Abstract
A recent study on a captive zebra finch population suggested that variation in digit ratio (i.e. the relative length of the second to the fourth toe) might be an indicator of the action of sex steroids during embryo development, as is widely assumed for human digits. Zebra finch digit ratio was found to vary with offspring sex, laying order of eggs within a clutch, and to predict aspects of female mating behaviour. Hence, it was proposed that the measurement of digit ratio would give insights into how an individual's behaviour is shaped by its maternal environment. Studying 500 individuals of a different zebra finch population I set out to: (1) determine the proximate causes of variation in digit ratio by means of quantitative genetics and (2) to search for phenotypic and genetic correlations between digit ratio, sexual behaviour and aspects of fitness. In contrast to the earlier study, I found no sexual dimorphism in digit ratio and no effect of either laying order or experimentally altered hatching order on digit ratio. Instead, I found that variation in digit ratio was almost entirely additive genetic, with heritability estimates ranging from 71 to 84%. The rearing environment (from egg deposition to independence) explained an additional 5–6% of the variation in digit ratio, but there was no indication of any maternal effects transmitted through the egg. I found highly significant phenotypic correlations (and genetic correlations of similar size) between digit ratio and male song rate (positive correlation) as well as between digit ratio and female hopping activity in a choice chamber (negative correlation). Rather surprisingly, the strength of these correlations differed significantly between subsequent generations of the same population, illustrating how quickly such correlations can appear and disappear probably due to genotype–environment interactions.
Keywords: genetic correlation, genotype–environment interaction, heritability, maternal environment, mating behaviour, morphology
1. Introduction
There is a large body of literature suggesting that, in humans, the relative length of the second to the fourth digit (digit ratio) may reflect the action of sex hormones during embryonic development (Manning 2002). Prenatal exposure to high levels of testosterone appears to be associated with relatively long fourth digits, and exposure to estrogens with relatively long second digits. Although, the evidence is only circumstantial, it is widely believed that digit ratio may be a proxy for steroid levels during times of brain organization, and may thereby provide a window into how the phenotype of an adult person is shaped by early hormonal effects. Consequently, there has been a boom of studies on digit ratio in humans, and many interesting correlations between digit ratio and various traits that tend to be affected by sex hormones have been found (Manning 2002; Putz et al. 2004).
Recently, Burley & Foster (2004) showed that also the zebra finch digit ratio (here the relative length of the second to the fourth toe measured on the right foot) displayed some interesting patterns. In their captive zebra finch population, digit ratio was found to be sexually dimorphic, to vary with the sequence of deposition of individual eggs within a clutch (‘laying order’, representing a maternal effect), and to correlate with the strength of female mating preferences in choice tests. It seems likely that this discovery may lead to a boom of digit ratio studies in birds, since digit ratio is easily measured and correlated with behaviour, attractiveness, fecundity, etc. The danger of this approach is that negative findings will rarely be reported, and that all positive findings will be interpreted as reflecting maternal effects even when the proximate mechanisms behind variation in digit ratio are unknown for the respective bird population.
Quantitative genetic analyses can help in understanding the evolutionary significance of variation in a trait. One can ask how much of the phenotypic variation is heritable, caused by maternal effects, or caused by the rearing environment. Likewise, phenotypic correlations such as between digit ratio and fitness can be decomposed. Does fitness vary with the additive genetic component of digit ratio (i.e. its breeding value) or with the environmental deviation from the breeding value? In the latter case, allele frequencies would not be affected by selection, but in the former case heritable variation in fitness could only be maintained if it were sexually antagonistic or if selection pressures fluctuated in space and/or time.
Quantitative genetic studies of digit ratio have not been undertaken in birds, but in humans one such study (Ramesh & Murty 1977) indicates that the trait is highly heritable (heritability around 58%). Ramesh and Murty found no indication of a maternal effect, since mothers did not resemble their offspring more than fathers, and since fullsibs were no more similar to each other than expected from midparent–offspring regressions. Genetic correlations with other traits have not yet been examined in any species.
In the zebra finch, Burley & Foster (2004) found that approximately 15% of the variation in digit ratio was attributable to laying order effects: digit ratio increased with laying order position, which is in line with the assumption that exposure to maternal testosterone decreases digit ratio and that the yolk testosterone concentration usually declines with laying order in the zebra finch (Gil et al. 1999; Gilbert et al. 2005; Rutkowska et al. 2005). However, strictly speaking this is not yet evidence for an early maternal effect mediated by egg components. Chicks tend to hatch in the same order in which eggs are laid, so that hatching order position within broods may alternatively have caused the variation in digit ratio (a rearing environment effect). Moreover, Burley & Foster (2004) suggested that the large amount of variation that could not be explained by laying order may be due to general maternal effects, i.e. individual mothers may differ in their overall maternal effect on offspring digit ratio. However, Burley and Foster did not test for such between-mother differences. It is the aim of the present study to fill these gaps.
First, the study is to establish the proximate causes of variation in digit ratio in a captive population of zebra finches by means of quantitative genetic analyses. This involves partitioning the phenotypic variance into its causal components, such as additive genetic variation (heritability), early maternal effects (via egg composition) and effects of the rearing environment (from early incubation to independence). A specific cross-fostering design allows me to separate these components and disentangle the effects of laying order from the effects of hatching order.
Second, I studied phenotypic and genetic correlations between digit ratio and sexual behaviour as well as some fitness parameters using large sample sizes. These sexual behaviours include aspects of female choosiness since the study of Burley & Foster (2004) suggested such a relationship. They speculated that this relationship might have resulted from variation in sexual attractiveness and fecundity. Hence, besides measuring various aspects of female mating behaviour, I also studied female fecundity. In males I studied song rate, aggressiveness and sexual attractiveness. Finally, I looked at beak colour, a sexually dimorphic trait that has received much attention in work with zebra finches.
2. Methods
(a) Breeding of zebra finches
This study is based on 258 male and 242 female zebra finches originating from a large captive population maintained at the University of Sheffield. Of these, 97 males and 104 females had already been the subjects of extensive study of their mating behaviour (Forstmeier 2004; Forstmeier & Birkhead 2004; Forstmeier et al. 2004), and these birds formed the ‘parental generation’ (see above references for details). The second generation came from 68 breeding pairs from the first generation that were maintained in individual cages. These pairs, each allowed two breeding attempts, resulted in 299 offspring (‘F1-generation’; 161 males and 138 females from 50 different pairs; 18 pairs were unsuccessful) who survived to the age where digit ratio could be measured (see below). Four out of 299 offspring were produced after mate switching, so I had to exclude them from those heritability analyses that cannot deal with half-sib relationships.
To separate early maternal effects from effects of the rearing environment, all offspring were cross-fostered among the pairs within 24 h of egg deposition. Fostering was done in such a way that nearly all eggs of a given clutch ended up with different fosters. Eggs were marked individually with pencil and chicks were marked within a few hours after hatching with coloured marker pens, which allowed me to recognize individuals up to the age of ringing (8–12 days post-hatch). Usually, for a given nest, one chick hatched per day, in the same order in which they had been fostered (one egg per day). In cases where two chicks hatched synchronously (14 individuals) I successfully used the following microsatellite markers to clarify the parentage: Ase50 (Richardson et al. 2000), INDIGO41 (Sefc et al. 2001) and Ppi2 (Martinez et al. 1999). For methods, see Dawson et al. (2005). Due to random fostering, a chick's hatching-order position within a brood raised by foster parents was not correlated with its original laying-order position within the clutch produced by the genetic mother (r=0.01; N=299; p=0.83). This allowed me to separate early maternal effects related to laying order from the effects of rearing conditions related to hatching order. For the 299 surviving offspring, mean clutch size of origin was 5.7±1.0 s.d. eggs and mean brood size of rearing was 3.8±1.2 s.d. chicks (smaller due to hatching failures and early chick mortality). After reaching independence, all offspring were housed in unisexual groups with visual and acoustic exposure to the other sex (gaps between opposite-sex cages/aviaries ranging from 0.5 to 1.2 m). This was done for consistency with earlier protocols (e.g. Forstmeier & Birkhead 2004). Although this rearing procedure is common practice in most laboratories, it might interfere with the development of male and female mating behaviours. Individual differences in sexual behaviour of birds reared under these conditions might not correspond perfectly to behavioural differences that would have arisen under more natural rearing conditions. Ongoing studies are exploring how this might have affected the results presented here.
(b) Digit ratio measurements
I measured the digit ratio of the parental generation at the age of 915±72 s.d. days (shortly after breeding the F1-generation) and that of the F1-generation at the age of 114±15 s.d. days (range 75–140). I used two different methods of measuring. First, I measured the lengths of the second (mean±s.d.=7.85±0.39 mm) and fourth (mean±s.d.=8.56±0.46 mm) toes with a ruler by pressing the second, third and fourth toes flat onto the ruler. The end of the ruler (starting with 0.0 mm) was pressed gently against the basal pad of the hind toe, while holding the hind toe perpendicularly to the other three toes. Both the second and the fourth toe were measured twice (3–4 min apart to avoid memory effects inflating repeatability) to the nearest 0.05 mm (the ruler had tick marks every 0.5 mm). Digit ratio measured in this way (the length of the second toe divided by the length of the fourth toe) was highly repeatable between the two independent measurements (repeatability R=0.875, F499,500=14.9; p<0.0001). Second, I measured with callipers (to the nearest 0.05 mm) the distance between the tip of the fourth and the tip of the second toe. To do this, I held the foot in a standardized fashion: looking from the ventral side, I lined up the second and fourth toe next to each other (holding the third digit further dorsally). Placing the callipers' inside legs onto the tip of the shorter toe I measured the distance to the tip of the longer toe. This distance measurement (negative if the fourth toe was shorter) was highly correlated with the calculated difference (length of fourth toe minus length of second toe) from the first method (r=0.88, N=500, p<0.0001). To reduce measurement error, I averaged these two differences, and then calculated digit ratio as (fourth toe−difference)/fourth toe.
This method of measuring was chosen for its high repeatability and time efficiency. Using a random sub-sample of 15 males and 15 females, I also tried the method of measuring from footprints as employed by Burley & Foster (2004). This method gives shorter toe length measurements than the previous method (mean±s.d. of second toe: 6.30±0.32 mm, fourth toe: 6.91±0.34 mm) since it does not include the gap between the basal pad of the focal toe and the basal pad of the hind toe. However, I found that this method of measuring from footprints was less repeatable (R=0.789; F29,30=8.5; p<0.0001) and much more time consuming. Nevertheless, the agreement between the two methods was still good (r=0.734; N=30; p<0.0001).
There were 504 birds to be measured on the right foot, but 13 of them had their right foot damaged by accident or mite infection. To see whether the left foot could be measured instead, I measured both feet in some fully intact individuals with very extreme digit ratios. The measurements from the two feet were highly correlated (r=0.97, N=5), so I decided to use the left foot if the right foot was damaged. Four birds had both feet damaged leaving me with N=500 individuals (by coincidence).
(c) Behavioural traits
I studied the sexual behaviour of the birds of the parental generation in three different test situations. These experiments were reported previously (Forstmeier 2004; Forstmeier & Birkhead 2004; Forstmeier et al. 2004), so here I give only a short summary.
I released eight males at the age of 284±77 s.d. days together with eight stimulus females into a large cage and observed the males during eight observation periods of 10 min for the frequency of directed song towards females (‘song 8’) and the frequency of aggression towards either sex (‘aggression’; Forstmeier & Birkhead 2004; Forstmeier et al. 2004).
I staged individual encounters lasting 5 min between one male and one female (eight tests for each individual tested at the age of 328±89 s.d. days). I measured the rate of directed singing by males (‘song 1’) and I scored the sexual responsiveness of females on a three-grade scale to reflect the female's readiness to copulate (Forstmeier 2004).
Females were tested twice (with different sets of stimulus males) for 3 h in a choice chamber, where four males were presented (females aged 392±96 s.d. days; Forstmeier & Birkhead 2004). I measured the number of hops and the time females spent on each of the two perches (one close and one further away) in front of each of the four males (eight perches in total). From this I extracted the following parameters: the total number of hops (‘total hops’; square-root transformed) and the total time on the eight perches (‘active time’), the proportion of hops and time on the four close perches as opposed to the four distant perches (‘proportion hops close’ and ‘proportion time close’), and the average deviation of hops and time from a random allocation of 25% to each of the males (‘time deviation from randomness’ and ‘hops deviation from randomness’; the former was termed ‘discrimination’ in Forstmeier et al. 2004). I also calculated preference functions with regard to five male traits (see Forstmeier & Birkhead 2004): preference for males with high aggression scores, preference for males with high song 1 scores, preference for males with dark red (as opposed to orange) beaks (see below), preference for males with high digit ratio, and preference for attractive males (as judged by seven other females than the focal female). All values were averaged between the two trials done by each female. Additional data was available for a 1 h acclimation period prior to each trial, during which males were hidden behind opaque dividers.
These behavioural tests were also conducted with all members of the F1-generation, yet with the following changes to the protocol published previously. (1) Song and aggression in groups of eight were measured during 10 observation periods and at a time when males were 132±8 s.d. days of age. (2) I made four measurements of song 1 and responsiveness when birds were 136±8 s.d. days of age, and another two measurements at the age of 316±43 s.d. days. The two means from different ages were averaged (individual repeatability between the two ages: song rate R=0.67, F158,159=5.0, p<0.0001; responsiveness R=0.52, F119,120=3.1, p<0.0001). (3) In the choice chamber 106 females of the F1-generation were tested with only one set of males and 27 females were tested with two sets of males (160 tests; mean age 304±46 s.d. days). All 160 males were used in four tests. Choice experiments and the second set of pair-wise encounters were conducted some months after transporting the birds to the Max Planck Institute at Seewiesen, Germany.
(d) Beak colour and attractiveness
I measured the beak colour of all males and females as previously described (Forstmeier & Birkhead 2004). The parental generation was measured at the age of 406±88 s.d. days, the F1-generation at the age of 322±43 s.d. days. Male attractiveness was measured as the proportion of active time that females spent close to them. Values were cube root arcsine transformed to avoid heteroscedasticity and were averaged between all the females judging each male.
(e) Female fecundity
After the completion of the choice-chamber tests, 86 females of the parental generation were paired up with a male they had ranked second or third in the choice chamber (designed for a different experiment reported elsewhere; Forstmeier, unpublished manuscript). Pairs were provided with a nest box for breeding, and I recorded the following fecundity parameters: (1) the latency to lay the first egg, (2) the mean volume (volume=0.5236×length×width2) of all eggs laid and (3) the mean clutch size of all clutches produced. Two females did not lay any eggs and were arbitrarily assigned a maximum latency of 100 days. Females that did not incubate their eggs were not scored for clutch size (missing values). All eggs were replaced by plastic eggs and were removed after 15 days of incubation. One hundred days after pair formation all pairs were split up (partners transferred to separate rooms).
Several months later, 68 of the females were paired up again for a maximum of 133 days. This time they were paired with a male they had also ranked second or third, but from the other set of stimulus males seen in the choice chamber. I measured the same three parameters as before. Two females did not lay any eggs and were assigned a latency of 133 days. This time, pairs were allowed to raise offspring (producing the F1-generation).
The latency to lay eggs was square-root log transformed to approach normality. To average the fecundity parameters between the two breeding rounds, I adjusted their mean values by using correction factors. This was done since not all females participated in both breeding rounds, and since in the first round females took longer to start laying eggs (18.3 versus 15.5 days; paired t-test: t68=2.9; p=0.006), females produced smaller eggs (1.13 versus 1.18 cm3; paired t63=5.9; p<0.0001) and smaller clutches (4.8 versus 5.2 eggs; paired t53=2.0; p=0.05). After adjustment, all three fecundity parameters showed significant individual repeatability between the two breeding rounds (latency: R=0.44; F68,69=2.6; p<0.0001; egg volume: R=0.83; F63,64=11.0; p<0.0001; clutch size: R=0.28; F53,54=1.8; p=0.019).
(f) Quantitative genetics
To estimate the heritability of digit ratio I followed the standard procedures for offspring–parent regression and fullsib analyses as outlined by Falconer & Mackay (1996) and Lynch & Walsh (1998). These analyses included 295 offspring from 48 families (excluding half-sibs produced after mate switching). For offspring–parent regressions I weighted families in relation to family size following Kempthorne & Tandon (1953). Since the weights depend on heritability, and the heritability estimate slightly changes with weights, the solution was found iteratively. For fullsib analyses, I used a restricted maximum likelihood (REML) approach implemented by R 2.0 (Free Software Foundation, Inc., Boston, Massachusetts, USA). This random-effect model approach is preferable to ANOVA since family sizes varied substantially (1–14 offspring). I used the model command m1<-lme(digitratio∼1, random=∼1|pair), and obtained the intra-class correlation and its standard error from summary(m1) and intervals(m1). I calculated the standard error of heritability estimates obtained from single-parent offspring regression by multiplying the standard error of the regression slope by 2/(1+r), where r=0.332 to correct for assortative mating (see below). I calculated the standard error of the difference between two heritability estimates following Lynch & Walsh (1998, p. 38).
I checked for assortative mating according to digit ratio between the parents and found that, unintentionally by experimental design, they were significantly positively correlated (r=0.332; N=48; p=0.021). This meant that assortative mating had to be accounted for in single-parent offspring regressions as well as in fullsib analyses (see Falconer & Mackay 1996; Lynch & Walsh 1998). However, this also raised the question whether assortative mating was due to coincidence or whether there were disassortative mating preferences (since all females were paired to an undesired partner, judging from choice-chamber experiments). Hence, I analysed female preferences for male digit ratio (see above).
Finally, I used an animal model implemented by REML-VCE 4.2.5 (Groeneveld 1998; see Forstmeier et al. 2004 for an example of application) to partition the phenotypic variance (VP) into four components: additive genetic variation (VA), variation due to maternal effects acting before fostering (VM), variation due to the rearing environment after fostering (VE), and residual variation (VR). The animal model extracts estimates of VA from phenotypic data in relation to a pedigree, which here covered only the two generations. Yet, given the high heritability of digit ratio (see below) the depth of the pedigree should be sufficient for the present purpose, which is to clarify the importance of VM and VE after accounting for the heritable component. To see how much of the variation in digit ratio was caused by VM and VE, mother identity (50 levels) was used as a random factor representing VM and foster identity (46 levels) as a random factor representing VE. To account for the possibility that maternal effects are not consistent between consecutive clutches by the same mother, and that foster environments are not stable between consecutive broods raised by the same fosters, I also used clutch identity (102 levels representing VM) and brood identity (90 levels representing VE) as random effects in another version of the model.
I also used REML-VCE to explore genetic correlations between digit ratio and those behaviours where I found strong phenotypic correlations. Given the relatively small sample size (approx. 250 individuals of each sex) and limited depth of the pedigree, the estimates of genetic correlation should be regarded cautiously.
3. Results
(a) Digit ratio in relation to sex and laying order
There was no sexual dimorphism in digit ratio (parental and F1-generation pooled: males: mean±s.d.=0.927±0.030; N=258; females: mean±s.d.=0.930±0.034; N=242; t498=1.2; p=0.24; Levene's test for equality of variances: F1,498=1.2; p=0.27). Given this sample size, I should have detected (with a power of 90%) any group difference larger than 0.26 s.d. (calculated by Gpower; Faul & Erdfelder 1992). For comparison, Burley & Foster (2004) found an effect size of more than 1 s.d. (estimated from their figure 1).
Figure 1.
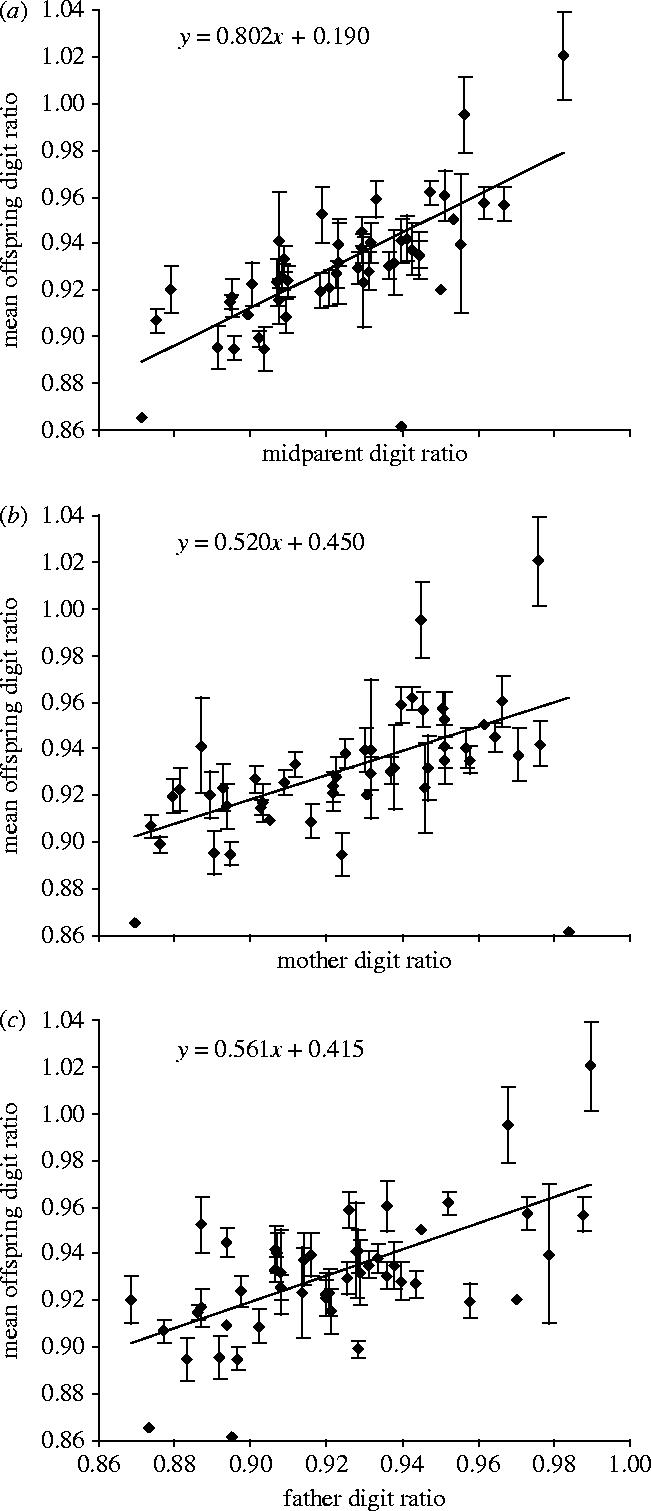
Mean digit ratio±s.e. of the offspring in 48 families in relation to (a) midparent digit ratio, (b) mother digit ratio and (c) father digit ratio. Regression lines and equations are for weighted regressions, where weights depend on family size (see Kempthorne & Tandon 1953).
Variation in digit ratio in the F1-generation depended strongly on the identity of the genetic parents (F49,202=6.0; p<0.0001), and to a lesser extent on the identity of the foster parents (F45,202=2.1; p=0.0002). However, digit ratio was not influenced by laying order within a clutch (F1,202=1.4; p=0.24), or by hatching order within a brood (F1,202=0.03; p=0.86; GLM accounting for all four effects simultaneously). Again, any laying-order effect larger than r=0.2 should have been detected with a power of 90% (Burley & Foster 2004 found r=0.39). There were no significant two- or three-way interactions between offspring sex, laying order and genetic parents, or between offspring sex, hatching order and foster parents, respectively (all p>0.15). Finally, the lack of an effect of laying order did not seem to result from the experimental dissociation of hatching order from laying order. In 62 offspring where the hatching order position was identical to the laying order position, digit ratio was also unaffected by laying order (r=−0.04; p=0.78).
(b) Heritability of digit ratio
A regression of mean offspring digit ratios on midparent values suggested a heritability of h2=0.802±0.096 (figure 1a; N=48 pairs). Separate offspring–mother and offspring–father regressions initially produced unrealistically high heritability estimates (mother: h2=2×B=1.039±0.193; figure 1b; father: h2=2×B=1.121±0.191; figure 1c; where B is the regression slope). I, therefore, checked for assortative mating between the partners of a pair, and in fact, the digit ratios of partners were positively correlated (r=0.332; N=48; p=0.021). Accounting for this bias, heritability estimates were close to the above estimate from midparent regression (mother: h2=2×B/1.332=0.780±0.145; father: h2=2×B/1.332=0.842±0.144). Notably with regard to possible maternal effects, the offspring did not resemble their mother more than their father (figure 2; difference in h2 estimates=−0.062±0.204).
Figure 2.
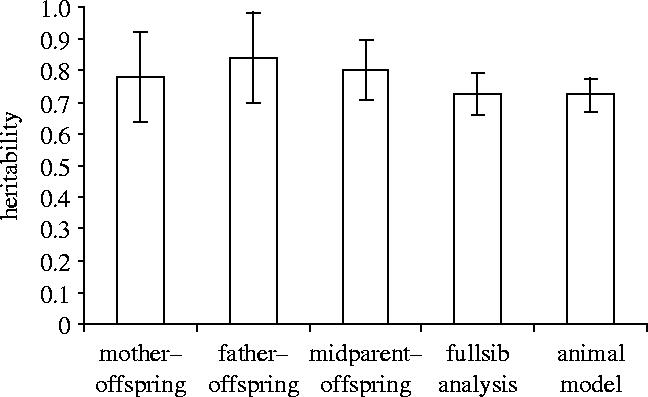
Heritability±s.e. of digit ratio as estimated by five different methods.
A fullsib analysis (REML estimation) yielded an intraclass correlation of t=0.448±0.049. Accounting for the inflating effect of assortative mating, this lead to a corrected heritability estimate of 0.723±0.066 (figure 2). According to quantitative genetics theory, this estimate is still inflated (as compared to the midparent regression) since it contains half the dominance variance and twice the maternal effect (before cross fostering). Assuming no dominance variance, the size of maternal effect can be estimated by subtracting the midparent-regression estimate from the fullsib estimate . Although, a negative variance component estimate is not biologically meaningful (negative estimates are constrained to zero in animal models; see below), it still indicates that the size of maternal effect must be small.
An animal model including additive genetic variation (500 individuals), maternal environment (mother identity with 50 levels for 299 individuals), and foster environment (foster-pair identity with 46 levels for 299 individuals) yielded the following variance component estimates: additive genetic variation VA/VP=0.723±0.052 (figure 2), maternal effect VM/VP=0 (negative estimates are constrained to zero), foster environment effect VE/VP=0.052±0.023, the latter being significant at p=0.024. An alternative model in which clutch of origin (102 levels) and brood of rearing (90 levels) were used as random effects (rather than mother and foster identities) led to similar results: additive genetic variation VA/VP=0.711±0.050, maternal effect VM/VP=0, foster environment effect VE/VP=0.064±0.025 (p=0.01).
(c) Digit ratio versus behaviour
In the parental generation, only the hopping activity of females in front of male cages during choice tests was significantly related to female digit ratio (table 1). Females with low digit ratios performed more hops per hour (figure 3a) and spent more time on the perches close to males (which are two correlated behavioural traits: r=0.53; N=234; p<0.0001) than females with high digit ratios (table 1). To examine whether this relationship reflected variation in sexual behaviour or general activity, I analysed the number of hops females made inside the choice chamber during the 1 h pre-testing periods where males were hidden behind opaque dividers. Females made fewer hops during pre-tests than during tests (82±108 s.d. hops per hour versus 165±123 s.d. hops per hour; paired t100=9.0; p<0.0001), showing that the presence of males stimulated female activity. However, more importantly, female digit ratio was equally related to hopping activity during pre-tests (r=−0.30; N=101; p=0.002) as to hopping activity during tests (r=−0.33; N=101; p=0.0007).
Table 1.
Phenotypic correlations between digit ratio and various male and female traits shown separately for two generations (parental generation and F1-generation).
| generation (g) | parental | parental | F1 | F1 | parental and F1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| method | correlation | lme | correlation | lme | lme | |||||
| effect | digit ratio (dr) | (dr) | (dr) | (dr) | (dr) | (dr×g) | ||||
| statistics | r | N | p | p | r | N | p | p | p | p |
| male song 1 | −0.05 | 92 | 0.63 | 0.88 | 0.27 | 160 | 0.001* | 0.003* | 0.031 | 0.054 |
| male song 8 | −0.07 | 96 | 0.53 | 0.75 | 0.21 | 160 | 0.007 | 0.011 | 0.072 | 0.073 |
| male aggression | 0.08 | 96 | 0.42 | 0.15 | 0.10 | 160 | 0.21 | 0.23 | 0.15 | 0.86 |
| male attractiveness | 0.05 | 91 | 0.66 | 0.67 | 0.07 | 160 | 0.36 | 0.41 | 0.35 | 0.78 |
| male beak colour | 0.14 | 91 | 0.20 | 0.28 | −0.09 | 160 | 0.25 | 0.20 | 0.90 | 0.095 |
| female responsiveness | 0.07 | 98 | 0.50 | 0.52 | −0.09 | 137 | 0.30 | 0.20 | 0.60 | 0.20 |
| female active time | −0.05 | 101 | 0.63 | 0.64 | −0.06 | 133 | 0.47 | 0.52 | 0.42 | 0.94 |
| female total hops | −0.33 | 101 | 0.001* | 0.001* | 0.005 | 133 | 0.96 | 0.69 | 0.021 | 0.029 |
| female time deviation from random | 0.13 | 101 | 0.19 | 0.13 | 0.07 | 122 | 0.44 | 0.37 | 0.11 | 0.75 |
| female hops deviation from random | 0.08 | 101 | 0.41 | 0.30 | 0.13 | 122 | 0.15 | 0.096 | 0.052 | 0.59 |
| female proportion time close | −0.32 | 101 | 0.001* | 0.002* | 0.03 | 133 | 0.73 | 0.74 | 0.12 | 0.011 |
| female proportion hops close | −0.21 | 101 | 0.034 | 0.026 | −0.01 | 133 | 0.91 | 0.92 | 0.13 | 0.093 |
| female aggressiveness preference | 0.02 | 101 | 0.88 | 0.56 | −0.16 | 132 | 0.060 | 0.061 | 0.15 | 0.18 |
| female song 1 preference | 0.04 | 98 | 0.73 | 0.99 | −0.13 | 132 | 0.14 | 0.14 | 0.31 | 0.23 |
| female beak colour preference | −0.04 | 101 | 0.68 | 0.73 | −0.02 | 132 | 0.80 | 0.80 | 0.65 | 0.87 |
| female digit ratio preference | −0.01 | 77 | 0.96 | 0.56 | −0.10 | 132 | 0.26 | 0.28 | 0.34 | 0.61 |
| female attractiveness preference | −0.02 | 101 | 0.85 | 0.86 | −0.17 | 132 | 0.051 | 0.19 | 0.20 | 0.37 |
| female beak colour | 0.07 | 101 | 0.50 | 0.50 | −0.18 | 133 | 0.041 | 0.15 | 0.81 | 0.18 |
| female latency to lay eggs | 0.28 | 84 | 0.010 | 0.010 | — | — | — | — | — | — |
| female clutch size | 0.20 | 80 | 0.08 | 0.049 | — | — | — | — | — | — |
| female egg size | 0.14 | 82 | 0.22 | 0.50 | — | — | — | — | — | — |
Linear mixed-effect models (lme) test for an effect of digit ratio (dr) on male and female traits while controlling for the identity of the mother as a random effect to partially account for the non-independence of data points due to genetic relatedness and shared maternal effects. These models were conducted for the parental generation, the F1-generation and for both generations together. In the latter case, I simultaneously tested for a digit ratio by generation interaction (dr×g). Bold print highlights significant p-values. An asterisk (*) indicates significance after Bonferroni correction for 21 traits (parental generation) or 18 traits (F1-generation), respectively.
Figure 3.
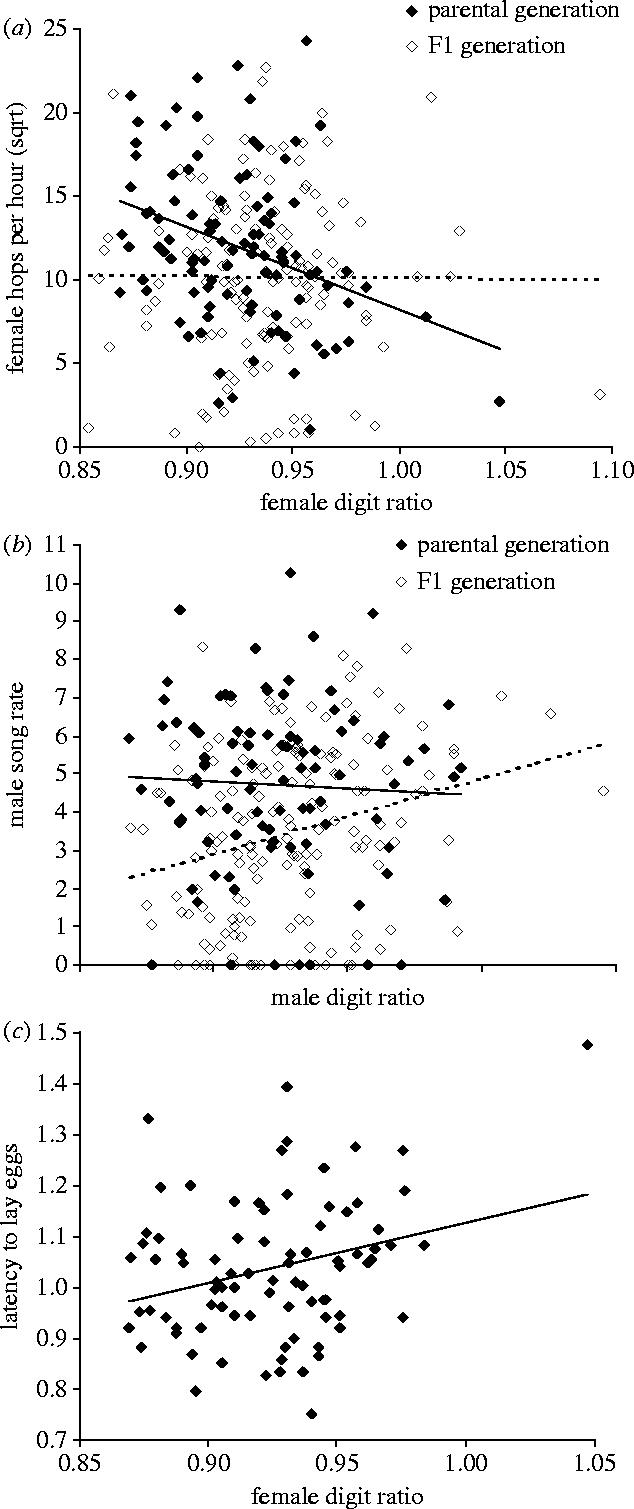
Phenotypic correlates of digit ratio: (a) digit ratio of 234 females from two subsequent generations and the number of hops (square-root transformed) made per hour during choice-chamber tests, (b) digit ratio of 252 males and their song rate in individual encounters with females (square-root transformed seconds of directed singing towards the female) and (c) digit ratio of 84 females from the parental generation and their latency to lay eggs (square-root log transformed number of days since pairing). Open symbols and dotted regression lines refer to the F1-generation, otherwise parental generation.
The statistical significance of this finding might be inflated by pseudoreplication, since genetically related individuals do not represent independent data points. Therefore, I used linear mixed-effect models (lme), where the identity of an individual's mother was entered as a random effect. This method largely accounts for the similarity of related individuals due to shared genes and shared maternal environment. However, these mixed-effect models confirmed the statistical significance of the correlation between female digit ratio and activity in the choice chamber (table 1).
Nevertheless, as multiple testing also increases the risk of statistical type I errors, I tried to replicate those findings with the F1-generation. Using even larger sample sizes, I found that in the F1-generation male song rate was positively correlated with digit ratio (lme accounting for mother identity F1,122=9.5; p=0.0025; Bonferroni-corrected for 18 tests: p=1−(1−0.0025)18=0.044), while this had not been the case in the parental generation (lme F1,29=0.02; p=0.88; figure 3b). On the contrary, the correlation between female digit ratio and hopping activity found in the parental generation (lme F1,45=12.2; p=0.0011; Bonferroni-corrected for 21 tests: p=1−(1−0.0011)21=0.023) was absent in the F1-generation (lme F1,86=0.2; p=0.69; figure 3a). In both cases (song rate and hopping), the two generations differed substantially in their digit-ratio ‘effects’ on behaviour, i.e. there were significant or nearly significant digit-ratio by generation interactions (table 1).
Using an animal model, I calculated for each individual its breeding value of digit ratio (estimated while including the focal individual) and its environmental deviation from the breeding value (residual phenotype). Female hopping activity in the parental generation was clearly predicted by the breeding value of digit ratio (standardized regression coefficient β=−0.34; t98=−3.5; p=0.0007), but not by the environmental deviation (β=0.01; t98=0.1; p=0.90). Likewise, male song rate in the F1-generation was predicted by the breeding value of digit ratio (β=0.28; t157=3.6; p=0.0004), but not by the environmental deviation (β=0.09; t157=1.1; p=0.27). A three-trait animal model indicated a positive genetic correlation between digit ratio and song rate (r=0.285±0.158; p=0.071) and a negative genetic correlation between digit ratio and female hopping activity (r=−0.244±0.123; p=0.047).
(d) Digit ratio versus male attractiveness and female fecundity
Male digit ratio was unrelated to male attractiveness as measured in choice tests, and digit ratio was not related to male beak colour (table 1). In females, I found no correlations between digit ratio, beak colour and egg volume (table 1). However, females with high digit ratios took longer to initiate a clutch (r=0.28; N=84; p=0.01; figure 3c). This correlation was largely due to the fact that the only female in this sample with a digit ratio larger than one was at the same time the only female in this sample that never laid an egg. When removing this (potentially interesting) outlier, there was not much of a correlation remaining (r=0.16; N=83; p=0.16). There was also a tendency for females with high digit ratios to produce larger clutches (table 1).
4. Discussion
(a) Proximate determination of digit ratio
Burley & Foster (2004) found that in their captive zebra finch population digit ratio was sexually dimorphic, and within each of the sexes digit ratio was related to laying order position within a clutch (explaining about 10% of the variation in males and 20% in females). The large amount of residual variation was suspected to result from general maternal effects but this possibility was not investigated.
The present study conducted on a different captive zebra finch population found no sexual dimorphism, no effect of laying order, and no general maternal effects on digit ratio. In contrast, the trait was highly heritable. The similarity of heritability estimates obtained from fullsib analysis and midparent regression suggested that there was little early maternal effect and also little dominance variance, which seems to be typical for morphological traits in general (Mousseau & Roff 1987). Hence, in terms of its proximate determination, I found little evidence that digit ratio behaved differently from other morphological traits and this also seems to apply to human digit ratio (Ramesh & Murty 1977). There was a small effect of the rearing environment, but I was unable to detect what aspect of the rearing environment caused this effect: digit ratio was not related to brood size, hatching order or mass at eight days of age (partly shown, partly unpublished data).
This difference in findings between the two zebra finch populations is unlikely to be due to chance, given the very high statistical power of both studies. Hence, it appears that the two populations differed in how digit ratio was determined. This could be either because the substance (e.g. steroid) that caused the maternal effect in Burley's population did not vary with laying order in my population, or because digit ratio was insensitive to this substance in my population. The former could easily be due to differences in breeding conditions (outdoor aviaries versus indoor cages), but there could also be genetic differences between the two captive populations resulting from likely genetic bottlenecks during the process of domestication. An ongoing population genetic study using microsatellite markers will shed light on this latter possibility, and an ongoing aviary experiment will help to clarify the role of environmental conditions. A comparative study on several human populations (Manning et al. 2000) suggests that population differences in what is reflected by digit ratio might be the rule rather than the exception.
The fact that digit ratio is determined by additive genetic variation rather than by maternal effects does not exclude the possibility that digit ratio is a proxy for steroid levels during brain organization. It only excludes maternal steroids, not steroids produced by the embryo itself, which might depend on the genes inherited from both parents and hence show up as additive genetic variation (see also Lutchmaya et al. 2004).
(b) Digit ratio versus behaviour and fitness
Burley & Foster (2004) found that female digit ratio correlated with female choosiness measured as the strength of preference for red-ringed (attractive) males as opposed to green-ringed (unattractive) males. Although highly significant (p=0.002), this finding was based on 15 females only.
In the present study, I did not specifically measure female preferences for red versus green colour rings, but digit ratio correlated neither with female preference for attractive versus unattractive males (which might be a similar trait) nor with discrimination behaviour, i.e. how strongly females deviated from randomness in their allocation of time (again a measure that is very close to what Burley and Foster measured). Instead, I found that digit ratio was correlated with general activity, both during and before choice-chamber experiments. It is possible that this latter relationship was responsible for the finding made by Burley & Foster (2004): if their choice chamber contained only few perches, it is possible that females with a higher general activity were classified as being less choosy since they distributed their time more equally between the two compartments (but note that this was not true for the four-compartment choice chamber used in the present study). However, there need not be a common explanation, since the proximate mechanisms determining digit ratio were not the same between the two populations either.
In their paper, Burley & Foster (2004) predict a positive relationship between digit ratio and female fecundity. Here, I found only little support for this hypothesis. The positive trend between digit ratio and clutch size is consistent with this prediction. However, there also seems to be the possibility that females with extremely high digit ratios may show reduced rather than enhanced fecundity, or at least may be harder to stimulate to breed (figure 3c). Further fitness measurements in the F1-generation will have to examine this.
The observed positive correlation between digit ratio and male song rate does not follow the expectation of high digit ratios indicating low levels of prenatal testosterone, unless prenatal testosterone would have negative effects on adult song rate. Irrespective of whether digit ratio is influenced by sex steroids or not, it seems noteworthy that there was a positive genetic correlation between digit ratio and male sexual activity and a negative genetic correlation between digit ratio and aspects of female activity. Hence, some of the genes that influence digit ratio seem to have some kind of opposing effect on male versus female behaviour. Most surprisingly, the effect of these genes on behaviour was observed in females in the first generation only, and in males in the second generation only. Since individual differences at least in song rate are stable for a lifetime (see §2c; unpublished data), the causes must lie somewhere early in life before the phenotype is fully established. Both generations were reared at Sheffield University, but in different housing facilities. The facilities differed in illumination (presence of daylight and flicker frequency of light bulbs) and type of cage (wooden versus plastic), but these differences seem rather minor compared to the differences that are usually found between labs.
Researchers studying the mating behaviour of captive zebra finch populations have often been daunted by the fact that their findings could not be repeated by other research groups (see e.g. Collins & ten Cate 1996; Jennions 1998). The present comparison with the study by Burley & Foster (2004) is another good example thereof. Possible genetic differences between lab populations have to be considered, but the present study seems to add an unexpected dimension to the problem: even two subsequent generations of the same population can differ in how genes influence sexual behaviour. I suspect that those genes that have pleiotropic effects on digit ratio and male and female sexual behaviour exert their influence on behaviour only under some but not all environmental conditions. In other words, there seem to be genotype–environment interactions.
The alternative explanation of statistical types I and II errors seems very unlikely. Even the statistically conservative mixed-effect models combined with strict Bonferroni correction revealed one significant correlation in each generation, and the probability of this happening by chance is very low.
5. Conclusion
It seems possible that digit ratio studies in birds will soon become as popular as they have become in the human field (Manning 2002; Putz et al. 2004). However, the present study calls for caution. Besides searching for behavioural and fitness correlates of digit ratio, studies should also try to establish the proximate mechanisms that determine digit ratio in the respective population, rather than assuming the presence of maternal effects by default. Generalizing between populations appears to be dangerous, even more so if subsequent generations of the same population can already differ in how digit ratio correlates with behaviour. More data from future generations may help with identifying the environmental conditions that cause these correlations to appear and disappear. Before that there seems to be little hope that the results obtained by different labs can be fully understood.
Acknowledgments
I thank Patricia Brekke, Jayne Pellatt, Nichola Roddis, Rachel Yeates and Phil Young for technical assistance at Sheffield University. Holger Schielzeth and Elisabeth Bolund conducted most of the choice experiments at Seewiesen. Kim Teltscher, Holger Schielzeth and Claudia Burger helped with the DNA analyses at Seewiesen. I thank Edith Bodendorfer, Annemarie Grötsch, Johann Hacker, Jenny Minshull and Agnes Türk for technical assistance at Seewiesen. Tim Birkhead provided birds and facilities at Sheffield and Bart Kempenaers provided facilities at Seewiesen. James Dale, Jarrod Hadfield, Kees van Oers, Francisco Pulido and Holger Schielzeth provided helpful comments and suggestions. This project was funded by an Emmy-Noether Fellowship (DFG: FO 340/1 and 340/2) and a Marie-Curie Fellowship (EU: HPMF-CT-2002-01871).
References
- Burley N.T, Foster V.S. Digit ratio varies with sex, egg order and strength of mate preference in zebra finches. Proc. R. Soc. B. 2004;271:239–244. doi: 10.1098/rspb.2003.2562. 10.1098/rspb.2003.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S.A, ten Cate C. Does beak colour affect female preference in zebra finches? Anim. Behav. 1996;52:105–112. 10.1006/anbe.1996.0156 [Google Scholar]
- Dawson D.A, Chittock J, Jehle R, Whitlock A, Nogueira D, Pellatt J, Birkhead T, Burke T. Identification of 13 polymorphic microsatellite loci in the zebra finch, Taeniopygia guttata (Passeridae, Aves) Mol. Ecol. Notes. 2005;5:298–301. 10.1111/j.1471-8286.2005.00907.x [Google Scholar]
- Falconer D.S, Mackay T.F.C. 4th edn. Pearson Education; Harlow, UK: 1996. Introduction to quantitative genetics. [Google Scholar]
- Faul F, Erdfelder E. Department of Psychology, University of Bonn; Bonn: 1992. GPOWER: a priori, post-hoc, and compromise power analyses for MS-DOS (Computer program) [Google Scholar]
- Forstmeier W. Female resistance to male seduction in zebra finches. Anim. Behav. 2004;68:1005–1015. 10.1016/j.anbehav.2004.02.003 [Google Scholar]
- Forstmeier W, Birkhead T.R. Repeatability of mate choice in the zebra finch: consistency within and among females. Anim. Behav. 2004;68:1017–1028. 10.1016/j.anbehav.2004.02.007 [Google Scholar]
- Forstmeier W, Coltman D.W, Birkhead T.R. Maternal effects influence the sexual behaviour of sons and daughters in the zebra finch. Evolution. 2004;58:2574–2583. doi: 10.1111/j.0014-3820.2004.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. 10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Gilbert L, Rutstein A.N, Hazon N, Graves J.A. Sex-biased investment in yolk androgens depends on female quality and laying order in zebra finches (Taeniopygia guttata) Naturwissenschaften. 2005;92:178–181. doi: 10.1007/s00114-004-0603-z. 10.1007/s00114-004-0603-z [DOI] [PubMed] [Google Scholar]
- Groeneveld E. Institute of Animal Husbandry and Animal Behaviour, Federal Research Center of Agriculture (FAL); Mariensee, Germany: 1998. VCE4 user's guide and reference manual. Version 1.3. [Google Scholar]
- Jennions M.D. The effect of leg band symmetry on female–male association in zebra finches. Anim. Behav. 1998;55:61–67. doi: 10.1006/anbe.1997.0579. 10.1006/anbe.1997.0579 [DOI] [PubMed] [Google Scholar]
- Kempthorne O, Tandon O.B. The estimation of heritability by regression of offspring on parent. Biometrics. 1953;9:90–100. [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning J.T. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum. Dev. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. 10.1016/j.earlhumdev.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Sinauer Association; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- Manning J.T. Rutgers University Press; New Brunswick, NJ: 2002. Digit ratio: a pointer to fertility, behavior, and health. [Google Scholar]
- Manning J.T, et al. The 2nd: 4th digit ratio, sexual dimorphism, population differences, and reproductive success: evidence for sexually antagonistic genes? Evol. Hum. Behav. 2000;21:163–183. doi: 10.1016/s1090-5138(00)00029-5. 10.1016/S1090-5138(00)00029-5 [DOI] [PubMed] [Google Scholar]
- Martinez J.G, Soler J.J, Soler M, Møller A.P, Burke T. Comparative population structure and gene flow of a brood parasite, the great spotted cuckoo (Clamator glandarius), and its primary host, the magpie (Pica pica) Evolution. 1999;53:269–278. doi: 10.1111/j.1558-5646.1999.tb05352.x. [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Roff D.A. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Putz D.A, Gaulin S.J.C, Sporter R.J, McBurney D.H. Sex hormones and finger length—what does 2D : 4D indicate? Evol. Hum. Behav. 2004;25:182–199. 10.1016/j.evolhumbehav.2004.03.005 [Google Scholar]
- Ramesh A, Murty J.S. Variation and inheritance of relative length of index finger in man. Ann. Hum. Biol. 1977;4:479–484. doi: 10.1080/03014467700002461. [DOI] [PubMed] [Google Scholar]
- Richardson D.S, Jury F.L, Dawson D.A, Salgueiro P, Komdeur J, Burke T. Fifty Seychelles warbler (Acrocephalus sechellensis) microsatellite loci polymorphic in Sylviidae species and their cross-species amplification in other passerine birds. Mol. Ecol. 2000;9:2226–2231. doi: 10.1046/j.1365-294x.2000.105338.x. 10.1046/j.1365-294X.2000.105338.x [DOI] [PubMed] [Google Scholar]
- Rutkowska J, Cichon M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity in zebra finches. Horm. Behav. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. 10.1016/j.yhbeh.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Sefc K.M, Payne R.B, Sorenson M.D. Characterization of microsatellite loci in village indigobirds Vidua chalybeata and cross-species amplification in estrildid and ploceid finches. Mol. Ecol. Notes. 2001;1:252–254. [Google Scholar]


