Abstract
Understanding how climate influences ecosystems represents a challenge in ecology and natural resource management. Although we know that climate affects plant phenology and herbivore performances at any single site, no study has directly coupled the topography–climate interaction (i.e. the climatological downscaling process) with large-scale vegetation dynamics and animal performances. Here we show how climatic variability (measured by the North Atlantic oscillation ‘NAO’) interacts with local topography in determining the vegetative greenness (as measured by the normalized difference vegetation index ‘NDVI’) and the body masses and seasonal movements of red deer (Cervus elaphus) in Norway. Warm springs induced an earlier onset of vegetation, resulting in earlier migration and higher body masses. Increasing values of the winter-NAO corresponded to less snow at low altitude (warmer, more precipitation results in more rain), but more snow at high altitude (colder, more precipitation corresponds to more snow) relative to winters with low winter-NAO. An increasing NAO thus results in a spatially more variable phenology, offering migrating deer an extended period with access to high-quality forage leading to increased body mass. Our results emphasize the importance of incorporating spring as well as the interaction between winter climate and topography when aiming at understanding how plant and animal respond to climate change.
Keywords: Cervus elaphus, red deer, normalized difference vegetation index, North Atlantic oscillation, body masses, dispersion patterns
1. Introduction
Understanding how climate influences vertebrate populations has become a major challenge in ecology and management facing the recent climatic changes (Walther et al. 2002; Stenseth et al. 2003). Whenever climate affects dynamics of keystone species, it may easily lead to trophic cascades having an impact far beyond that particular species (Post et al. 1999). We know that both mean temperature and precipitation have increased and will likely increase further in the northern hemisphere (IPCC 2001). However, we have currently a rather poor understanding of how the overall mean averages may change due to local climate–topography interactions, the so-called climatological ‘downscaling process’, which actually determines the prevalent weather conditions at the scale of the plants and animals. This is a critical step in order to understand the impact of climate on ecological system, especially for systems involving migratory animals, such as those including large herbivores.
Climate introduces considerable temporal and spatial variations in plant quality (Chapin et al. 1995). Increased access to highly nutritious forage is generally regarded as the most important driving force in the evolution of migration in large herbivores (Fryxell & Sinclair 1988; Mysterud et al. 2001a). Even slight changes in plant quality may affect body growth of ruminants substantially. Feeding on high quality forage gives the ruminant both more energy and protein per time unit, but also more feeding time as less time is used for rumination. Thus more can be eaten of high quality, and this ‘multiplier’ effect represents a link between foraging ecology and demography of ruminants (White 1983). The vertical movement from a low elevation winter range to a high elevation summer range is a typical pattern of migration (Demarais & Krausman 2000; Mysterud et al. 2001a) assumed to be a strategy to increase energy intake in northern-temperate cervids. Plant phenology is a good proxy of plant quality, as young plants are generally of high nutritional value with low levels of secondary plant chemicals (Demment & Van Soest 1985; Van Soest 1994). A key mechanism linking plant development and herbivore performance is thus that a spatially variable plant phenology leads to a prolonged period with access to newly available, high quality forage. Trophic interactions are expected to be particularly important ecological processes affected by climate change (Schmitz et al. 2003). Specifically, the start of vegetation growth, which is assumed to be strongly determined by snow depth and spring conditions (Langvatn et al. 1996; Post & Stenseth 1999), is then regarded as the key period (Illius & O'Connor 2000) as herbivores have to recover from winter harshness and face the energetically most demanding period of lactation (Clutton-Brock et al. 1982).
Using 20 years of data on body mass of red deer (Cervus elaphus) from three populations inhabiting a topographically highly variable area along the west coast of Norway, timing of migration, and satellite derived data on plant vegetation dynamics (timing of greenness as assessed by the Normalized Difference Vegetation Index (NDVI); Justice et al. 1985) collected at the 64 km2 scale covering the entire study area (about 65 000 km2), we explored in this study processes linking vegetation phenology and red deer body mass and migration patterns regarding local topography and climatic fluctuations (as measured by the North Atlantic Oscillation (NAO); Hurrell et al. 2003; Stenseth et al. 2003). More specifically, we (i) explored how climate and topography determined vegetation onset as assessed by satellite data in our study area. From previous work (Mysterud et al. 2001a,b), we expected topography to interact with global climatic conditions in winter determining the local snow conditions and thus the future onset of vegetation the following spring. Then we aimed at quantifying to what extent an early spring could affect (ii) body masses and (iii) migration departure date of red deer in Norway.
2. Material and methods
(a) Data
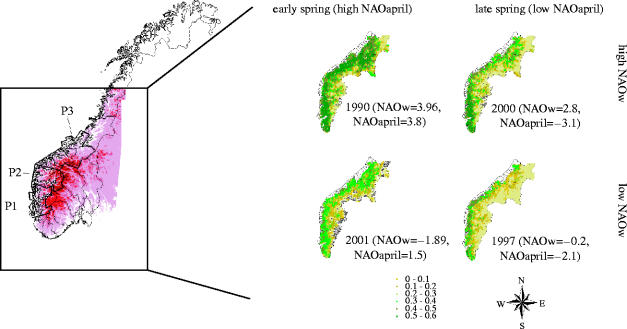
The study area is the west coast in the southern part of Norway, encompassing the main distribution area of Norwegian red deer (Mysterud et al. 2001a). Three populations live in topographically variable habitats and were hence included in the analyses: Rogaland and Hordaland counties are referred to as population P1, Sogn and Fjordane as population P2, Møre og Romsdal and Sør-Trøndelag as population P3 (figure 2). A detailed description of the study area is given elsewhere (Mysterud et al. 2001a,b).
Figure 2.
The location of the study site (in bold) in Norway and the topographic variability within the study area, and the NDVI on the 1st of May in a year with a low and a high NAO in winter (NAOw) in years of early and late spring (assessed by the NAO in April).
(i) Climate data
As an index of global winter and spring climate, we used the NAO (Hurrell et al. 2003; Stenseth et al. 2003) index for winter (Dec–Mar) and April. Data on snow depth, precipitation and temperature were obtained from 123 meteorological stations along the west coast of Norway through The Norwegian Meteorological Institute, Oslo. The average length of the time series was 25 years (range 10–28).
(ii) Topographic data
Data on municipality borders were retrieved as vector data, while data on altitude (in a terrain model) were obtained as raster data with a resolution of 100×100 m (Mysterud et al. 2001a). We obtained all other spatial information from the National Mapping agency of Norway (Statens Kartverk) in a format that was used directly in a Geographical Information System (GIS; ESRI 1996). Topographic variables linked to red deer performance were calculated at the municipality scale and are described in detail elsewhere (Mysterud et al. 2001a).
(iii) Vegetation data
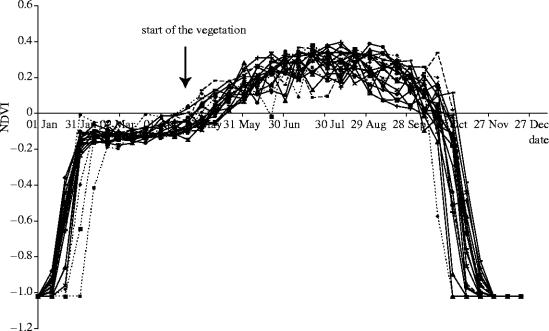
Data collected by the National Oceanic and Atmospheric Administration satellites have recently been made available to ecologists (http://eosdata.gsfc.nasa.gov/). From these, NDVI values (ranging from −1 to 1; Justice et al. 1985; Chapin et al. 1995) have been produced from visible and near-infrared reflectance measurements (NDVI=(NIR−RED)/(NIR+RED), where NIR is the Near InfraRed light reflected by the vegetation, and RED the Red visible light reflected by the vegetation). The spatial scale of resolution (pixel size) is 64 km2 and a NDVI value is available for the entire study area on a 10-day basis, from the 13th of July 1981 to 21st September 2001. NDVI has already been described as a good proxy of vegetation dynamics (Myneni et al. 1997). As a measure of the greenness in spring, we chose to use NDVI on 1st of May (which is the average date of vegetation start in the study area over the 20 years considered; figure 1, Albon & Langvatn 1992), the average date in Europe being the April 23 (Chmielewski & Rötzer 2002). Other measures (for example the slope from the 1st of May to the 21st of June) were highly correlated and provide a similar conclusion.
Figure 1.
Yearly variation in the vegetation development represented as 10 days spatial averages in the NDVI over the study area and during the considered period (1982–2001, based on 1019 pixels).
Coupling information on topography and vegetation was done through a tessellation procedure (http://arcscripts.esri.com) in GIS (ESRI 1996) obtaining a network of 1019 polygons. By using overlays, we calculated the different spatial covariates of topography for each vegetation polygon. We calculated for each polygon the proportion of ‘low’ altitude habitats between 0 and 700 m (approximate tree line in the study area; Mysterud et al. 2001a). We used the geographic longitude to estimate for each vegetation pixel the distance to the coast.
(iv) Red deer data
We used a subset of the red deer data relative to previous studies (Mysterud et al. 2000, 2001a,b) covering the period 1982–2001, this being the period covered by the NDVI data (see below). This includes 12 046 male and 10 223 female red deer (>1 yr old) harvested between September 10 and November 15 in 94 Norwegian municipalities. For each individual, sex, age, date, location (municipality) and dressed body mass are known (Mysterud et al. 2001a). Moreover, between 1983 and 1999, 28 females (>1 yr old) belonging to two different winter range areas near the Trondheim fjord (Population ‘P3’: winter range area 1:63.38′ N and 8.75′ E, winter range area 2:63.46 N, 9.50′E) were radio-monitored from February to November in order to study migration patterns. Sixty-one migration events were monitored during this period. For each event, it was possible to estimate the date of the first fix where the individual was located out of its winter range (checking that the following fixes were also out of the winter range).
(b) Statistical analyses
(i) General approach
We used linear mixed models (LME; Pinheiro & Bates 2000) to analyse variations in snow depth, temperature and precipitations in April, NDVI on the 1st of May, body mass of female and male red deer and migration date, after initial use of additive models (AM; Pinheiro & Bates 2000) with smoothing splines to explore possible non-linear relationships. Polynomials were chosen for modelling non-linear relationships. Since the year 1996 came out as an outlier in previous analyses (Mysterud et al. 2001a,b), it was excluded, the effect of the winter-NAO being then modelled as linear. All statistical analyses were performed in the statistical package SPlus (Pinheiro & Bates 2000) vs. 6.0.
In order to facilitate the interpretation of polynomial terms and assess interactions between two continuous predictors, variables were standardized (mean 0, variance 1; Mysterud et al. 2000). Furthermore, only multiplicative terms were considered for defining interactions.
The data are covering quite a wide geographical range (see §2a), and areas close to each other may be more similar than those far apart (i.e. spatial autocorrelation). Ignoring positive spatial autocorrelation in residuals may lead to too small confidence intervals. Spatial models such as geostatistical models are recommended in such cases and were used; however, in our cases (i.e. large data sets and complex models), this procedure did not work because of convergence problems and thus unreliable estimates. We thus accounted for spatial autocorrelation among residuals from the retained LME models by performing spatial bootstrapping (Efron & Tibshirani 1993; Lahiri 2003). Due to some imbalance in the sampling design (we do not have data every year for all municipalities, the smallest spatial unit), we grouped data into spatial blocks (see electronic supplementary material, figure 1A) to provide reliable estimates for all years. The size of blocks was chosen to fit the observed autocorrelation structure of the residuals. Varying the number of spatial blocks considered did not affect the presented results. Similarly, temporal effects of individual years (e.g. effects of the NAO) should be based on bootstrapping years too, since consecutive years may be more similar than those far apart (i.e. temporal autocorrelation). We therefore used a bootstrapping procedure based on spatial blocks in a given year (i.e. a spatiotemporal bootstrapping). A total of 500 bootstrap samples were used.
(ii) Climate
We expected topography and global climatic conditions in winter to interact in determining snow depth. For snow depth modelling, we thus updated a former analysis of snow depth (Mysterud et al. 2000) as a function of the winter-NAO, altitude, degree of latitude and degree of longitude with LME (and year as random effect). The need for transformation of snow depth was assessed using the Box–Cox transformation family, the parameters being estimated using profile log-likelihood (Burnham & Anderson 1998). We therefore transformed snow depth as ‘log (snow depth+4)’ (Mysterud et al. 2000).
To interpret NAO in April variations, we modelled temperature and precipitation in spring as a function of latitude, altitude, and NAO in April with LME (and year as random effect). Biological interpretations of results are presented in table 1, while detailed results of both analyses are presented in the electronic supplementary material, table 1.
(iii) NDVI—1st of May
In order to model the vegetation start in Norway based on satellite data, we considered all the available variables and interactions expected to affect vegetation onset (winter climatic conditions, topography, spring climatic conditions). This first step was crucial in order to check the reliability of our remote sensing proxy, by confirming expected patterns. The vegetation start is expected when the NDVI turns positive (Justice et al. 1985). Initial screening of the NDVI data showed that variation in greenness was adequately modelled using variation on the 1st of May. Model selection and the use of Akaike's Information Criterion (AIC; Burnham & Anderson 1998) were in this case non-adapted as no a priori small set of models was available. Our main goal is in effect to use a biologically reasonable model to estimate the strength of the main effects and their interactions. We accordingly determined the relationship between the NDVI and the NAO in winter, latitude, distance to the sea, diversity of altitudes, diversity of aspects, and the proportion of altitudes between 0 and 700 m. We also included the NAO in April (r=0.11 from 1982 to 2001 for NAO winter vs April), as spring conditions are highly important for early plant growth (Langvatn et al. 1996; Post & Stenseth 1999). Interactions between NAO in winter and the proportion of low altitudes or the distance to the coast were expected to affect vegetation start from previous studies (Mysterud et al. 2001a,b). Interactions between spring conditions and geographical position were also considered, as did the interactions among spatial variables (which allowed more complex spatial patterns in plant phenology to be accounted for). We here present estimates from the spatial bootstrapping considering 500 bootstrap samples and 29 spatial blocks. Varying the number of blocks (from 16 to 73) did not affect the presented results.
(iv) Red deer body mass
To explore to what extent an early spring might affect body masses, we simply added NDVI in the 1st of May at the municipality scale as a covariable within the already known model describing body mass variations of red deer in Norway (Mysterud et al. 2001a, Yoccoz et al. 2002). As previously described (Mysterud et al. 2001a, Yoccoz et al. 2002), body mass was thus ln-transformed to stabilize the variance. Because the relationship between age and body mass is markedly different in males and females (as well as their interactions with other factors), we replicated the analysis by sex. For females, we used age categories 1, 2, 3, 4 and 5–19 years, as female weight is stable until 20 years of age when they reach prime-age at 5 years of age (cf. Mysterud et al. 2001c). We excluded females aged 20 years or older due to low sample size and senescence at this stage. Males cannot be modelled the same way, since they do not have a stable weight during prime-age. Rather they grow fast until prime-age is reached (and later than for females), and then they decrease in weight with age (before and faster than females). Since the increase in weight with age before prime-age is fast, while the decrease in weight with age after prime-age is comparably slower, we modelled male age as a sixth order polynomial (Yoccoz et al. 2002). A symmetrical second order polynomial is insufficient in capturing these necessary details (see Yoccoz et al. 2002 for further biological reasons for these choices), but the 6th order polynomial does not appear ‘complex’—it is also a bell-shaped curve—but that is not symmetrical (cf. Yoccoz et al. 2002). The current analyses include only years 1982–2001 for which the NDVI was available and only for the three topographic heterogeneous populations (P1–3; Mysterud et al. 2001a,b). Our starting point was thus the model retained in a previous study (Yoccoz et al. 2002). We then added population specific averages for NDVI on 1st of May, and the spatial residual, which was the municipality (local) minus population yearly average, as well as interaction terms with population.
We present estimates from the spatial bootstrapping considering 500 bootstrap samples and 14 spatial blocks, although the results from the simple LME are the same (estimates and confidence intervals), as no spatial autocorrelation among residuals was found.
We included all covariates known to affect body mass, in order to reduce possible bias due to imbalanced sampling design. Fitting too complex a model generally results in estimates with lower bias but lower precision. Since avoiding bias is a more important issue than precision in observational studies (Rosenbaum 1995), we thus included several covariates that may or may not significantly affect body weight, but that nevertheless may help avoid bias. This approach may thus be considered as a conservative approach, as it lowers power in testing. In order to demonstrate the robustness of our approach (i.e. that the results are not dependent on inclusion of a large number of covariates), we fitted also a much simpler model (only four terms (age, density, population and distance from the coast) in addition to the ones related to the NAO and NDVI): the parameter estimates were similar to those obtained on the basis of the more complex model. Therefore, the presented results are not sensitive to other factors being included—and we can certainly say that those included are biologically important.
(v) Red deer migration
To explore the relationship between the starting date of the spring migration and the greenness (indexed by NDVI value in the winter area in the 1st of May) we used LME (with individual as random effect). Data were derived for 2×6 pixels encompassing the two red deer winter ranges. For each year the relative NDVI value in the 1st of May per winter range according to the average NDVI value during the sampled years in both sites was determined.
3. Results
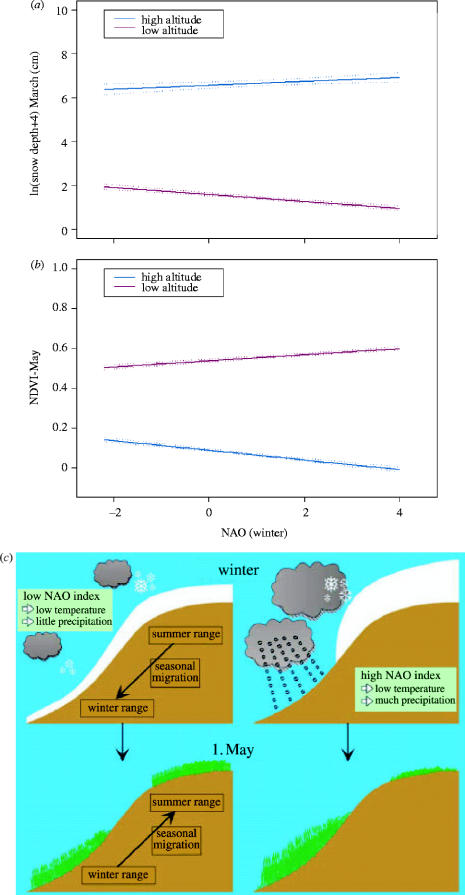
Spring conditions were the main determinant of the vegetation onset. High temperature in April directly led to an early greenness on 1st of May (table 1). The green-ness was earlier in the lowland, near the coast and at low latitude, as would be expected from the spatial pattern of temperature variation at this time of the year along the west coast of Norway (figure 2). Indirect effects of winter harshness on vegetation onset were also reported, which confirm previous results (Mysterud et al. 2001a). Winter-NAO interacted with local topography in determining patterns of spatial snow accumulation (figure 3a; see electronic supplementary material, table 2). An increasing winter-NAO index is correlated with higher temperature, but also more precipitation (Hurrell et al. 2003). Temperature decreases with altitude, and is a main determinant of whether precipitation falls as rain or snow. As coastal winter temperatures are often around 0 °C, there was a negative correlation between the winter-NAO and snow depth in March at low altitude, but a positive correlation at high altitude (figure 3a,c). The NDVI in May was accordingly more delayed with increasing altitude following high NAO winters than following low NAO winters (figure 3b,c). Due to this contrasting effect of the winter-NAO on snow accumulation at high and low altitude, no overall effect of the winter-NAO on spring phenology across all sites was found, but a strong interacting effect with altitude was evident. Also, the indirect effect of the winter-NAO on NDVI was more pronounced along the coast than in the inland, as would be expected since the winter-NAO has a weaker influence on weather when approaching the eastern parts of Norway.
Table 1.
Relevant factors influencing (a) snow depth, (b) NDVI for the 1st of May, body mass of adult (c) female and (d) male red deer, (e) date of migration start.
| parameter | biological interpretation |
|---|---|
| (a) snow depth | |
| altitude | more snow with increasing altitude |
| NAO in winter×altitude | less snow depth with higher NAO-values during winter at low altitude, while more snow at high altitude |
| (b) NDVI-greenness | |
| NAO in winter×proportion of low-altitude habitats | greenness is delayed with high NAO-values during winter at high altitude, but less so at low altitude |
| NAO in April×proportion of low-altitude habitats | greenness is delayed with high NAO-values during April at high altitude, but less so at low altitude |
| (c) body mass adult female red deer | |
| NAO in winter | high NAO-values during winter corresponds to higher body mass |
| NDVI (yearly) | early vegetation start in a year corresponds to higher body mass |
| NDVI (spatial) | early vegetation start in one place comparing to others corresponds to higher body mass |
| (d) body mass adult male red deer | |
| NAO in winter | high NAO-values during winter corresponds to higher body mass |
| NDVI (yearly) | early vegetation start in a year corresponds to higher body mass |
| NDVI (spatial) | early vegetation start in one place comparing to others corresponds to higher body mass |
| (e) migration date | |
| NDVI | migration begins earlier when vegetation green-ness starts earlier in the winter range |
All factors reported below were significant, and the detailed results are presented in the electronic supplementary material, tables 2 and 3.
Figure 3.
The relationship between the winter NAO and (a) snow depth during March at high (above 700 m) and low (sea level) altitudes (predicted values from analysis in the electronic supplementary material, table 2) and (b) NDVI on the 1st of May the consecutive spring at high (p=1) and low (p=0) proportion of low altitudes (predicted values from analysis in the electronic supplementary material, table 3). (c) A schematic presentation of the results as it relates to the migration pattern of red deer in Norway.
Early vegetation start had then a positive effect on red deer condition in the subsequent autumn (table 1; see electronic supplementary material, figure 3A). The effect of annual variation in the NDVI was the same for all three populations. An increase from 0 to 0.5 in NDVI on 1st of May between years could generate a 4 kg increase in a 2-yr-old female under ‘average’ conditions (around 8% increase), while an increase in the NAO in winter from −2 to 4 generated a 2 kg or ∼4% increase in body mass. The effect of spatial variation in NDVI (within populations) was positive and similar for populations ‘P1’ and ‘P2’ in both males and females, while there was no significant effect in population ‘P3’.
Finally, an early onset had a positive effect on the start of red deer migration (t=−3.53, df=32, P<0.001, R2=0.19). Depending on the NDVI value within the winter range, the starting date of migration would in our study area vary up to 20 days (see electronic supplementary material, figure 2A).
This provides to our knowledge the first explicit link of how a climatological downscaling process affects the biology of animals (but see discussion in Mysterud et al. 2000).
4. Discussion
The clearly most novel insight from our study is that topography–climate interactions, i.e. the climatological downscaling process, introduces a spatially variable phenology affecting performance and behaviour of red deer. The only (albeit quite remotely) similar finding is the famous one in the Serengeti showing that non-synchronous greenness affects herbivore migration. In the Serengeti, migratory herds of ungulates move over ‘vast’ geographical regions, following a rainfall gradient affecting plant quality in different seasons (McNaughton 1990). In contrast, at northern latitudes, plant quality gradients determine migration and herbivore body mass within the same geographical region due to the climate–topography interface in heterogeneous landscapes: in our study area, spatial asynchrony in phenology results from topography–climate interactions at the scale of a few km. The current climate models used by IPCC (Climate Change 2001) refer to global means and variability of climate; however, the ‘downscaling’ process at which the ecological processes are operating is critically influenced by climate–topography interactions—of the kind reported in our paper. Here we explicitly show that any temporal variation in the average climatic variables could have profoundly different consequences for vegetation dynamics or ungulate performance according to the location and the topography of the considered area. Our study demonstrates thus the importance of the exploration of climate change impacts at lower scales (i.e. the ‘downscaling process’), and will therefore be of great importance for understanding critical ecological processes relating to climate change (Gosz & Sharpe 1989; Stenseth et al. 2002).
Our study is also the first one to couple long-term, high-quality data on wild herbivore population dynamics at a large spatial scale with long-term vegetation dynamics data and climate. Most of the causal links between plants and herbivorous mammals presented here are known. Earlier studies have generally relied on time-series data on the phenology of single plant species (Albon & Langvatn 1992; Post & Stenseth 1999): the problem with those kinds of studies is the local scale of the series that are often not from the same area, which prevent taking fully into account this large spatial variation characterizing the vegetation dynamics features demonstrated in this study.
Previous work in that study area had already shown that 1—the percentage in crude protein in red deer forage was maximal in spring, at high altitude and at high distance from the coast and 2—that the percentage in crude protein affected directly red deer body mass (Albon & Langvatn 1992). Here we show that 1—NDVI in the winter area determined migration start to higher altitudes 2—that NDVI affected directly and positively red deer body mass. Seen together, satellite derived NDVI data thus constitute an excellent proxy to monitor vegetation quality dynamics at a large spatial scale, both confirming obvious patterns with a later start at high altitude and latitude, while revealing also mechanistic links between climate and plants (Justice et al. 1985; Chapin et al. 1995; Myneni et al. 1997; Lenart et al. 2002). Previous studies have underlined the interest of remote sensing data in global ecology and climatic change problems (Kerr & Ostrovsky 2003). Here we demonstrate also the interest of those kinds of data in population dynamics and management.
Resource management is often falling short of predicting even short-term population changes, because they fail to account for climate. Management plans are usually updated with a short time horizon where climate often is more important than slower density changes for population changes. Clearly, knowing this year's winter-NAO will able managers to predict following autumn body weight (∼2 kg or ∼4% weight increase resulting from an increase in the winter NAO from −2 to 4 in a 2-yr-old female under ‘average’ conditions), while the next year winter-NAO can be predicted correctly with a probability of 0.45 (Hurrell et al. 2003). The use of NDVI dramatically increases the predictive potential (∼4 kg or ∼8% weight increase resulting from an increase in the NDVI from 0 to 0.5). Such weight changes will have a major impact on population dynamics, as a 1 kg (∼2%) increase in weight increase the proportion of female calves surviving by 0.13 (Loison et al. 1999) and the proportion of primiparous females ovulating by 0.07 (Langvatn et al. 2004). The longer-term periodicity of the NAO (such as decadal scale oscillations) has been more difficult to predict (Hurrell et al. 2003). However, most climate models simulate significant increases in the NAO in response to increasing concentrations of greenhouse gases—suggesting an increase of average to about 4 for year 2050 (Hurrell et al. 2003). Future scenarios will likely be strongly influenced by changes in other factors such as population density and land use as well, but this provides a promising starting point for managers in foresee population performance in a not so far future subject to climate change.
Acknowledgments
We gratefully acknowledge comments and help from Jean Michel Gaillard, Robert Weladji, Geir Ottersen, Dag Hjermann, Leif Egil Loe, Christophe Bonenfant and Leif Christian Stige to previous drafts, and the financial support of the Research Council of Norway (to AM and NCS). This work has furthermore been supported by the French Ministry of foreign Affairs (Bourse Lavoisier) and by the Marie Curie Fellowship to NP.
Supplementary Material
References
- Albon S.D, Langvatn R. Plant phenology and the benefits of migration in a temperate ungulate. Oikos. 1992;65:502–513. [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; Berlin: 1998. Model selection and inference: a practical information-theoretic approach. [Google Scholar]
- Chapin F.S, Shaver G.R, Giblin A.E, Nadelhoffer K.J, Laundre J.A. Responses of Artic Tundra to experimental and observed changes in climate. Ecology. 1995;76:694–711. [Google Scholar]
- Chmielewski F.M, Rötzer T. Annual and spatial variability of the beginning of growing season in Europe in relation to air temperature changes. Clim. Res. 2002;19:257–264. [Google Scholar]
- Climate Change . Cambridge University Press; Cambridge: 2001. Third assessment report of the intergovernmental panel on climate change IPCC. [Google Scholar]
- Clutton-Brock T.H, Guinness F.E, Albon S.D. University of Chicago Press; Chicago: 1982. Red deer: behaviour and ecology of two sexes. [Google Scholar]
- Demarais S, Krausman P.R. Prentice Hall; Upper Saddle River, NJ: 2000. Ecology and management of large mammals in North America. [Google Scholar]
- Demment M.W, Van Soest P.J. A nutritional explanation for body-size patterns of ruminant and non ruminant herbivores. Am. Nat. 1985;125:641–672. 10.1086/284369 [Google Scholar]
- Efron B, Tibshirani R.J. Chapman & Hall; London: 1993. An introduction to the bootstrap. [Google Scholar]
- ESRI Arcview GIS . Environmental Systems Research Institute; New York: 1996. The geographical information system for everyone. [Google Scholar]
- Fryxell J.M, Sinclair A. R.E. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 1988;3:237–241. doi: 10.1016/0169-5347(88)90166-8. 10.1016/0169-5347(88)90166-8 [DOI] [PubMed] [Google Scholar]
- Gosz J.R, Sharpe P. J.H. Broad scale concepts for interactions of climate, topography, and biota at biome transitions. Landsc. Ecol. 1989;3:229–243. 10.1007/BF00131541 [Google Scholar]
- Hurrell J.W, Kushnir Y, Ottersen G, Visbeck M. The north Atlantic oscillation: climate significance and environmental impact. Geophys. Monogr. Ser. 2003:134. [Google Scholar]
- Illius A.W, O'Connor T.G. Resource heterogeneity and ungulate population dynamics. Oikos. 2000;89:283–294. 10.1034/j.1600-0706.2000.890209.x [Google Scholar]
- Justice C.O, Townshend J. R.G, Holben B.N, Tucker C.J. Analysis of the phenology of global vegetation using meteorological satellite data. Int. J. Rem. Sens. 1985;6:1271–1318. [Google Scholar]
- Kerr J.T, Ostrovsky M. From space to species: ecological applications for remote sensing. Trends Ecol. Evol. 2003;18:299–305. 10.1016/S0169-5347(03)00071-5 [Google Scholar]
- Lahiri S.N. Springer; Berlin: 2003. Resampling methods for dependent data. [Google Scholar]
- Langvatn R, Albon S.D, Burkey T, Clutton-Brock T.H. Climate, plant phenology and variation in age at first reproduction in a temperate herbivore. J. Anim. Ecol. 1996;65:653–670. [Google Scholar]
- Langvatn R, Mysterud A, Stenseth N.C, Yoccoz N.G. Timing and synchrony of ovulation in red deer constrained by short northern summers. Am. Nat. 2004;163:763–772. doi: 10.1086/383594. [DOI] [PubMed] [Google Scholar]
- Lenart E.A, Bowyer R.T, Van Hoef J, Ruess R.W. Climate change and caribou: effects of summer weather on forage. Can. J. Zool. 2002;80:664–678. 10.1139/z02-034 [Google Scholar]
- Loison A, Langvatn R, Solberg E.J. Body mass and winter mortality in red deer calves: disentangling sex and climate effects. Ecography. 1999;22:20–30. [Google Scholar]
- McNaughton S.J. Mineral nutrition and seasonal movements of African migratory ungulates. Nature. 1990;345:613–615. 10.1038/345613a0 [Google Scholar]
- Myneni R.B, Keeling C.D, Tucker C.J, Asrar G, Nemani R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. 10.1038/386698a0 [Google Scholar]
- Mysterud A, Yoccoz N.G, Stenseth N.C, Langvatn R. Relationships between sex ratio, climate and density in red deer: the importance of spatial scale. J. Anim. Ecol. 2000;69:959–974. 10.1046/j.1365-2656.2000.00454.x [Google Scholar]
- Mysterud A, Langvatn R, Yoccoz N.G, Stenseth N.C. Plant phenology, migration and geographic variation in body weight of a large herbivore: the effect of a variable topography. J. Anim. Ecol. 2001a;70:915–923. 10.1046/j.0021-8790.2001.00559.x [Google Scholar]
- Mysterud A, Stenseth N.C, Yoccoz N.G, Langvatn R, Steinheim G. Nonlinear effects of large-scale climatic variability on wild and domestic herbivores. Nature. 2001b;410:1096–1099. doi: 10.1038/35074099. 10.1038/35074099 [DOI] [PubMed] [Google Scholar]
- Mysterud A, Yoccoz N.G, Stenseth N.Chr, Langvatn R. Effects of age, sex and density on body weight of Norwegian red deer: evidence of density-dependent senescence. Proc. R. Soc. B. 2001c;268:911–919. doi: 10.1098/rspb.2001.1585. 10.1098/rspb.2001.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; Berlin: 2000. Mixed-effects models in S and S-plus. [Google Scholar]
- Post E, Stenseth N.C. Climatic variability, plant phenology, and northern ungulates. Ecology. 1999;80:1322–1339. [Google Scholar]
- Post E, Peterson R.O, Stenseth N.C, McLaren B.E. Ecosystem consequences of wolf behavioural response to climate. Nature. 1999;401:905–907. 10.1038/44814 [Google Scholar]
- Rosenbaum P.R. Springer; New York: 1995. Observational studies. [Google Scholar]
- Schmitz O.J, Post E, Burns C.E, Johnstone K.M. Ecosystem responses to global climate change: moving beyond colour mapping. BioScience. 2003;53:1199–1205. [Google Scholar]
- Stenseth N.C, Mysterud A, Ottersen G, Hurrell J.W, Chan K.S, Lima M. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. 10.1126/science.1071281 [DOI] [PubMed] [Google Scholar]
- Stenseth N.C, Ottersen G, Hurrell J.W, Mysterud A, Lima M, Chan K.S, Yoccoz N, Ådlandsvik B. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Nino Oscillation and beyond. Proc. R. Soc. B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. 10.1098/rspb.2003.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P.J. Cornell University Press; New York: 1994. Nutritional ecology of the ruminant. [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. 10.1038/416389a [DOI] [PubMed] [Google Scholar]
- White R.G. Foraging patterns and their multiplier effects on productivity of northern ungulates. Oikos. 1983;40:377–384. [Google Scholar]
- Yoccoz N.G, Mysterud A, Langvatn R, Stenseth N.C. Age- and density-dependent reproductive effort in male red deer. Proc. R. Soc. B. 2002;269:1523–1528. doi: 10.1098/rspb.2002.2047. 10.1098/rspb.2002.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.