama5 –/– and Lama5 +/+ grafts was equivalent, approaching 100%, and with a lack of significant apoptosis in the absence of laminin-10. Therefore, hypoxia as a sole explanation for lack of hair follicle development in Lama5 –/– grafts seems unlikely. Similarly, no differences in apoptosis were seen in β1 integrin-deficient hair follicles (Brakebusch et al., 2000).
Materials and methods
Antibodies
The anti-human laminin α5 chain mAb 4C7 (Engvall et al., 1986), kindly provided by Dr Eva Engvall, Burnham Institute, La Jolla, CA, was purified from IgG-free hybridoma medium using protein G–Sepharose chromatography. Peak antibody elution fractions were then dialyzed extensively against PBS, and passed through a 0.2 µm filter for cell/animal studies, coupled to activated CL-4B Sepharose (Pharmacia, Uppsala, Sweden) or directly conjugated to fluorescein using the manufacturer’s protocol (Pierce, Rockford, IL). Anti-mouse laminin α5 chain rabbit antisera (Miner et al., 1997) and anti-laminin-5/6 rabbit antisera (Marinkovich et al., 1992b) have been characterized previously. Anti-mouse laminin β2 rabbit antiserum (Sasaki et al., 2002) was generously provided by Dr Rupert Timpl, Max Plank Institut, Martinsreid, Germany. The mAb 545 directed against the laminin β1 chain has been described previously (Marinkovich et al., 1992a). Rat mAbs directed against α6 integrin, laminin γ1 and α1 chains, and affinity-purified goat anti-type IV collagen antibody were obtained from Chemicon, Temecula, CA. Anti-α3 integrin rabbit antiserum (Hodivala-Dilke et al., 1998) was generously provided by Dr Kairbaan Hodivala-Dilke, St Thomas’ Hospital, London. Affinity-purified rabbit polyclonal antibodies directed against loricrin and keratin 1 were obtained from Covance, Berkeley, CA. Rat mAb anti-Ki67 was obtained from Dako, Carpenteria, CA. The mAb P5D2 is a blocking antibody directed against the β1 integrin subunit (Wayner et al., 1993), and P5D2 hybridoma cells were obtained from the Iowa Developmental Studies Hybridoma Bank.
Cell attachment assay
Purified laminin-10 or purified laminin-5 in PBS was coated at a concentration of 10 µg/ml overnight on 96-well tissue culture dishes; the dishes were then blocked with 1% BSA for 2 h at room temperature. Primary human keratinocytes were isolated from neonatal foreskin and cultured in serum-free medium according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). First-passage keratinocytes were briefly trypsinized, plated onto laminin-10- or laminin-5-coated 96-well dishes at 105 cells/well, and incubated at 37°C. Cell attachment after 30 min was quantified, three sets of three wells per condition, by a colorimetric assay as described previously (Marinkovich et al., 1995).
Purification of laminin-10
Laminin-10 was isolated from A549 lung carcinoma cell conditioned medium by affinity chromatography using a 4C7-Sepharose column as described previously (Kikkawa et al., 1998). Peak fractions were further purified by passage over a Mono-Q column (Pharmacia, Uppsala, Sweden) followed by elution with a linear NaCl gradient, and extensive dialysis against PBS. After this final purification step, laminin-10 purity was assessed by SDS–PAGE using 3–5% acrylamide gradient gels followed by Coomassie Blue total protein staining. Western blotting was performed on purified laminin-10 as described previously (Marinkovich et al., 1992a). Purified laminin-10 concentration was assessed by Bio-Rad total protein assay (Bio-Rad, Hercules, CA). Laminin-5 was purified as described previously (Marinkovich et al., 1992b).
Human skin grafting studies
Sections (2 × 1 cm) of full-thickness second trimester human scalp skin were sutured into 2 × 1 cm full-thickness skin defects created on the lateral dorsal surface of nude mice and covered with antibiotic ointment and non-adherent dressings. After 3 weeks, mice (six mice per condition) were given 2 mg injections of either mouse IgG (Sigma, St Louis, MO), mAb P5D2 or mAb 4C7. Subsequent 1 mg booster injections were administered at weekly intervals. Circulating antibody titers between 1:1000 and 1:3000 were maintained throughout the period of antibody injections, as assessed using sera obtained from grafted mice 1 day prior to weekly injections, analyzed by IDIF on frozen sections of human neonatal foreskin. After 4 weeks of injections, grafts were harvested, fixed in 10% formalin, embedded in paraffin, and analyzed by hematoxylin and eosin staining.
Mouse skin grafting studies
Sections (1.5 × 1.5 cm) of full-thickness E16.5 embryonic skin from Lama5 –/– mutant mice or wild-type sibling controls were grafted to 1.5 × 1.5 cm full-thickness skin defects created on the lateral dorsal surface of nude mice. Grafts were covered with antibiotic ointment and non-adherant dressings, and harvested at 9 or 30 day intervals (six mice per each condition). In separate experiments, E16.5 Lama5 –/– mouse skin specimens were incubated in laminin-10/11 (40 µg/ml in PBS) or PBS overnight at 4°C prior to grafting, and grafts were harvested at 5 and 9 days (six mice per condition). Grafts were sectioned and stained with hematoxylin and eosin, and additionally were analyzed using transmission electron microscopy, immunofluorescence microscopy and in situ hybridization.
Quantitative hair histomorphometry
This technique was performed as described previously (St-Jacques et al., 1998). Briefly, the number of hair follicles per millimeter of epidermal length was calculated in cryostat and paraffin sections of mouse back skins of E16.5 skin grafts from Lama5 –/– mutants and compared with those of wild-type littermates (each group n = 3–5). Serial sections were cut and every tenth section was collected. A total of 40–50 microscopic fields from each group were analyzed and quantified by Student’s t-test. All sections were analyzed at 100× magnification; means and SEM were calculated from pooled data. A P-value <0.05 was considered as significant.
In situ hybridization
In situ hybridization was performed as described previously (Wilkinson et al., 1987) with some modifications. Briefly, fresh skin from embryos and grafts was fixed in 4% paraformaldehyde in PBS at 4°C overnight, washed with PBS, dehydrated in a graded ethanol series and embedded in paraffin. Sections (5–6 µm) were cut, dewaxed, rehydrated and fixed with 4% paraformaldehyde in PBS for 20 min. The sections were then digested with 20 µg/ml proteinase K in 50 mM Tris–HCl pH 7.4, 5 mM EDTA for 8 min, rinsed in 0.2% glycine in PBS and PBS, then re-fixed with 4% paraformaldehyde in PBS for 5 min. The sections were then treated with acetic anhydride, dehydrated with graded ethanol and hybridized to 106 c.p.m. [35S]UTP-labeled riboprobes at 55°C overnight under high stringency conditions in a solution containing 50% formamide, 0.3 M NaCl, 5 mM EDTA, 20 mM Tris pH 7.4, 5 mM sodium phosphate pH 8, 1× Denhardt’s, 10% dextran sulfate, 0.5 mg/ml yeast tRNA and 100 mM DTT. Unhybridized probe was removed by washes, and sections were dehydrated with graded ethanols. Slides were then exposed to Kodak NTB2 emulsion for 4–6 weeks at 4°C in the dark and developed with Kodak D19 developer. Sections were photographed under both bright-field and dark-field illumination. Gli1 (Hui et al., 1994) and Shh (Oro et al., 1997) riboprobes were synthesized using an in vitro transcription kit according to the manufacturer’s instructions (Stratagene). Briefly, riboprobes were generated by in vitro transcription in 20 µl reactions containing 100 µCi of [35S]UTP, 1 µl of 10 mM each rATP, rCTP and rGTP, 40 U of RNase inhibitor and 10 U of T3 or T7 RNA polymerases. Unincorporated nucleotides were removed on a Sephadex G50 column (Boehringer Mannheim). A sense probe was used as an internal negative control.
Other methods
IDIF of 5 µm thick frozen skin sections was performed as described previously (Marinkovich et al., 1997) using fluorescein-conjugated goat anti-rabbit IgG and Texas Red-conjugated goat anti-rat IgG secondary antibodies (Sigma). In some instances, Hoechst stain was applied to visualize nuclei. All sections were visualized and photographed using a Zeiss Axiovert 100 inverted microscope. TUNEL assay and Ki67 immunohistochemistry were performed on paraffin-embedded skin sections according to the manufacturer’s instructions (Roche Diagnostic Corporation, Indianapolis, IN) using alkaline phosphatase substrate with a methyl green counterstain. Transmission electron microscopy of skin specimens was performed as described previously (Keene et al., 1987).
Acknowledgments
Acknowledgements
Correspondence should be addressed to M.P.M. The authors wish to thank Sara Tufa for excellent technical assistance with electron microscopy. This investigation was supported by funding from the Office of Research, Palo Alto VA Health Care System and NIH grants AR47223 and AR44012 (M.P.M.).
References
- Brakebusch C. et al. (2000) Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J., 19, 3990–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R.E. and Christiano,A.M. (1997) The dermal–epidermal junction. Curr. Opin. Cell Biol., 9, 651–658. [DOI] [PubMed] [Google Scholar]
- Burgeson R.E. et al. (1994) A new nomenclature for the laminins. Matrix Biol., 14, 209–211. [DOI] [PubMed] [Google Scholar]
- Callahan C.A. and Oro,A.E. (2001) Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling. Curr. Opin. Genet. Dev., 11, 541–546. [DOI] [PubMed] [Google Scholar]
- Chiang C. et al. (1999) Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol., 205, 1–9. [DOI] [PubMed] [Google Scholar]
- DiPersio C.M., van der Neut,R., Georges-Labouesse,E., Kreidberg,J.A., Sonnenberg,A. and Hynes,R.O. (2000) α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci., 113(Pt 17), 3051–3062. [DOI] [PubMed] [Google Scholar]
- Doi M. et al. (2002) Recombinant human laminin-10 (α5β1γ1). Production, purification and migration-promoting activity on vascular endothelial cells. J. Biol. Chem., 277, 12741–12748. [DOI] [PubMed] [Google Scholar]
- Dowling J., Yu,Q.C. and Fuchs,E. (1996) β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol., 134, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom M., Falk,M., Salmivirta,K., Durbeej,M. and Ekblom,P. (1998) Laminin isoforms and epithelial development. Ann. N. Y. Acad. Sci., 857, 194–211. [DOI] [PubMed] [Google Scholar]
- Engvall E. and Wewer,U.M. (1996) Domains of laminin. J. Cell Biochem., 61, 493–501. [DOI] [PubMed] [Google Scholar]
- Engvall E., Davis,G.E., Dickerson,K., Ruoslahti,E., Varon,S. and Manthorpe,M. (1986) Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite promoting site. J. Cell Biol., 103, 2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J.D. et al. (2000) Revised classification system for inherited epidermolysis bullosa: Report of the Second International Consensus Meeting on diagnosis and classification of epidermolysis bullosa. J. Am. Acad. Dermatol., 42, 1051–1066. [PubMed] [Google Scholar]
- Fuchs E. and Raghavan,S. (2002) Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet., 3, 199–209. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti,E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Hardy M.H. (1992) The secret life of the hair follicle. Trends Genet., 8, 55–61. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Mochizuki,M., Nomizu,M., Uchinuma,E., Yamashina,S. and Kadoya,Y. (2002) Inhibition of hair follicle growth by a laminin-1 G-domain peptide, RKRLQVQLSIRT, in an organ culture of isolated vibrissa rudiment. J. Invest. Dermatol., 118, 712–718. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke K.M., DiPersio,C.M., Kreidberg,J.A. and Hynes,R.O. (1998) Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol., 142, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C., Slusarski,D., Platt,K.A., Holmgren,R. and Joyner,A.L. (1994) Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2 and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during post-implantation development. Dev. Biol., 162, 402–413. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Keene D.R., Sakai,L.Y., Lunstrum,G.P., Morris,N.P. and Burgeson,R.E. (1987) Type VII collagen forms an extended network of anchoring fibrils. J. Cell Biol., 104, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Sanzen,N. and Sekiguchi,K. (1998) Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. Laminin-10/11 mediates cell adhesion through integrin α3β1. J. Biol. Chem., 273, 15854–15859. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Sanzen,N., Fujiwara,H., Sonnenberg,A. and Sekiguchi,K. (2000) Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α3β1, α6β1 and α6β4 integrins. J. Cell Sci., 113, 869–876. [DOI] [PubMed] [Google Scholar]
- Klinowska T.C., Soriano,J.V., Edwards,G.M., Oliver,J.M., Valentijn,A.J., Montesano,R. and Streuli,C.H. (1999) Laminin and β1 integrins are crucial for normal mammary gland development in the mouse. Dev. Biol., 215, 13–32. [DOI] [PubMed] [Google Scholar]
- Lazarova Z., Hsu,R., Yee,C. and Yancey,K.B. (2000) Human anti-laminin 5 autoantibodies induce subepidermal blisters in an experimental human skin graft model. J. Invest. Dermatol., 114, 178–184. [DOI] [PubMed] [Google Scholar]
- Li D.Y., Brooke,B., Davis,E.C., Mecham,R.P., Sorensen,L.K., Boak,B.B., Eichwald,E. and Keating,M.T. (1998) Elastin is an essential determinant of arterial morphogenesis. Nature, 393, 276–280. [DOI] [PubMed] [Google Scholar]
- Li S., Harrison,D., Carbonetto,S., Fassler,R., Smyth,N., Edgar,D. and Yurchenco,P.D. (2002) Matrix assembly, regulation and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol., 157, 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta M., Virtanen,I., Burgeson,R. and Autio-Harmainen,H. (2001) Comparative analysis of the distribution of laminin chains in the basement membranes in some malignant epithelial tumors: the α1 chain of laminin shows a selected expression pattern in human carcinomas. J. Histochem. Cytochem., 49, 711–726. [DOI] [PubMed] [Google Scholar]
- Marinkovich M.P., Lundstrum,G.P., Keene,D.R. and Burgeson,R.E. (1992a) The dermal–epidermal junction of human skin contains a novel laminin variant. J. Cell Biol., 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich M.P., Lunstrum,G.P. and Burgeson,R.E. (1992b) The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J. Biol. Chem., 267, 17900–17906. [PubMed] [Google Scholar]
- Marinkovich M.P., Meneguzzi,G., Burgeson,R.E., Blanchet-Bardon,C., Holbrook,K.A., Smith,L.T., Christiano,A.M. and Ortonne,J.P. (1995) Prenatal diagnosis of Herlitz junctional epidermolysis bullosa by amniocentesis. Prenat. Diagn., 15, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Marinkovich M.P. et al. (1997) LAD-1 is absent in a subset of junctional epidermolysis bullosa patients. J. Invest. Dermatol., 109, 356–359. [DOI] [PubMed] [Google Scholar]
- Marinkovich M.P., Herron,G.S., Khavari,P.A. and Bauer,E.A. (1999) Inherited epidermolysis bullosa. In Freedberg,I.M., Eisen, A.Z., Wolff,K., Austen,K.F., Goldsmith,L.A., Katz,S.I. and Fitzpatrick,T.B. (eds), Dermatology in General Medicine. McGraw-Hill, New York, NY.
- Martin D., Zusman,S., Li,X., Williams,E.L., Khare,N., DaRocha,S., Chiquet-Ehrismann,R. and Baumgartner,S. (1999) wing blister, a new Drosophila laminin α chain required for cell adhesion and migration during embryonic and imaginal development. J. Cell Biol., 145, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K.A. and Marinkovich,M.P. (2000) Laminins and human disease. Microsc. Res. Tech., 51, 262–279. [DOI] [PubMed] [Google Scholar]
- Millar S.E. (2002) Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol., 118, 216–225. [DOI] [PubMed] [Google Scholar]
- Miner J.H. and Li,C. (2000) Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol., 217, 278–289. [DOI] [PubMed] [Google Scholar]
- Miner J.H., Lewis,R.M. and Sanes,J.R. (1995) Molecular cloning of a novel laminin chain, α5 and widespread expression in adult mouse tissues. J. Biol. Chem., 270, 28523–28526. [DOI] [PubMed] [Google Scholar]
- Miner J.H., Patton,B.L., Lentz,S.I., Gilbert,D.J., Snider,W.D., Jenkins,N.A., Copeland,N.G. and Sanes,J.R. (1997) The laminin α chains: expression, developmental transitions and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11 and cloning of a novel α3 isoform. J. Cell Biol., 137, 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.H., Cunningham,J. and Sanes,J.R. (1998) Roles for laminin in embryogenesis: exencephaly, syndactyly and placentopathy in mice lacking the laminin α5 chain. J. Cell Biol., 143, 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba D., Hieda,Y. and Nakanishi,Y. (2000) Remodeling of desmosomal and hemidesmosomal adhesion systems during early morphogenesis of mouse pelage hair follicles. J. Invest. Dermatol., 114, 171–177. [DOI] [PubMed] [Google Scholar]
- Noakes P.G., Gautam,M., Mudd,J., Sanes,J.R. and Merlie,J.P. (1995) Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature, 374, 258–262. [DOI] [PubMed] [Google Scholar]
- Oro A.E. and Scott,M.P. (1998) Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell, 95, 575–578. [DOI] [PubMed] [Google Scholar]
- Oro A.E., Higgins,K.M., Hu,Z., Bonifas,J.M., Epstein,E.H.,Jr and Scott,M.P. (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science, 276, 817–821. [DOI] [PubMed] [Google Scholar]
- Raghavan S., Bauer,C., Mundschau,G., Li,Q. and Fuchs,E. (2000) Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation and hair follicle invagination. J. Cell Biol., 150, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P., Lunstrum,G.P., Keene,D.R. and Burgeson,R.E. (1991) Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J. Cell Biol., 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.C., Lee,K., Miyashita,Y. and Carter,W.G. (1999) Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J. Cell Biol., 145, 1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Mann,K., Miner,J.H., Miosge,N. and Timpl,R. (2002) Domain IV of mouse laminin β1 and β2 chains. Eur. J. Biochem., 269, 431–442. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Zhang,X., Zhang,J., Leontovich,A., Fei,K., Yan,L. and Sarras,M.P.,Jr (2002) Epithelial morphogenesis in hydra requires de novo expression of extracellular matrix components and matrix metalloproteinases. Development, 129, 1521–1532. [DOI] [PubMed] [Google Scholar]
- Skoven I. and Drzewiecki,K.T. (1979) Congenital localized skin defect and epidermolysis bullosa hereditaria letalis. Acta Derm. Venereol., 59, 533–537. [PubMed] [Google Scholar]
- Sorokin L.M., Pausch,F., Frieser,M., Kröger,S., Ohage,E. and Deutzmann,R. (1997) Developmental regulation of the laminin α5 chain suggests a role in epithelial and endothelial cell maturation. Dev. Biol., 189, 285–300. [DOI] [PubMed] [Google Scholar]
- St-Jacques B. et al. (1998) Sonic hedgehog signaling is essential for hair development. Curr. Biol., 8, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Timpl R., Tisi,D., Talts,J.F., Andac,Z., Sasaki,T. and Hohenester,E. (2000) Structure and function of laminin LG modules. Matrix Biol., 19, 309–317. [DOI] [PubMed] [Google Scholar]
- Wayner E.A., Gil,S.G., Murphy,G.F., Wilke,M.S. and Carter,W.G. (1993) Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for α3β1 positive T lymphocytes. J. Cell Biol., 121, 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D.G., Bailes,J.A., Champion,J.E. and McMahon,A.P. (1987) A molecular analysis of mouse development from 8 to 10 days post coitum detects changes only in embryonic globin expression. Development, 99, 493–500. [DOI] [PubMed] [Google Scholar]
- Willem M., Miosge,N., Halfter,W., Smyth,N., Jannetti,I., Burghart,E., Timpl,R. and Mayer,U. (2002) Specific ablation of the nidogen-binding site in the laminin γ1 chain interferes with kidney and lung development. Development, 129, 2711–2722. [DOI] [PubMed] [Google Scholar]