Abstract
Ecologists have greatly advanced our understanding of the processes that regulate trophic structure and dynamics in ecosystems. However, the causes of systematic variation among ecosystems remain controversial and poorly elucidated. Contrasts between aquatic and terrestrial ecosystems in particular have inspired much speculation, but only recent empirical quantification. Here, we review evidence for systematic differences in energy flow and biomass partitioning between producers and herbivores, detritus and decomposers, and higher trophic levels. The magnitudes of different trophic pathways vary considerably, with less herbivory, more decomposers and more detrital accumulation on land. Aquatic–terrestrial differences are consistent across the global range of primary productivity, indicating that structural contrasts between the two systems are preserved despite large variation in energy input. We argue that variable selective forces drive differences in plant allocation patterns in aquatic and terrestrial environments that propagate upward to shape food webs. The small size and lack of structural tissues in phytoplankton mean that aquatic primary producers achieve faster growth rates and are more nutritious to heterotrophs than their terrestrial counterparts. Plankton food webs are also strongly size-structured, while size and trophic position are less strongly correlated in most terrestrial (and many benthic) habitats. The available data indicate that contrasts between aquatic and terrestrial food webs are driven primarily by the growth rate, size and nutritional quality of autotrophs. Differences in food-web architecture (food chain length, the prevalence of omnivory, specialization or anti-predator defences) may arise as a consequence of systematic variation in the character of the producer community.
Keywords: bottom-up versus top-down control, cross ecosystem comparisons, nutrient stoichiometry, allometry and size-structured food webs, trophic cascade, biomass turnover
1. Introduction
The search for commonalities and contrasts among ecosystems has yielded some of the most informative patterns and insights in ecology. Ideas about trophic structure, diversity, energy flow and nutrient cycles percolate freely across systematic and disciplinary boundaries. However, large differences in emphasis persist among ecologists working in different environments. For instance, evidence for the role of bottom-up factors (abiotic resources like nutrients, energy and water) in controlling terrestrial primary productivity is unequivocal, while that for trophic interactions is much more sparse. Aquatic ecologists have long recognized the importance of bottom-up forces, but have also shown major influence of top-down processes like herbivory and indirect effects of higher trophic levels (e.g. trophic cascades). The different histories and trajectories of aquatic and terrestrial ecology suggest either that different processes are at work in these systems, or that social and disciplinary forces constrain the thinking of scientists and lead to divergent lines of inquiry. Ecologists have often claimed that ecosystems vary in their underlying structure and the processes that govern their dynamics (Elton 1927; Lindeman 1942; Strong 1992; Hairston & Hairston 1993; Chase 2000). However, only recently has sufficient data for direct quantitative comparison become available (Cyr & Pace 1993; Cyr et al. 1997; Cebrian 1999; Elser et al. 2000; Shurin et al. 2002; Cebrian 2004; Cebrian & Lartigue 2004).
Elton (1927) first proposed a ‘pyramid of numbers’, where primary producers dominate and consumer densities decrease as organisms become more remote from the base of production. This generality apparently applies well to most terrestrial systems, but aquatic ecosystems often violate Elton's rule with inverted biomass pyramids, or ratios of heterotroph-to-autotroph biomass (H : A) greater than 1 (Del Giorgio et al. 1999). To explain the differences in biomass partitioning between aquatic and terrestrial ecosystems, Lindeman (1942) hypothesized systematic contrasts in trophic efficiency and energy flow by observing the successional transitions of lakes from lacustrian to bog mats to terrestrial states.
The relative absence of massive supporting tissues in plankters and the very rapid completion of their life cycle exert a great influence on the differential productivities of terrestrial and aquatic systems. The general convexity of terrestrial systems as contrasted with the concavity of aquatic substrata results in striking trophic and successional differences. (Lindeman 1942, p. 402)
Lindeman identified two salient system properties that may generate contrasts in trophic transfer efficiency and biomass partitioning among different parts of the food web. The first is that primary producers in pelagic systems (and some benthic habitats) are predominantly unicellular, whereas terrestrial plants are multicellular and structurally complex. This contrast in organismal size between phytoplankton and plants has major implications for life history parameters, rates of biomass turnover and allocation to tissues with different chemical compositions and nutritional qualities (Peters 1983; Brown & West 2000). The second difference Lindeman proposes is that aquatic systems lie at low positions in the landscape and, therefore, accumulate nutrients and detritus through runoff, whereas limiting mineral elements like nitrogen (N) and phosphorus (P) leach out of soil and into lakes, streams and ultimately the oceans. Aquatic systems may therefore be more nutrient-rich and receive more inputs of allochthonous detritus than their terrestrial counterparts.
Despite this long-standing interest in contrasting trophic structure between ecosystems, until recently there have been surprisingly few quantitative comparisons. Whereas many ecologists agree on the existence of strong differences between terrestrial and aquatic systems, few have quantified the variation in different trophic pathways. Our review synthesizes current knowledge of patterns of trophic interactions between aquatic and terrestrial environments. Well-established contrasts between the two types of ecosystems include the following.
Size structure. Pelagic food webs are more strongly size-structured than terrestrial, with clear positive correlations between organismal body size and trophic position. Terrestrial consumers range in size from much larger (e.g. ungulate grazers) to much smaller (e.g. forest lepidoptera) than the plants they consume. Benthic food webs share characteristics of both pelagic and terrestrial, with some multicellular (e.g. macrophytes) and some unicellular (e.g. benthic diatoms) producers.
Growth rate. Producer communities in different ecosystems fix carbon at similar absolute rates; however, less material is stored in living biomass in phytoplankton communities than in forests or grasslands (Cebrian 1999). Primary producers therefore replace their tissues at a faster rate in water than on land. Macrophytes have higher mass-specific growth rates than terrestrial plants, indicating that the contrast is not solely a product of allometry.
Nutrient stoichiometry. Because they lack structural and transport tissues, phytoplankton are composed almost entirely of nutrient-rich (high N and P) photosynthetic material. Heterotrophs in all systems have high demands for N and P relative to supply in primary producers and therefore face nutritional deficit. However, terrestrial consumers experience greater imbalance than those in aquatic systems (Elser et al. 2000).
We propose that the above demonstrated contrasts lead to a number of emergent properties that constrain the pattern of feeding links in food webs, the degree of omnivory, the distribution of body sizes, the vertical flow of materials from producers to consumers, and reciprocal top-down effects of consumers and predators. They also have implications for global chemical cycles and the responses of aquatic and terrestrial ecosystems to anthropogenic changes like N deposition or elevated CO2.
2. The patterns
(a) Bottom-up control
Ideas about trophic flow of energy and materials can be traced to classic studies from both terrestrial and aquatic systems (Elton 1927; Lindeman 1942; Odum & Odum 1955; Hutchinson 1959; Odum et al. 1962). These studies share the perspective that the configuration of food webs (the number and identities of important pools and fluxes, their relative sizes and the connections among them) is an emergent property of the supply of energy or nutrients entering the system, and the efficiencies of trophic transfer among the compartments. According to this view, apparent contrasts between aquatic and terrestrial ecosystems arise from differences in energy or nutrient availability, or the efficiency with which energy or materials are exchanged through trophic linkages. Although rates of net primary production are similar across ecosystems (Cebrian 1999), herbivorous zooplankton in lakes remove a three to four times greater proportion of primary productivity than grazers in terrestrial systems (Cyr & Pace 1993; Hairston & Hairston 1993; Cebrian 1999), and aquatic consumers can be anywhere from six to sixty times more abundant on an areal basis within similar body size classes (Cyr et al. 1997). These data suggest that systematic variation in trophic structure is not due to differences in the amounts of energy or nutrients supplied by photosynthesis. Rather, the efficiencies of herbivores at removing plant material and converting it to their own biomass are greatest in lake plankton, lowest in forests and intermediate in grasslands. These patterns imply that differences in the plant–herbivore link rather than the overall supply of energy govern trophic structure variation across systems.
Hairston & Hairston (1993) present a contrasting view that the number of trophic levels present and the partitioning of biomass among them are not constrained by energetics or nutrition, but are consequences of evolutionary traits such as body size and feeding mode. Hairston & Hairston (1993) argue that terrestrial food webs contain only three functional trophic levels (plants, herbivores and primary predators), while the pelagic zones of lakes often have abundant piscivorous fishes that occupy a fourth trophic level. They invoke size-structured predation and gape limitation as explanations for varying numbers of trophic levels. Grazing zooplankton remove a greater fraction of primary productivity than terrestrial herbivores (Cyr & Pace 1993; Cebrian 1999), and may suffer lower levels of predatory losses (Hairston & Hairston 1993). Hairston & Hairston (1993) argue that these differences occur because terrestrial primary predators suppress carbon flow through the herbivorous pathway, causing more biomass to be diverted toward detrital accumulation and away from the classical food chain. They based their arguments on the largest data compilation available at the time, which was limited to a few studies in temperate forest, grassland and lentic systems. More recent syntheses of larger data sets have upheld their conclusion that the rate of grazing differs substantially between aquatic and terrestrial systems. However, their contention that the grazing contrast reflects differences in food chain length is not supported by evidence from trophic cascade experiments (see §2b).
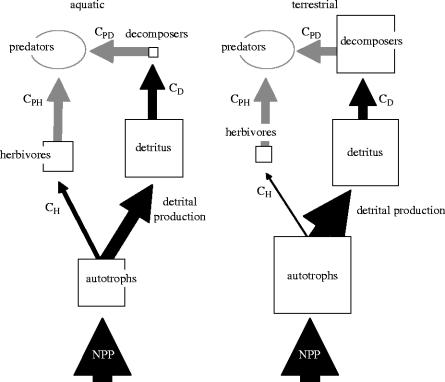
Cebrian and co-workers (Cebrian 1999, 2004; Cebrian & Lartigue 2004) synthesized an extensive data set on the fate of carbon fixed by primary productivity across ecosystem types. The data reveal marked contrasts between aquatic and terrestrial environments in a number of important trophic pathways (figure 1). First, net primary productivity ranges over more than two orders of magnitude across all systems, but does not vary consistently between aquatic and terrestrial environments. Second, pools of both detritivore and herbivore biomass accumulate with increasing primary productivity. The slope of the scaling relationship is similar across ecosystems but the intercept varies considerably. The patterns of biomass partitioning among food-web components are therefore consistent along productivity gradients. Thus, the entire food web swells as more inorganic resources become available at the base. The different components increase at similar rates that vary consistently between the aquatic and terrestrial spheres. These differences persist across the entire global range of primary productivity from deserts and oligotrophic lakes and oceans to productive forests and eutrophic aquatic systems.
Figure 1.
Differences in pathways of carbon flow and pools between aquatic and terrestrial ecosystems. The figure summarizes the patterns demonstrated in Cebrian (1999, 2004) and Cebrian & Latrigue (2004). The thickness of the arrows (flows) and the area of the boxes (pools) correspond to the magnitude. The size of the pools are scaled as log units since the differences cover four orders of magnitude. The C's indicate consumption terms (i.e. CH is consumption by herbivores). Ovals and arrows in grey indicate unknown quantities.
Rates of carbon flux between producer, herbivore and detritivore pools also contrast markedly among ecosystems and show consistent variation across levels of basal productivity. Cebrian (1999) showed that, on average across levels of productivity, the turnover rate of phytoplankton is on the order of 1000 times that of forests, 100 times faster than grasslands and 10 times faster than multicellular aquatic producers. Since net primary productivity does not vary by system, less carbon is stored in the living autotroph biomass pool and producer biomass is consumed by aquatic herbivores at four times the terrestrial rate. Although detritivores consume similar quantities of detrital carbon in the two ecosystems (figure 1), decomposers are much more abundant in terrestrial systems. This suggests that aquatic decomposers suffer greater losses to predation and/or recycle nutrients into the inorganic pool at faster rates as they accumulate less biomass despite similar consumption levels. Energy flow from the detrital loop to consumers with higher trophic positions (e.g. zooplankton eating bacteria) has been proposed as one explanation for steeper biomass pyramids in oligotrophic than eutrophic lakes (Del Giorgio et al. 1999; Prairie et al. 2002). The patterns suggest that terrestrial decomposers may be nutrient limited and, therefore, less efficient than their aquatic counterparts (Swift et al. 1979). The detrital pathway may also be more of a dead end from the perspective of higher trophic levels on land (e.g. accumulation of refractory carbon).
(b) Top-down control
Evidence for bottom-up control is shown by correlations in abundance or biomass between consumers and their resources, and in rates of fluxes along productivity gradients. Ideas about top-down control are more difficult to evaluate. For instance, top-down control cannot operate the same way in herbivorous and detritivorous chains because decomposers cannot influence the renewal rate of the detritus except by indirect means (e.g. nutrient recycling; Moore et al. 2003). The rate of biomass movement from one pool to another is one measure of the strength of top-down control by consumption. However, the rate of flux is not necessarily a good indicator of a consumer's effect on standing biomass of its resource. Consumption can either stimulate or suppress production of the prey population (De Mazancourt et al. 1998), or have no impact (i.e. donor-control; De Angelis 1975). Consumption rate and population impact measure different aspects of interaction strength (Berlow et al. 1999). Field measurements indicate that consumption of living plant biomass by herbivores is three to four times greater in water than on land, and that aquatic decomposers consume more than ten times as much detritus on a mass-specific basis (figure 1). Top-down effects are greater in water in the sense that first-order consumers (herbivores and decomposers) remove carbon at a faster rate than those on land (Cebrian 1999). Their effects on standing stocks can be assessed by removal experiments. Below we review evidence for systematic differences in top down control by predators via herbivores from trophic cascade experiments.
Whether the top-down impact of consumers and trophic cascades (indirect effects of predators) vary among ecosystems is a subject of active debate in ecology (Strong 1992; Polis & Strong 1996; Polis 1999; Chase 2000; Polis et al. 2000; Schmitz et al. 2000; Halaj & Wise 2001). Recent meta-analyses of the literature on trophic cascade experiments found considerable variation among ecosystems and between habitats within systems (Shurin et al. 2002; Borer et al. 2005). The biomass response of plant communities to removal of primary predators was larger in aquatic systems than terrestrial. This result supports evidence from observational measurements of the flow and accumulation of carbon through trophic links that aquatic and terrestrial food webs differ systematically in their structure and function (figure 1). Greater herbivory in aquatic habitats leads to stronger impact of consumption on the standing stock of primary producers, and larger indirect effects of predators. Lesser top-down control observed in terrestrial ecosystems is a consequence of weakness in the herbivore–plant link. That is, terrestrial predators have comparable impacts on their herbivore prey to those in many aquatic systems; however, the reduced grazer community elicits a relatively weak response at the level of primary producers. This result contrasts with the contention of Hairston & Hairston (1993) that longer aquatic food chains drive aquatic–terrestrial contrasts. If primary predators on land are under weaker top-down regulation (fewer secondary predators), then we expect their removal to have smaller effects on herbivores. Examples of webs with four functional trophic levels have been shown in freshwater (Drenner & Hambright 2002), marine (Estes et al. 1998) and terrestrial (Letourneau & Dyer 1998) ecosystems; however, empirical quantification of their dynamical significance remains to be performed.
The meta-analyses of trophic cascade experiments also reveal large variability within systems, and several limitations and biases in the existing experimental literature. First, aquatic systems vary considerably in the magnitude of the expression of trophic cascades (see figure 1 in Shurin et al. 2002). Marine and freshwater benthic habitats have some of the strongest cascades, whereas marine plankton shows negligible phytoplankton responses to planktivore removal. Observed differences among marine and freshwater pelagic systems may arise from greater omnivory by calanoid copepods in the ocean than by the cladocerans that dominate zooplankton in many lakes (Stibor et al. 2004). Second, the terrestrial literature is limited in the range of habitats where predator manipulations have been attempted, and where effects are measured at the level of primary producer biomass. Nearly all studies where plant community biomass was assessed occurred in grassland and agricultural systems (Shurin et al. 2002). Studies in forests are rare due to methodological and timescale difficulties, and generally measure response by single plant species (‘species cascades’; sensu Polis et al. 2000) or responses such as leaf damage which are not directly comparable with other systems (Schmitz et al. 2000). Although the present literature indicates stronger trophic cascades in water, the range of terrestrial systems considered is limited. Moreover, there has been little attention accorded to trophic structure in belowground systems (but see Mikola & Setälä 1998; Moore et al. 2003), even though underground standing biomass and primary production can exceed the levels aboveground (Jackson et al. 1997).
Data syntheses indicate prominent differences in the strengths of top-down and bottom-up forces between aquatic and terrestrial environments. The studies of Cebrian (1999, 2004) and Cebrian & Lartigue (2004) show clear variation in carbon flow and accumulation, but not assimilation from the inorganic pool. Synthesis of trophic cascade experiments indicates that reciprocal top-down control via herbivores and indirect effects of predators are also greater in aquatic ecosystems (Shurin et al. 2002; Borer et al. 2005). Thus, aquatic herbivores remove more carbon from the autotroph community, exert greater influence on the biomass of primary producers and transmit stronger indirect effects from higher trophic levels. We now turn our attention to evaluating candidate hypotheses for the striking contrasts in food-web structure across ecosystems.
3. The mechanisms
(a) Size
Lindeman's (1942) first proposed cause of aquatic–terrestrial variation is that unicellular producers dominate many aquatic ecosystems but are virtually absent on land. Size clearly has different implications for ecological performance in the two environments. Large phytoplankton suffer greater losses due to sinking and have less surface area (per unit biomass) over which to absorb nutrients from their environment (Sommer 1989). However, size also confers resistance to planktonic herbivores that are often gape-limited in the maximum particle size they can ingest. This may explain why more productive pelagic environments in lakes and the ocean are dominated by larger phytoplankton (Watson & McCauley 1988; Sommer 2000; Stibor et al. 2004). Under oligotrophic conditions, large algae are at a disadvantage because their low surface-to-volume ratio reduces their capacity to absorb limiting nutrients, whereas grazing losses become more important in productive environments. By contrast, large terrestrial plants may be better able to compete for nutrients or water in the soil and for light by overtopping their neighbours (Falster & Westoby 2003). Size may be less of a defence against herbivory on land since most grazers consume parts of plants rather than entire individuals. Competition for resources therefore creates selection for small size in planktonic autotrophs and large size in land plants.
Autotroph size also places unique selection on herbivores in water versus land. Pelagic herbivores are virtually all larger than the phytoplankton they consume (Cohen et al. 2003), whereas terrestrial herbivores range from much smaller (e.g. forest lepidoptera) to much larger (e.g. ungulates) than their plant resources. On balance, predators in all systems are generally larger than their prey (Cohen et al. 1993), although parasites, pathogens and cooperative hunters are obvious exceptions. The correlation between body size and trophic position extends across all organisms in pelagic systems, but breaks down at the autotrophic and herbivorous end of terrestrial webs. The large size of terrestrial plants has a number of important consequences that may influence the structure of the entire web. Terrestrial plants are less productive per unit standing biomass because growth rate declines with size in primary producers (Nielsen et al. 1996). Allometric constraints may explain the faster turnover time of phytoplankton relative to terrestrial plants. However, aquatic macrophytes also exhibit faster growth than terrestrial plants (Cebrian 1999). This suggests that allometry is not the whole explanation for aquatic–terrestrial differences in turnover rates (see §3b).
Allometric considerations make predictions about how relative sizes of producers and consumers should affect the vertical flow of energy and top-down impact of consumption. Since small producers have high mass-specific growth rates (Nielsen et al. 1996; Niklas & Enquist 2001), consumers derive greater nutritional benefit from them. Biomass that is removed from a fast-growing plant community is replaced at a greater rate, therefore faster turnover times can sustain more secondary productivity. Larger consumers, similarly, have lower mass-specific metabolic rates (Peters 1983) and therefore are more efficient at converting food to their own tissues. Metabolic rate is also greater in vertebrates than invertebrates, and in endotherms than ectotherms. A metabolically constrained food chain model derived by Yodzis & Innes (1992) predicts that the strength of herbivore control over producers and trophic cascades are greatest when the ratio of consumer-to-producer size is highest (Shurin & Seabloom 2005). Since pelagic herbivores are virtually all larger than their algal resources (Cohen et al. 2003), this condition is common in many aquatic ecosystems. In addition, the largest terrestrial herbivores are endotherms (mammals) with high metabolic demands. The energetics of size, therefore, may help understand why aquatic food webs support higher secondary production, steeper biomass pyramids and stronger trophic cascades.
Size also has important implications for the spatial scale of patchiness at which organisms experience their environment (Ritchie & Olff 1999), which in turn may influence differences in food-web structure and strength of interactions between systems. Since many terrestrial plants are larger than their herbivores, they may respond to spatial patchiness at broader scales. For instance, a tree is affected by the nutrient conditions encountered by its roots and the light reaching its leaves, whereas a folivorous insect may live its entire life on one leaf. In aquatic ecosystems, trophic position is positively correlated with both body size and scale of individual movement. McCann et al. (2005) showed that more spatially confined consumers exerted stronger top-down effects than wider ranging ones that encounter multiple dispersed prey populations. Pelagic cascades tend to be stronger in lakes than in marine systems (Shurin et al. 2002), perhaps explained in part by the relative confines of space for top predators (McCann et al. 2005). Moreover, home ranges of piscivorous fish tend to be smaller, and increase with body size more slowly, than mammals or birds of similar biomass (Cyr et al. 1997). Differences in the scale of patchiness between aquatic and terrestrial environments may have consequences for energy flow and the transmission of top-down effects that have not yet been fully explored.
(b) Stoichiometry
A second consequence of life in aquatic environments lies in the chemical composition of autotrophs. Terrestrial plants have prominent structural and transport (xylem and phloem) tissues that consist largely of cellulose and lignin and are, therefore, carbon-rich (Polis & Strong 1996). Unicellular aquatic producers, macrophytes and macro-algae contain much more photosynthetic tissue that is rich in N and P (Cebrian 1999; Sterner & Elser 2002; Cebrian & Lartigue 2004). Since heterotrophs in all systems have high demands for N and P, terrestrial grazers face a greater nutritional imbalance than those in water (Elser et al. 2000). Low food quality may explain why herbivores consume less plant matter and decomposers degrade less detritus on land than in water. Producer nutrient content (percentage N and P) and the rate of herbivory are positively correlated both within and among systems, as are detritus quality and the rate of decomposition (Cebrian & Lartigue 2004). Thus, autotroph nutritional quality stands out as a consistent indicator of the importance of first-order consumers (herbivores and detritivores) as pathways for carbon flow relative to refractory detrital accumulation. These patterns suggest that differences between aquatic and terrestrial systems are driven greatly by characteristics of the primary producer community, the relative similarity of its elemental composition to that of the consumers, and the quality of detritus that it produces.
4. Emergent properties: food-web topology and complexity
(a) Food-web topology
The characterization of food webs as discrete trophic levels originally introduced by Elton (1927), Lindeman (1942) and Hairston et al. (1960) has been in equal parts influential and criticized in ecology (Murdoch 1966; Ehrlich & Birch 1967; Cousins 1987; Burns 1989; Polis 1991; Strong 1992; Polis & Strong 1996; Chase 2000; Polis et al. 2000). Strong (1992) proposed that the depiction of food webs with small numbers of aggregated feeding guilds (e.g. trophic levels) by Hairston et al. (1960), applies only to simple aquatic ecosystems in lakes. He argued that terrestrial food webs resemble a ‘trophic tangle’ which prevents community-wide trophic effects on primary producers (trophic cascades). Differences in food-web configuration or trophic complexity between ecosystems are intriguing possibilities that are surprisingly difficult to subject to empirical evaluation.
One criticism of the concept of trophic levels and simplified food-web diagrams is that omnivory blurs the distinction between trophic levels, affects the vertical flow of energy and materials, and dampens top-down control (Polis 1991). Gruner (2004) showed that effects of bird predators in tropical forests were dampened by omnivory and did not cascade to tree biomass. Stibor et al. (2004) suggested that greater omnivory leads to weaker trophic cascades in marine plankton than freshwater, as meta-analysis has shown (Shurin et al. 2002). However, community-wide cascades have been observed in other speciose systems with abundant omnivores. Terborgh et al. (2001) found that mammalian carnivore exclusion on small islands increased herbivore density and plant damage in tropical forests with many omnivores. Frank et al. (2005) showed cascading effects of over-fishing of cod in the Scotian Shelf through four trophic levels despite rampant omnivory at every stage. Omnivory clearly influences the expression of trophic cascades and bottom-up control in many cases. However, analysis of food-web data provide no indication that its prevalence varies systematically between aquatic or terrestrial ecosystems (Thompson et al. submitted).
One way of assessing the community-wide importance of omnivory is to examine the distribution of trophic position in food webs. Thompson et al. (submitted) used 60 published webs from marine pelagic, stream, lake and terrestrial ecosystems to test whether discrete trophic levels were apparent in topological food webs (maps of patterns of feeding links among species) or if trophic position varied continuously with no tendency to aggregate around particular values. They found that discrete trophic levels occurred among plants and herbivores while omnivory was more common among higher trophic positions, leading to a more continuous distribution of trophic positions among predators. Trophic positions tended to occur near integer values (trophic levels) more often in real data than in randomizations of the food webs, indicating that real webs are non-randomly structured. The degree of structure or discreteness varied among ecosystems, but was not consistently greater in water than on land. Omnivory was most common in marine pelagic systems, least common in streams, and intermediate in lakes and terrestrial systems. However, topological webs that contain no information on abundance or interaction strength may overemphasize the importance of rare interactions for the structure or dynamics of food webs. A recent analysis of several well-resolved energetic webs argued for the utility of trophic levels, and suggested that omnivory is functionally less important than topological webs might suggest (Williams & Martinez 2004). The available data on topological food webs provide no indication that terrestrial food webs are more structurally complex, or that omnivory is more prevalent on land.
(b) Food-web diversity
Related to food-web topology is the question of whether aquatic and terrestrial food webs differ in diversity within trophic levels. There is remarkably little evidence for such a difference, mainly due to difficulties in reliably estimating species richness and trophic links in the unicellular and small metazoan parts of aquatic (Schmid-Araya et al. 2002) and terrestrial invertebrate and soil food webs (Mikola & Setälä 1998). These uncertainties inhibit a direct comparison of species richness across ecosystems. However, indirect evidence suggests that terrestrial food webs contain more species. First, the most speciose plant (angiosperms) and animal (insects) phyla are primarily terrestrial. Second, terrestrial systems show steeper species-area curves than aquatic ones (Cyr et al. 1997; Drakare et al. in press) and terrestrial latitudinal gradients on local scales are steeper than their aquatic counterparts (Hillebrand 2004), both indicating higher species turnover through space in terrestrial systems. Finally, higher terrestrial diversity may reflect a greater degree of trophic specialization. If terrestrial environments are in fact more diverse, this could have important implications for the transmission of top-down and bottom-up effects. Recent studies highlight the important role of plant or herbivore diversity for the strength of trophic interactions in aquatic (Leibold et al. 1997; Duffy et al. 2003; Hillebrand & Cardinale 2004; Bruno & O'Connor 2005; Gamfeldt et al. 2005; Steiner et al. 2005) and terrestrial food webs (Mikola & Setälä 1998; Finke & Denno 2004).
(c) Specialization, defences and edibility
Specialization may impede the vertical flow of energy if consumers feed on only a limited subset of species or tissues within individuals. Many aquatic consumers (e.g. filter-feeding mollusks, planktivorous and piscivorous fish) discriminate mainly on the basis of prey size and are therefore trophic generalists. Although there are examples of generalist terrestrial consumers (e.g. ungulates), many terrestrial metazoans feed on a restricted set of potential resources in a given ecosystem. For example, lepidopteran larvae often specialize on a single plant family (Novotný & Basset 2005), and many hymenopteran parasitoids are specific to a single host species (Godfray 1994). It is therefore possible that aquatic food webs contain more generalists, and that terrestrial webs are more specialized. A second possibility is that terrestrial plants are better defended than aquatic autotrophs (Strong 1992). Variable edibility and defensive properties of prey species have the effect (similar to specialization) of dampening the strength of trophic interactions at the community level. Unicellular algae may have limited structural or chemical defences against herbivores compared to terrestrial plants that can elaborate long-lived tissues and accumulate secondary compounds over longer periods. Aquatic macrophytes have abundant chemical defences (Toth et al. 2005) but limited structural defences. In comparison, terrestrial plants have both abundant chemical and structural defence strategies (Koricheva et al. 2004). Coley et al. (1985) suggested that allocation to defensive compounds and structures is favoured when biomass turnover is low, i.e. when lost biomass is costly to replace. If this argument is correct, then aquatic autotrophs, which show high biomass turnover (figure 1), should have limited defensive ability compared to terrestrial plants. However, this possibility remains to be demonstrated.
(d) Habitat coupling and subsidies
Lindeman's (1942) second hypothesis for emergent structural differences between ecosystems suggests that aquatic systems may receive more allocthonous resource subsidies (both organic and inorganic) than terrestrial because they lie low in the landscape. Although the rates of net primary production are similar in the two systems, detritus and nutrients arriving in downstream habitats represent a second source of energy for higher order consumers in addition to local primary production. Pelagic habitats in lakes are linked to littoral and benthic food webs through predation and nutrient relocation by mobile predators (Schindler & Scheuerell 2002), and to terrestrial habitat by detrital input from plants (Pace et al. 2004). Some terrestrial systems also receive resource inputs such as marine wrack and seabird guano deposited to littoral and island systems (Polis et al. 1997; Sánchez-Piñero & Polis 2000), and emerging aquatic insects to insectivorous birds in riparian systems (Nakano & Murakami 2001). Externally derived detritus may support higher levels of secondary production and contribute to steeper biomass pyramids in aquatic ecosystems (Del Giorgio et al. 1999; Pace et al. 2004) and to stronger top-down control of autotrophs (Vander Zanden et al. 2005). If such resource subsidies are more important in water (as Lindeman's ‘concavity’ argument suggests), then they may contribute to the tendency for greater secondary production and consumption in water. Decomposers accumulate much less biomass in water than on land (Cebrian 2004; figure 1), suggesting that aquatic detritivores may support more predators in the classical food web and are more efficient at recycling detritus.
5. Conclusions
Syntheses of data across ecosystems indicate that aquatic and terrestrial food webs show unambiguous differences in their structure and function. Aquatic producers support more consumption and are regulated by top-down forces to a greater degree. Two categories of explanation for these patterns have been proposed. The first is that autotrophs in water and on land differ in size, allocation to different tissues, growth rate, chemical composition and nutritional quality. Evidence for these contrasts are compelling, and have profound implications for food-web structure which are just recently beginning to be explored. The second class of explanations is that systems differ in patterns of feeding links, their degree of trophic complexity, omnivory, defences and specialization. This is an intriguing suggestion, but one that has proven remarkably difficult to test. We argue that aquatic–terrestrial differences in the strength of top-down and bottom-up forces reflect variation in the selective constraints imposed on the producers. Differences due to food-web architecture and complexity are intriguing possibilities that remain to be tested.
Acknowledgments
This paper benefited from comments from Just Cebrian, Nelson Hairston Jr, Allyson Longmuir, Ross Thompson and Spencer Wood. J.B.S. was supported by the National Science and Engineering Research Council of Canada, D.S.G. was supported by NSF DEB-0315289. H.H. was supported by the German Research Council (DFG Hi848 4-1). This work was conducted as part of the Trophic Structure Comparisons Working Group supported by the National Center for Ecological Analysis and Synthesis, a Center funded by NSF (grant #DEB-0072909), the University of California, and the Santa Barbara campus. This is contribution number 2274, Bodega Marine Lab, University of California at Davis.
References
- Berlow E.L, Navarrete S.A, Briggs C.J, Power M.E, Menge B.A. Quantifying variation in the strengths of species interactions. Ecology. 1999;80:2206–2224. [Google Scholar]
- Borer E.T, Seabloom E.W, Shurin J.B, Anderson K, Blanchette C.A, Broitman B, Cooper S.D, Halpern B.S. What determines the strength of a trophic cascade? Ecology. 2005;86:528–537. [Google Scholar]
- Brown J.H, West G.B. Oxford University Press; Oxford, UK: 2000. Scaling in biology. [Google Scholar]
- Bruno J.F, O'Connor M.I. Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Lett. 2005;8:1048–1056. doi:10.1111/j.1461-0248.2005.00808.x [Google Scholar]
- Burns T.P. Lindeman's contradiction and the trophic structure of ecosystems. Ecology. 1989;70:1355–1362. [Google Scholar]
- Cebrian J. Patterns in the fate of production in plant communities. Am. Nat. 1999;154:449–468. doi: 10.1086/303244. doi:10.1086/303244 [DOI] [PubMed] [Google Scholar]
- Cebrian J. Role of first-order consumers in ecosystem carbon flow. Ecol. Lett. 2004;7:232–240. doi:10.1111/j.1461-0248.2004.00574.x [Google Scholar]
- Cebrian J, Lartigue J. Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecol. Monogr. 2004;74:237–259. [Google Scholar]
- Chase J.M. Are there real differences among aquatic and terrestrial food webs? Trends Ecol. Evol. 2000;15:408–412. doi: 10.1016/s0169-5347(00)01942-x. doi:10.1016/S0169-5347(00)01942-X [DOI] [PubMed] [Google Scholar]
- Cohen J.E, Pimm S.L, Yodzis P, Saldana J. Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 1993;62:67–78. [Google Scholar]
- Cohen J.E, Jonsson T, Carpenter S.R. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA. 2003;100:1781–1786. doi: 10.1073/pnas.232715699. doi:10.1073/pnas.232715699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley P.D, Bryant J.P, Chapin F.S. Resource availability and antiherbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- Cousins S. The decline of the trophic level concept. Trends Ecol. Evol. 1987;2:312–316. doi: 10.1016/0169-5347(87)90086-3. doi:10.1016/0169-5347(87)90086-3 [DOI] [PubMed] [Google Scholar]
- Cyr H, Pace M.L. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature. 1993;361:148–150. doi:10.1038/361148a0 [Google Scholar]
- Cyr H, Peters R.H, Downing J.A. Population density and community size structure: comparison of aquatic and terrestrial systems. Oikos. 1997;80:139–149. [Google Scholar]
- De Angelis D.L. Stability and coexistence in food web models. Ecology. 1975;56:238–243. [Google Scholar]
- De Mazancourt C, Loreau M, Abbadie L. Grazing optimization and nutrient cycling: when do herbivores enhance plant production? Ecology. 1998;79:2242–2252. [Google Scholar]
- Del Giorgio P.A, Cole J.J, Caraco N.F, Peters R.H. Linking planktonic biomass and metabolism to net gas fluxes in northern temperate lakes. Ecology. 1999;80:1422–1431. [Google Scholar]
- Drakare S, Lennon J.J, Hillebrand H. The imprint of the geographical, evolutionary and ecological context on species–area relationships. Ecol. Lett. In press doi: 10.1111/j.1461-0248.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- Drenner R.W, Hambright K.D. Piscivores, trophic cascades and lake management. Sci. World. 2002;2:284–307. doi: 10.1100/tsw.2002.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.E, Richardson J.P, Canuel E.A. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol. Lett. 2003;6:637–645. doi:10.1046/j.1461-0248.2003.00494.x [Google Scholar]
- Ehrlich P, Birch L.C. Evolutionary history and population biology. Nature. 1967;214:349–352. doi: 10.1038/214349a0. [DOI] [PubMed] [Google Scholar]
- Elser J.J, et al. Nutritional constraints in terrestrial and freshwater food webs. Nature. 2000;408:578–580. doi: 10.1038/35046058. doi:10.1038/35046058 [DOI] [PubMed] [Google Scholar]
- Elton C. Sidgwick and Jackson; London, UK: 1927. Animal ecology. [Google Scholar]
- Estes J.A, Tinker M.T, Williams T.M, Doak D.F. Killer whale predation on sea otters linking oceanic and nearshore environments. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. doi:10.1126/science.282.5388.473 [DOI] [PubMed] [Google Scholar]
- Falster D.S, Westoby M. Plant height and evolutionary games. Trends Ecol. Evol. 2003;18:337–343. doi:10.1016/S0169-5347(03)00061-2 [Google Scholar]
- Finke D.L, Denno R.F. Predator diversity dampens trophic cascades. Nature. 2004;429:407–410. doi: 10.1038/nature02554. doi:10.1038/nature02554 [DOI] [PubMed] [Google Scholar]
- Frank K.T, Petrie B, Choi J.S, Leggett W.C. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. doi:10.1126/science.1113075 [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J. Princeton University Press; Princeton, NJ: 1994. Parasitoids: behavioural and evolutionary ecology. [Google Scholar]
- Gamfeldt L, Hillebrand H, Jonsson P.R. Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol. Lett. 2005;8:698–703. doi:10.1111/j.1461-0248.2005.00765.x [Google Scholar]
- Gruner D.S. Attenuation of top-down and bottom-up forces in a complex terrestrial community. Ecology. 2004;85:3010–3022. [Google Scholar]
- Hairston N.G, Smith F.E, Slobodkin L.B. Community structure, population control, and competition. Am. Nat. 1960;44:421–425. doi:10.1086/282146 [Google Scholar]
- Hairston N.G.J, Hairston N.G.S. Cause–effect relationships in energy-flow, trophic structure, and interspecific interactions. Am. Nat. 1993;142:379–411. doi:10.1086/285546 [Google Scholar]
- Halaj J, Wise D.H. Terrestrial trophic cascades: how much do they trickle? Am. Nat. 2001;157:262–281. doi: 10.1086/319190. doi:10.1086/319190 [DOI] [PubMed] [Google Scholar]
- Hillebrand H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004;163:192–211. doi: 10.1086/381004. doi:10.1086/381004 [DOI] [PubMed] [Google Scholar]
- Hillebrand H, Cardinale B.J. Consumer effects decline with prey diversity. Ecol. Lett. 2004;7:192–201. doi:10.1111/j.1461-0248.2004.00570.x [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalina, or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. doi:10.1086/282070 [Google Scholar]
- Jackson R.B, Mooney H.A, Schulze E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl Acad. Sci. USA. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. doi:10.1073/pnas.94.14.7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koricheva J, Nykanen H, Gianoli E. Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all? Am. Nat. 2004;163:E64–E75. doi: 10.1086/382601. doi:10.1086/382601 [DOI] [PubMed] [Google Scholar]
- Leibold M.A, Chase J.M, Shurin J.B, Downing A.L. Species turnover and the regulation of trophic structure. Annu. Rev. Ecol. Syst. 1997;28:467–494. doi:10.1146/annurev.ecolsys.28.1.467 [Google Scholar]
- Letourneau D.K, Dyer L.A. Expermental test in lowland tropical forest shows top-down effects through four trophic levels. Ecology. 1998;79:1678–1687. [Google Scholar]
- Lindeman R. The trophic-dynamic aspect of ecology. Ecology. 1942;23:399–418. [Google Scholar]
- McCann K.S, Rasmussen J.B, Umbanhowar J. The dynamics of spatially coupled food webs. Ecol. Lett. 2005;8:513–523. doi: 10.1111/j.1461-0248.2005.00742.x. doi:10.1111/j.1461-0248.2005.00742.x [DOI] [PubMed] [Google Scholar]
- Mikola J, Setälä H. No evidence of trophic cascades in an experimental microbial-based soil food web. Ecology. 1998;79:153–164. [Google Scholar]
- Moore J.C, McCann K, Setala H, de Ruiter P.C. Top-down is bottom-up: does predation in the rhizosphere regulate aboveground dynamics? Ecology. 2003;84:846–857. [Google Scholar]
- Murdoch W.W. “Community structure, population control, and competition”: a critique. Am. Nat. 1966;100:219–226. doi:10.1086/282415 [Google Scholar]
- Nakano S, Murakami M. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl Acad. Sci. USA. 2001;98:166–170. doi: 10.1073/pnas.98.1.166. doi:10.1073/pnas.98.1.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.L, Enriquez S, Duarte C.M, SandJensen K. Scaling maximum growth rates across photosynthetic organisms. Funct. Ecol. 1996;10:167–175. [Google Scholar]
- Niklas K.J, Enquist B.J. Invariant scaling relationships for interspecific plant biomass production rates and body size. Proc. Natl Acad. Sci. USA. 2001;98:2922–2927. doi: 10.1073/pnas.041590298. doi:10.1073/pnas.041590298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotný V, Basset Y. Host specificity of insect herbivores in tropical forest. Proc. R. Soc. B. 2005;272:1083–1090. doi: 10.1098/rspb.2004.3023. doi:10.1098/rspb.2004.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum H.T, Odum E.P. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol. Monogr. 1955;25:291–320. [Google Scholar]
- Odum E.P, Connell C.E, Davenport L.B. Population energy flow of three primary consumer components of old-field ecosystems. Ecology. 1962;43:88–96. [Google Scholar]
- Pace M.L, Cole J.J, Carpenter S.R, Kitchell J.F, Hodgson J.R, Van de Bogert M.C, Bade D.L, Kritzberg E.S, Bastviken D. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature. 2004;427:240–243. doi: 10.1038/nature02227. doi:10.1038/nature02227 [DOI] [PubMed] [Google Scholar]
- Peters R.H. Cambridge University Press; Cambridge, UK: 1983. The ecological implications of body size. [Google Scholar]
- Polis G.A. Complex trophic interactions in deserts: an empirical critique of food-web theory. Am. Nat. 1991;138:123–155. doi:10.1086/285208 [Google Scholar]
- Polis G.A. Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos. 1999;86:3–15. [Google Scholar]
- Polis G.A, Strong D.R. Food web complexity and community dynamics. Am. Nat. 1996;147:813–846. doi:10.1086/285880 [Google Scholar]
- Polis G.A, Anderson W.B, Holt R.D. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Ann. Rev. Ecol. Syst. 1997;28:289–316. doi:10.1146/annurev.ecolsys.28.1.289 [Google Scholar]
- Polis G.A, Sears A.L.W, Huxel G.R, Strong D.R, Maron J. When is a trophic cascade a trophic cascade? Trends Ecol. Evol. 2000;15:473–475. doi: 10.1016/s0169-5347(00)01971-6. doi:10.1016/S0169-5347(00)01971-6 [DOI] [PubMed] [Google Scholar]
- Prairie Y.T, Bird D.F, Cole J.J. The summer metabolic balance in the epilimnion of southeastern Quebec lakes. Limnol. Oceanogr. 2002;47:316–321. [Google Scholar]
- Ritchie M.E, Olff H. Spatial scaling laws yield a synthetic theory of biodiversity. Nature. 1999;400:557–560. doi: 10.1038/23010. doi:10.1038/23010 [DOI] [PubMed] [Google Scholar]
- Sánchez-Piñero F, Polis G.A. Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology. 2000;81:3117–3132. [Google Scholar]
- Schindler D.E, Scheuerell M.D. Habitat coupling in lake ecosystems. Oikos. 2002;98:177–189. doi:10.1034/j.1600-0706.2002.980201.x [Google Scholar]
- Schmid-Araya J.M, Hildrew A.G, Robertson A, Schmid P.E, Winterbottom J. The importance of meiofauna in food webs: evidence from an acid stream. Ecology. 2002;83:1271–1285. [Google Scholar]
- Schmitz O.J, Hamback P.A, Beckerman A.P. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 2000;155:141–153. doi: 10.1086/303311. doi:10.1086/303311 [DOI] [PubMed] [Google Scholar]
- Shurin J.B, Seabloom E.W. The strength of trophic cascades across ecosystems: predictions from allometry and energetics. J. Anim. Ecol. 2005;74:1029–1038. doi:10.1111/j.1365-2656.2005.00999.x [Google Scholar]
- Shurin J.B, Borer E.T, Seabloom E.W, Anderson K, Blanchette C.A, Broitman B, Cooper S.D, Halpern B.S. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 2002;5:785–791. doi:10.1046/j.1461-0248.2002.00381.x [Google Scholar]
- Sommer U, editor. Plankton ecology: succession in plankton communities. Springer; Berlin: 1989. [Google Scholar]
- Sommer U. Scarcity of medium sized phytoplankton in the northern Red Sea explained by strong bottom-up and weak top-down control. Mar. Ecol. Prog. Ser. 2000;197:19–25. [Google Scholar]
- Steiner C.F, Darcy-Hall T.L, Dorn N.J, Garcia E.A, Mittelbach G.G, Wojdak J.M. The influence of consumer diversity and indirect facilitation on trophic level biomass and stability. Oikos. 2005;110:556–566. doi:10.1111/j.0030-1299.2005.13665.x [Google Scholar]
- Sterner R.W, Elser J.J. Princeton University Press; Princeton, NJ: 2002. Ecological stoichiometry. [Google Scholar]
- Stibor H, et al. Copepods act as a switch between alternative trophic cascades in marine pelagic food webs. Ecol. Lett. 2004;7:321–328. doi:10.1111/j.1461-0248.2004.00580.x [Google Scholar]
- Strong D.R. Are trophic cascades all wet? Differentiation and donor control in speciose ecosystems. Ecology. 1992;73:747–754. [Google Scholar]
- Swift M.J, Heal O.W, Anderson J.M. Blackwell Scientific; Oxford, UK: 1979. Decomposition in terrestrial ecosystems. [Google Scholar]
- Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. doi:10.1126/science.1064397 [DOI] [PubMed] [Google Scholar]
- Thompson, R. M., Hemberg, M., Starzomski, B. M. & Shurin, J. B. Submitted. Trophic levels and trophic tangles: the prevalence of omnivory in real food webs. [DOI] [PubMed]
- Toth G.B, Langhamer O, Pavia H. Inducible and constitutive defenses of valuable seaweed tissues: consequences for herbivore fitness. Ecology. 2005;86:612–618. [Google Scholar]
- Vander Zanden M.J, Essington T.E, Vadeboncoeur Y. Is pelagic top-down control in lakes augmented by benthic energy pathways? Can. J. Fish. Aquat. Sci. 2005;62:1422–1431. doi:10.1139/f05-042 [Google Scholar]
- Watson S, McCauley E. Comparing patterns of net- and nanoplankton production and biomass among lakes. Can. J. Fish. Aquat. Sci. 1988;45:915–920. [Google Scholar]
- Williams R.J, Martinez N.D. Limits to trophic levels and omnivory in complex food webs. Am. Nat. 2004;163:458–468. doi: 10.1086/381964. doi:10.1086/381964 [DOI] [PubMed] [Google Scholar]
- Yodzis P, Innes S. Body size and consumer-resource dynamics. Am. Nat. 1992;139:1151–1175. doi:10.1086/285380 [Google Scholar]