Abstract
A demographic understanding of population dynamics requires an appreciation of the processes influencing survival—a demographic rate influenced by parameters varying at the individual, maternal and cohort level. There have been few attempts to partition the variance in demography contributed by each of these parameter types. Here, we use data from a feral population of Soay sheep (Ovis aries), from the island of St Kilda, to explore the relative importance of these parameter types on early survival. We demonstrate that the importance of variation occurring at the level of the individual, and maternally, far outweighs that occurring at the cohort level. The most important variables within the individual and maternal levels were birth weight and maternal age class, respectively. This work underlines the importance of using individual based models in ecological demography and we, therefore, caution against studies that focus solely on population processes.
Keywords: cohort effects, maternal provisioning, demography, population dynamics
1. Introduction
Juvenile survivorship can be a critical density-dependent process regulating wild animal populations (Gaillard et al. 2000). Therefore, an appreciation of the factors that influence early survival is crucial if the population dynamics of wild animals are to be understood. Alongside individual-level parameters (e.g. birth weight and sex), and population density, other density-independent variables, such as weather severity, are increasingly being considered in analyses of population dynamics and demography (e.g. Coulson et al. 2001; Forchhammer et al. 2002; Saether et al. 2004; Barbraud & Weimerskirch 2005; Schwartz & Armitage 2005). These variables are typically experienced by the whole population and may generate cohort effects (Lindstrom & Kokko 2002). From these and other studies it is clear that individual-, maternal- and cohort-level processes all influence demographic rates. What is less clear is the relative importance of such processes.
A variety of factors may influence the juvenile survival of mammals: offspring-specific characters such as birth weight, sex and the number of individuals in the litter (Morris 1996; Keech et al. 2000); maternal characteristics such as the age and condition of the mother (Keech et al. 2000); and environmental factors such as weather severity and food availability (Forchhammer et al. 2001) which typically influence the whole cohort.
Numerous studies have found that birth weight is a major determinant of early survival of many mammal species, with heavier offspring being more likely to survive both to nutritional independence (weaning), and to sexual maturity, than light offspring (Clutton-Brock et al. 1992; Loison et al. 1999). Furthermore, in many mammal populations, heavier females tend to give birth to heavier offspring than lighter females (Georges & Guinet 2000; Côté & Festa-Bianchet 2001).
Maternal malnutrition is a major predisposing factor influencing juvenile mortality, initially via its negative effect on birth weight (Rhind et al. 1998), due to the allocation of resources to the foetus during gestation, and then via its effect on lactational quality (Robinson et al. 2000). Because of increased grazing pressure, such effects are likely to be exacerbated at high population densities. In addition, there may be competition for other resources such as shelter.
The influence of weather conditions on herbivore forage availability and quality are well established (Lenart et al. 2002). However, weather conditions may also have an influence on foraging behaviour. The negative effects of severe weather, such as high wind speeds, rain and low temperatures, can limit the time available for foraging. There is a trade-off between the need to obtain nutrients to maintain condition and the increased rate of heat loss (and hence reduced condition) caused by exposure to the severe weather. In this case, the effects on early survival are manifested via the mother; by reducing the time available for the mother to feed, nutritional stress is placed on the growing foetus or suckling lamb. The newborn lamb will also be directly affected by adverse weather and, because of its small size, the threshold weather severity is likely to be lower and, therefore, the effects greater than on adults.
Parasite burden is also likely to be an important factor because it affects the balance between resource allocation to self-maintenance and to maternal care. The main metabolic effects of ungulate gastrointestinal parasites are increased endogenous protein loss, increased mucoprotein secretion and damage to the gut tissue, which can result in losses into the gastrointestinal tract and reduced nutrient absorption (Coop & Kyriazakis 1999). These losses and inefficiencies exert a potentially heavy cost on the host because nutrients and protein synthesis are diverted away from production processes, such as growth and muscle or fat deposition and milk production, into homeostatic responses, like blood protein synthesis, digestive tract and immune defence maintenance (Coop & Kyriazakis 1999).
In this study, we use detailed long-term data from St Kilda's Soay sheep population to disentangle the relative roles of cohort-, maternal- and individual-level covariates on early survival, an important regulatory component of many wild animal populations (although not necessarily of the Soay sheep population). We show that, in determining early survival, the importance of variation at the level of the individual far outweighs the importance of variation operating at the cohort level (e.g. weather or density effects experienced by the whole cohort).
2. Material and methods
(a) Study area and population
Data were collected from a free-ranging population of Soay sheep (Ovis aries L.) from Hirta, part of Scotland's St Kilda archipelago (57°49′N 08°34′W). Hirta supports a sheep population that fluctuates between ∼600 and ∼2000 individuals, and the study area supports about one-third of this total. Soay sheep are precocious breeders and participate in the rut within their first year of life (at around seven months of age), and some ewes give birth at 1 year of age.
The population was studied intensively in the 1960s (Jewell et al. 1974) and since 1985 (Clutton-Brock & Pemberton 2004). In this study, we used a sub-set of 829 individuals of both sexes that had both known mothers and known survival status. These were born between 1989 and 2002. In 2001, a mainland outbreak of foot and mouth disease (Ferguson et al. 2001) restricted data collection to dead individuals. To avoid potential bias the 2001 data were excluded from the analyses.
(b) Birth date and survival
Birth dates were determined by daily observation of the population between early March and early May. The lambs were considered to have survived if they survived until the population was rigorously censused in August of the year of birth.
(c) Morphometric and parasite burden measurements
The mothers of all animals used in the analyses were caught in August of the year prior to birth, allowing measurements of maternal body mass to be made. Faecal samples were also taken at this time to assess maternal parasite burden using a modified version of the McMaster faecal egg count (FEC) technique which estimates the number of parasite eggs per gram of fresh faeces (MAFF 1971). Only strongyles, the main pathogenic parasite of the Soay sheep on Hirta (Gulland 1992), were considered.
Lambs were caught soon after birth and ear tagged and weighed (93% within 5 days). In 1988–1989, samples of lambs were repeatedly caught and weighed to give an estimated growth rate of 0.108 g d−1 in early life (Robertson et al. 1992). This figure was used to control for the effect of catch date, by standardizing body mass to that expected at birth.
(d) Environmental variables
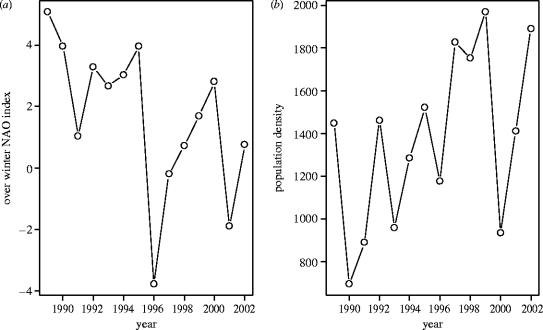
On Hirta there is significant inter-year variation in environmental parameters including, among others, population density and weather severity (figure 1). It is a mixture of these influences that give rise to the cohort effects that are apparent in the Soay sheep population (Coltman et al. 1999; Forchhammer et al. 2001). In this study, we tried both including these parameters explicitly by breaking down the effect of this inter-year variation into mechanistic components (weather severity, population density), and implicitly, by fitting cohort as a factor. The latter method allows evaluation of the amount of variation in survival probability explained by the numerous environmental parameters that vary on a year-to-year basis as a whole. The advantage of doing this, as opposed to fitting various environmental parameters separately, is that it does not require an a priori decision about which of the many environmental components are influential, or what functional forms any relationships take. Nor does it have the problems associated with high correlations between some environmental parameters (e.g. forage biomass and island population density). We present the results of both approaches.
Figure 1.
Examples of environmental parameters that experience considerable variation from year to year. (a) Overwinter NAO index, a measure of weather severity. (b) Whole island sheep population.
Whole-island population counts of adults and lambs were made between May and August of each year. Most lambs are born in April (in year t+1) and, therefore, a count from year t provides an estimate of population density between April in year t and April in year t+1.
We use the winter North Atlantic oscillation (NAO) index as a measure of weather severity. This index is a composite measure based on the atmospheric pressure gradient between Iceland and Portugal between December and March. It encapsulates of a number of variables, such that a low index signifies dry, cold and still winter weather, whereas a high index signifies wet, windy and warm winter weather (Hurrell 1995).
(e) Statistical methods
We constructed models that could include three categories of variable. These were: (i) individual parameters including sex, birth date, twin status and birth weight; (ii) maternal parameters such as maternal weight, parasite burden and age and (iii) cohort or environmental parameters (population density and NAO index). The amount of variation contributed by each of these categories was assessed by: (i) examining the change in residual deviance that results from the removal of the term from the model and (ii) using a hierarchical partitioning (HP) approach (MacNally 2002; Walsh & MacNally 2003). HP quantifies the independent and joint contribution of each predictor variable (which we first identified by the generalized linear model (GLM) procedure described below) to variation in the response variable. The HP method avoids the problem of multi-colinearity between the explanatory variables when assessing their contribution to variation.
The model fitting for survival was done using a logistic GLM with a binomial error structure and logit link function (Agresti 1990). The binary response variable was survival to August 31 (0, did not survive; 1, survived), and the explanatory variables were birth weight, sex, twin status, maternal age class (fitted as a three level factor: (i) lamb at conception, (ii) yearling at conception and (iii) adult at conception), maternal weight, and log(maternal strongyle parasite egg count+1). Maternal age classes were selected after a graphical analysis of the distribution of offspring birth weight with maternal age in years.
The model was fitted with main effects and first-order interactions (defined as products). Variables were then removed one at a time, using a backwards selection procedure based on the Akaike information criterion (AIC) (Burnham & Anderson 2002). The AIC is defined as the deviance +2*p, where p is the number of parameters of the model. The backwards elimination was continued until a minimum adequate model (in which all the variables retained were significant) was produced (Crawley 2002). We were aware that the probability of survival of individual lambs with the same mother might not be independent. We tried fitting logistic mixed effects models with maternal identity as a random effect but these proved to be unstable. However, because a large proportion of ewes (52%) only contributed a single lamb to the dataset and only 25% of them contributed more than two lambs, we are confident that this maternal identity effect was negligible.
By adding year (i.e. cohort) as a main effect we control for inter-year differences in survival (which are likely to be caused by inter-year variation in environmental conditions) and estimate the relative importance of the cohort effect. All terms were assessed for nonlinearity by examination of the residuals and by test fitting of continuous variables as quadratic terms. A bias-corrected r2 for the model was estimated by resampling cross-validation of the regression model with 45 repetitions using the ‘validate’ procedure from the ‘Design’ library of R. The ‘hier.part’ procedure from the ‘hier.part’ package was used to do the HP. All statistics were carried out using R v.2.0.1 (R Development Core Team 2004).
3. Results
In the final model that included the cohort term, the cohort term was highly significant (F-value=3.284, d.f.=12, p<0.001) indicating important temporal (between year) variation in early survival. The model had an estimated r2-value of 0.597 and the individual, maternal and cohort terms explained 42.8, 33.0 and 24.2% of this variation, respectively, when using the first method, and 80.1, 19.3 and 0.6% of the variation, respectively, when using the HP method. The results were qualitatively similar when individual environmental parameters (NAO and population density) were used instead of the cohort term: the individual, maternal and environmental terms explained 48.5, 31.7 and 19.8% of the variation using the first method and 72.7, 20.9 and 6.4% of the variation using the HP method.
The final model was relatively complex and included six interactions in addition to the main effects (table 1; figures 2 and 3). Individual level parameters were the most important, but most of these interacted with other maternal or environmental terms. Apart from cohort, sex was the only term to be present without occurring in an interaction and showed that females had a slightly higher probability of survival than males (females: 0.85±0.02; males: 0.81±0.02).
Table 1.
Summary of the generalized linear model for early survival of Soay lambs born between 1989 and 2002.
| type | main effect terms | estimate | s.e. |
|---|---|---|---|
| (intercept; 1989: n=5) | −57.89 | 20.10 | |
| I | birth weight | 7.83 | 2.62 |
| I | sex (male) | −0.82 | 0.27 |
| I | twin status (twin) | 2.81 | 2.90 |
| I | Julian birthdate | 0.44 | 0.17 |
| M | maternal age class (Y'ling) | 31.39 | 21.16 |
| M | maternal age class (Adult) | 37.20 | 19.98 |
| M | maternal weight | 0.41 | 0.20 |
| M | log(FEC+1) | −0.48 | 0.24 |
| C | year (1990: n=17) | −1.28 | 1.73 |
| C | year (1991: n=36) | 0.54 | 1.68 |
| C | year (1992: n=58) | −0.74 | 1.18 |
| C | year (1993: n=87) | 0.13 | 1.23 |
| C | year (1994: n=95) | 0.31 | 1.17 |
| C | year (1995: n=58) | −0.02 | 1.20 |
| C | year (1996: n=69) | 0.26 | 1.28 |
| C | year (1997: n=103) | 0.75 | 1.21 |
| C | year (1998: n=103) | 1.44 | 1.21 |
| C | year (1999: n=57) | 0.67 | 1.25 |
| C | year (2000: n=95) | 1.10 | 1.22 |
| C | year (2002: n=51) | 0.76 | 1.19 |
| interaction terms | |||
| I | birth weight: twin | 3.36 | 1.04 |
| I and M | birth weight: maternal age class (Y'ling) | 4.63 | 2.90 |
| I and M | birth weight: maternal age class (Adult) | −0.08 | 2.58 |
| I and M | birth weight: maternal weight | −0.23 | 0.12 |
| I and M | twin: maternal weight | −0.28 | 0.13 |
| I and M | Julian birth date: maternal age class (Y'ling) | −0.35 | 0.18 |
| I and M | Julian birth date: maternal age class (Adult) | −0.35 | 0.17 |
| M | maternal age class (Y'ling): log(FEC+1) | 0.47 | 0.30 |
| M | maternal age class (Adult): log(FEC+1) | 0.39 | 0.25 |
A logit link and binomial error structure were used and interactions were defined as products. Coefficients are given along with their standard errors. Treatment contrasts were used and the significance of the main effects and interaction terms was <0.05. The residual deviance was 406.56 on 799 d.f. The estimated r2-value was 0.597 and individual (I), maternal (M) and cohort (C) terms explained 42.8%, 33.0% and 24.2% of this variation, respectively. Where interactions were between two different types of variable, the amount of variation was partitioned equally between the two types. Sample sizes within each year are given after the year (e.g. 1992; n=58). Hierarchical variance partitioning gave the same ranking classification both using cohort (I=80.1%, M=19.3%, C=0.6%) and when using NAO and population density as individual environmental parameters (I=72.7%, M=20.9%, C=6.4%).
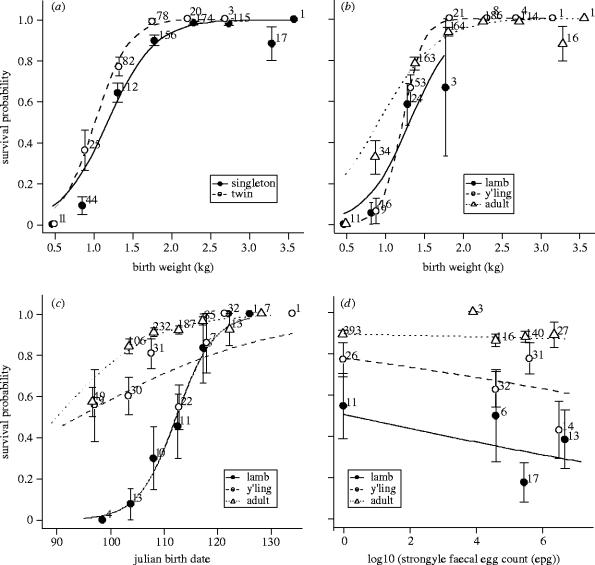
Figure 2.
The influence of (a) birth weight and twin status, (b) birth weight and maternal age class, (c) Julian birth date and maternal age class, and (d) maternal parasite burden and maternal age class on the probability of early survival of Soay lambs born between 1989 and 2002. Lines represent prediction from the GLM, points represent data, error bars represent ±1 s.e.m. The numbers next to the points indicate the number of observations represented by each point.
Figure 3.
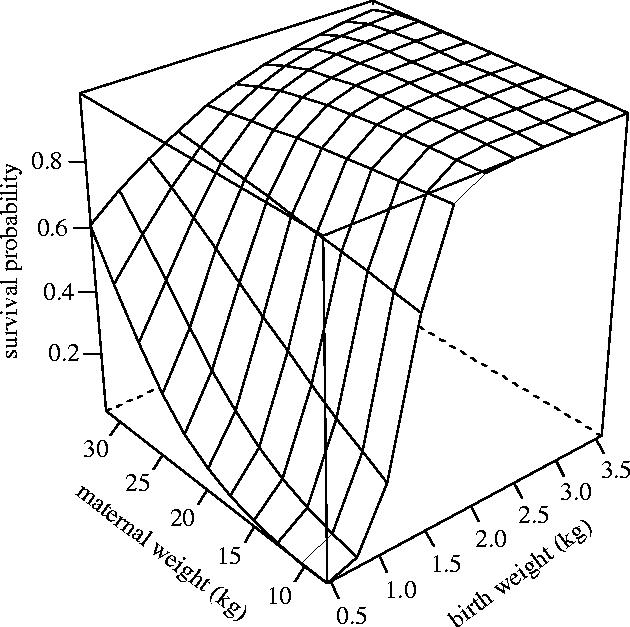
The interacting effects of maternal weight and birth weight upon the probability of early survival of Soay sheep lambs born between 1989 and 2002. Although it appears that survival decreases slightly with maternal weight, at very high birth weights care should be taken not to overinterpret the figure in this region, where there was relatively little data compared to the central region of parameter space.
Birth weight was an important factor but interacted with twin status (figure 2a), maternal age (figure 2b), and maternal weight (figure 3). Overall, birth weight was strongly positively associated with survival probability but the effect size was slightly larger for twins than it was for singletons (i.e. birth weight had more influence on the survival of twins than it had on the survival of singletons). The interaction between birth weight and maternal age was subtle but showed that light lambs born to adults had a higher probability of survival than those of a similar weight born to either lambs or yearlings (figure 2b). The interaction between maternal weight and birth weight (figure 3) indicated that the effect of maternal weight was generally positive but that it was largest for lambs with lower birth weights. The interaction between Julian birth date and maternal age was more striking (figure 2c) and showed that the timing of birth was more important for the offspring of lambs than for those of older age classes.
Maternal parasite burden occurred in a significant interaction with maternal age and showed that burden did not have a strong effect on the reproductive success of adult ewes but had a fairly strong negative influence on the success of yearlings and lambs (figure 2d).
4. Discussion
The detailed nature of our 14 year dataset, which included cohort information in addition to individual- and maternal-level parameters varying at the level of the individual, allowed us to explore the relative influence of these parameter types on early survival. It also enabled us to evaluate the mechanisms influencing this demographic rate. Earlier work on the juvenile survival of Soay sheep by Clutton-Brock et al. (1992) spanned a period of just 7 years (1985–1992) and did not include a measure of maternal condition or parasite burden. Our work includes a greater variety of maternal parameters over a longer time period (thus covering a greater range of environmental conditions) and includes cohort in an effort to encompass all sources of environmental variation. We, therefore, had more power to assess the relative influence of any effects that operate in this system.
(a) Early survival
Our model, which explained more than half of the variation in early survival probability (r2=0.597), showed that early survival was influenced by a complex range of parameters. Further, we demonstrated that, over the period studied, most of the variation in early survival resulted from factors varying at the maternal and individual level. Cohort-level variation, although significant, explained less than a quarter of the variation explained by the model. Due to the filtering effect of natural selection, individuals tend to show greater phenotypic variation in early life than in later life. This may explain the dominance of individual- and maternal-level variation here and we would expect that the influence of individually varying parameters to decrease as the animals age. Nevertheless, this clearly emphasizes the importance of using individual-based models (Deangelis & Gross 1992) in ecological demography.
As with previous work (e.g. Clutton-Brock 1988), we show that birth weight has a large influence on early survival. However, most previous work demonstrated fairly simple relationships between birth weight and survival. Our results differ in that the effect of birth weight is mediated by three interactions: with twin status, maternal age and maternal weight. The interaction between birth weight and twin status was peculiar because it seemed to show that for a given birth weight twins had a higher probability of survival than singletons. We had expected that this work would concur with work on other taxa showing that survival rate decreases as litter size increases (e.g. Clutton-Brock 1988). In effect, twins compete for resources from their mother and, if these resources are scarce, one or both of the twins may perish. The effect found in this study may be a statistical artefact brought about by bias in our dataset introduced because we restricted ourselves to live-born lambs: a significant proportion of twins conceived may die in utero, leaving only those which were conceived and born under conditions that favour survival. Alternatively, mothers of twins may be of higher quality and/or more experienced than mothers of singletons, so that their offspring survival was higher. Although this effect has been touched upon by Wilson et al. (2005) further work is required to resolve this issue.
The effect of birth weight was dependent on maternal age but apparently only for lower birth weights. For lambs with birth weights of <1.25 kg, maternal age was important, with the offspring of adults being more likely to survive than those of either lambs or yearlings. This can be explained by the level of maternal experience in rearing offspring, with more experienced mothers being better able to care for light-weight and, therefore, vulnerable, lambs, than those with less experience. Alternatively this could relate to the ability of the mother to invest in her offspring, i.e. for primiparous ewes there are fitness benefits to invest proportionally more in personal survival than in the survival of offspring (Festa-Bianchet & Jorgenson 1998).
As with other ungulates (Birgersson & Ekvall 1997; Keech et al. 2000), birth date was an important determinant of survival. Work examining the association between phases of rapid plant growth and parturition has shown increased juvenile survivorship at these times (Rubin et al. 2000). However, because body size is a major determinant of overwinter survival (Milner et al. 1999), there is also a cost to being born late and not having enough time to grow before entering the winter. Theoretically, therefore, we would expect a modal birth date–survival relationship. However, we did not detect such an effect. In this system, the importance of birth date depended both on maternal age and on population density. Although birth date was positively associated with survival for all three maternal age classes, the effect was most pronounced for the offspring of lambs. Without information on conception dates it is impossible to determine whether this effect is due to forage abundance or premature birth. However, lactation is an energetically costly process and, although it has little effect on the survival of mothers (Clutton-Brock et al. 1996), it is likely that ewes that conceived as lambs are often unable to allocate sufficient resources to milk production unless forage is abundant. The fact that the effect of birth date was greater in high-density years when there was more competition for both food and shelter emphasizes the importance of competitive processes during early development. The interaction between birth weight and maternal weight emphasizes the importance of these two parameters and shows the catastrophic results of being both born light and having a light-weight mother.
Over longer time periods, the survivorship of males is less than that of females, mainly as a result of the higher costs of reproduction for males in comparison to females (Jewell 1997). The fact that there are small sex differences in survival at such an early stage indicates that there may be other costs to being a male, possibly related to the higher post-parturition growth rate and corresponding higher nutritional demands of males. This is contrary to the findings of previous studies that have failed to detect sex-biased survival at this stage in other ungulate species (Clutton-Brock & Albon 1989; Fairbanks 1993; Côté & Festa-Bianchet 2001).
Previous work has shown that weather severity and population density influence the over-winter survival of Soay sheep (Milner et al. 1999; Coulson et al. 2001). Here, we have also shown that there is significant inter-year variation in early survival in spring–summer, which is also likely to be caused by a combination of environmental sources including weather severity, population density and forage availability. Given the cost of parasitism (Coop & Kyriazakis 1999), it is not surprising that maternal parasite burden had a negative influence upon offspring survival for lambs and yearlings. It is likely that, because of their larger body mass and stronger immune systems, adults are able to withstand greater burdens than young animals. Furthermore, since it is known that ewes are flexible in their resource allocation patterns (LeBlanc et al. 2001), it is possible that adults are more flexible in this regard than young animals and, although parasites also influence adult maternal condition, these effects are not great enough in adults to be passed on to influence the gestating or growing lamb which may take priority over self-maintenance (Coop & Kyriazakis 1999).
These results highlight the complexity of the relationships that exist between early survival and a hierarchy of parameters ranging from exogenous cohort-level parameters (which include weather severity, forage availability and population density) to parameters that vary at the level of the individual such as maternal-level factors (e.g. maternal weight) and individual-level parameters (e.g. sex and birth date). Perhaps of particular importance to population dynamics is the empirical demonstration of cohort-based and maternal time-lagged effects which are known to have implications in generating complex dynamics (Lande et al. 2003). Although we would certainly not condemn population level analyses outright, we make the case that they may not perform as well as models that use individual level data for parameterization because much of the variation in individual performance is attributed to the individual level rather than population level. A similar conclusion was reached by Coulson et al. (2001) who showed that age–sex structured models perform better than models averaging over the whole population. The effects described in this paper, alongside our demonstration of the relative amount of variation explained by individually varying versus cohort-level parameters, underline the importance of using individual based models in ecological demography.
Acknowledgments
We are grateful to the National Trust for Scotland and Scottish Natural Heritage for permission to work on St Kilda and their assistance in many aspects of the work. Much assistance and logistical support was also given by the Royal Artillery, SERCo and QinetiQ. For access to data on birth weight and juvenile survival collected between 1985 and 1995, we are grateful to Tim Clutton-Brock and members of the St Kilda sheep project including A. Robertson, D. Green and A. MacColl. A large number of volunteers have also worked on the project, for which we are grateful. Thanks to Tim Coulson, Giacomo Tavecchia, Nigel Yoccoz, Marco Festa-Bianchet and Tim Clutton-Brock and anonymous referees for useful comments on earlier drafts of this manuscript. The Soay sheep project is supported by grants from NERC, BBSRC and the Wellcome Trust (UK). O.R.J. was supported by a NERC studentship.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Agresti A. Wiley; New York: 1990. Categorical data analysis. [Google Scholar]
- Barbraud C, Weimerskirch H. Environmental conditions and breeding experience affect costs of reproduction in blue petrels. Ecology. 2005;86:682–692. [Google Scholar]
- Birgersson B, Ekvall K. Early growth in male and female fallow deer fawns. Behav. Ecol. 1997;8:493–499. [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York: 2002. Model selection and inference: a practical information-theoretic approach. [Google Scholar]
- Clutton-Brock T. University of Chicago Press; Chicago, IL: 1988. Reproductive success. Studies of individual variation in contrasting breeding systems. [Google Scholar]
- Clutton-Brock T, Price O, Albon S, Jewell P. Early development and population fluctuations in Soay sheep. J. Anim. Ecol. 1992;61:381–396. [Google Scholar]
- Clutton-Brock T, Stevenson I, Marrow P, MacColl A, Houston A, McNamara J. Population fluctuations, reproductive costs and life-history tactics in female Soay sheep. J. Anim. Ecol. 1996;65:675–689. [Google Scholar]
- Clutton-Brock T.H, Albon S.D. Blackwell Scientific Publications; Oxford, UK: 1989. Red deer in the highlands. [Google Scholar]
- Clutton-Brock T.H, Pemberton J.M. Cambridge University Press; Cambridge, UK: 2004. Soay sheep: dynamics and selection in an island population. [Google Scholar]
- Coltman D.W, Smith J.A, Bancroft D.R, Pilkington J, MacColl A.D.C, Clutton-Brock T.H, Pemberton J.M. Density-dependent variation in lifetime breeding success and natural and sexual selection in Soay rams. Am. Nat. 1999;154:730–746. doi: 10.1086/303274. 10.1086/303274 [DOI] [PubMed] [Google Scholar]
- Coop R.L, Kyriazakis I. Nutrition–parasite interaction. Vet. Parasitol. 1999;84:187–204. doi: 10.1016/s0304-4017(99)00070-9. 10.1016/S0304-4017(99)00070-9 [DOI] [PubMed] [Google Scholar]
- Coulson T, Catchpole E.A, Albon S.D, Morgan B.J.T, Pemberton J.M, Clutton-Brock T.H, Crawley M.J, Grenfell B.T. Age, sex, density, winter weather, and population crashes in Soay sheep. Science. 2001;292:1528–1531. doi: 10.1126/science.292.5521.1528. 10.1126/science.292.5521.1528 [DOI] [PubMed] [Google Scholar]
- Côté S, Festa-Bianchet M. Birthdate, mass and survival in mountain goat kids: effects of maternal characteristics and forage quality. Oecologia. 2001;127:230–238. doi: 10.1007/s004420000584. 10.1007/s004420000584 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2002. Statistical computing: an introduction to data analysis using S-Plus. [Google Scholar]
- Deangelis D.L, Gross L.J, editors. Individual-based models and approaches in ecology: populations, communities and ecosystems. Routledge, Chapman & Hall; New York: 1992. [Google Scholar]
- Fairbanks W. Birth date, birth weight and survival in pronghorn fawns. J. Mammal. 1993;74:123–135. [Google Scholar]
- Ferguson N.M, Donnelly C.A, Anderson R.M. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science. 2001;292:1155–1160. doi: 10.1126/science.1061020. 10.1126/science.1061020 [DOI] [PubMed] [Google Scholar]
- Festa-Bianchet M, Jorgenson J. Selfish mothers: reproductive expenditure and resource availability in bighorn ewes. Behav. Ecol. 1998;9:144–150. [Google Scholar]
- Forchhammer M.C, Clutton-Brock T.H, Lindstrom J, Albon S.D. Climate and population density induce long-term cohort variation in a northern ungulate. J. Anim. Ecol. 2001;70:721–729. 10.1046/j.0021-8790.2001.00532.x [Google Scholar]
- Forchhammer M.C, Post E, Stenseth N.C, Boertmann D.M. Long-term responses in arctic ungulate dynamics to changes in climatic and trophic processes. Popul. Ecol. 2002;44:113–120. 10.1007/s101440200013 [Google Scholar]
- Gaillard J.M, Festa-Bianchet M, Yoccoz N.G, Loison A, Toigo C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 2000;31:367–393. 10.1146/annurev.ecolsys.31.1.367 [Google Scholar]
- Georges J.Y, Guinet C. Early mortality and perinatal growth in the subantarctic fur seal (Arctocephalus tropicalis) on Amsterdam Island. J. Zool. 2000;251:277–287. 10.1017/S0952836900007019 [Google Scholar]
- Gulland F.M.D. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- Hurrell J.W. Decadal trends in the north-atlantic oscillation—regional temperatures and precipitation. Science. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- Jewell P.A. Survival and behaviour of castrated Soay sheep (Ovis aries) in a feral island population on Hirta, St. Kilda, Scotland. J. Zool. 1997;243:623–636. [Google Scholar]
- Jewell P.A, Boyd J.M, Milner C, editors. Island survivors: the ecology of the Soay sheep of St. Kilda. Athlone Press; London: 1974. [Google Scholar]
- Keech M.A, Bowyer R.T, Ver Hoef J.M, Boertje R.D, Dale B.W, Stephenson T.R. Life-history consequences of maternal condition in Alaskan moose. J. Wildl. Manag. 2000;64:450–462. [Google Scholar]
- Lande R, Engen S, Saether B.-E. Oxford series in ecology & evolution. Oxford University Press; Oxford, UK: 2003. Stochastic population models in ecology and conservation: an introduction. [Google Scholar]
- LeBlanc M, Festa-Bianchet M, Jorgenson J.T. Sexual size dimorphism in bighorn sheep (Ovis canadensis): effects of population density. Can. J. Zool.-Revue Canadienne De Zoologie. 2001;79:1661–1670. 10.1139/cjz-79-9-1661 [Google Scholar]
- Lenart E.A, Bowyer R.T, Hoef J.V, Ruess R.W. Climate change and caribou: effects of summer weather on forage. Can. J. Zool.-Revue Canadienne De Zoologie. 2002;80:664–678. 10.1139/z02-034 [Google Scholar]
- Lindstrom J, Kokko H. Cohort effects and population dynamics. Ecol. Lett. 2002;5:338–344. 10.1046/j.1461-0248.2002.00317.x [Google Scholar]
- Loison A, Langvatn R, Solberg E.J. Body mass and winter mortality in red deer calves: disentangling sex and climate effects. Ecography. 1999;22:20–30. [Google Scholar]
- MacNally R. Multiple regression and inference in ecology and conservation biology: further comments on retention of independent variables. Biodivers. Conserv. 2002;11:1397–1401. 10.1023/A:1016250716679 [Google Scholar]
- MAFF . HMSO; London: 1971. Manual of veterinary laboratory parasitological techniques. [Google Scholar]
- Milner J.M, Elston D.A, Albon S.D. Estimating the contributions of population density and climatic fluctuations to interannual variation in survival of Soay sheep. J. Anim. Ecol. 1999;68:1235–1247. 10.1046/j.1365-2656.1999.00366.x [Google Scholar]
- Morris D.W. State-dependent life histories, Mountford's hypothesis, and the evolution of brood size. J. Anim. Ecol. 1996;65:43–51. [Google Scholar]
- R Development Core Team 2004 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. See http://www.R-project.org
- Rhind S.M, Elston D.A, Jones J.R, Rees M.E, McMillen S.R, Gunn R.G. Effects of restriction of growth and development of Brecon Cheviot ewe lambs on subsequent lifetime reproductive performance. Small Ruminant Res. 1998;30:121–126. 10.1016/S0921-4488(98)00103-5 [Google Scholar]
- Robertson A, Hiraiwahasegawa M, Albon S, Clutton-Brock T. Early growth and sucking behavior of Soay sheep in a fluctuating population. J. Zool. 1992;227:661–671. [Google Scholar]
- Robinson J.J, McEvoy T.G, Sinclair K.D. Maternal nutrition and foetal growth. Reprod. Domest. Anim. 2000;6:14–19. [Google Scholar]
- Rubin E.S, Boyce W.M, Bleich V.C. Reproductive strategies of desert bighorn sheep. J. Mammal. 2000;81:769–786. 10.1644/1545-1542(2000)081%3C0769:RSODBS%3E2.3.CO;2 [Google Scholar]
- Saether B.E, Sutherland W.J, Engen S. Birds and climate change. vol. 35. 2004. Climate influences on avian population dynamics. pp. 185–209. [Google Scholar]
- Schwartz O.A, Armitage K.B. Weather influences on demography of the yellow-bellied marmot (Marmota flaviventris) J. Zool. 2005;265:73–79. 10.1017/S0952836904006089 [Google Scholar]
- Walsh, C. & MacNally, R. 2003 The hier.part package. Hierarchical Partitioning. R project for statistical computing. http://cran.r-project.org/
- Wilson A.J, Coltman D.W, Pemberton J.M, Overall A.D.J, Byrne K.A, Kruuk L.E.B. Maternal genetic effects set the potential for evolution in a free-living vertebrate population. J. Evol. Biol. 2005;18:405–414. doi: 10.1111/j.1420-9101.2004.00824.x. 10.1111/j.1420-9101.2004.00824.x [DOI] [PubMed] [Google Scholar]