Abstract
Yolk androgens affect offspring hatching, begging, growth and survival in many bird species. If these effects are sex-specific, yolk androgen deposition may constitute a mechanism for differential investment in male and female offspring. We tested this hypothesis in zebra finches. In this species, females increase yolk-testosterone levels and produce male-biased sex ratios when paired to more attractive males. We therefore predicted that especially sons benefit from elevated yolk androgens. Eggs were injected with testosterone or sesame oil (controls) after 2 days of incubation.
Testosterone had no clear effect on sex-specific embryonic mortality and changed the pattern of early nestling mortality independent of offspring sex. Testosterone-treated eggs took longer to hatch than control eggs. Control males begged significantly longer than females during the first days after hatching and grew significantly faster. These sex differences were reduced in offspring from testosterone-treated eggs due to prolonged begging durations of daughters, enhanced growth of daughters and reduced growth of sons. The results show that variation in maternal testosterone can play an important role in avian sex allocation due to its sex-specific effects on offspring begging and growth.
Keywords: maternal hormones, testosterone, sex allocation, survival, begging, growth
1. Introduction
Birds have been found to adjust investment in male relative to female offspring with respect to factors that differentially affect the fitness of sons and daughters, such as food availability, mate attractiveness, season or laying order (reviewed by Hasselquist & Kempenaers 2002; West et al. 2002). While the actual mechanisms of sex allocation are unknown (Krackow 1995; Pike & Petrie 2003), differential sex allocation may be achieved by varying the numbers of male and female eggs (Dijkstra et al. 1990; Heinsohn et al. 1997), or the investment in males and females, reflected in e.g. size of male and female eggs (Mead et al. 1987; Anderson et al. 1997), egg composition (Petrie et al. 2001; Saino et al. 2003) or differential feeding of sons and daughters (Stamps et al. 1987; Lessells 1998).
A variety of factors that influence avian sex allocation also influence maternal yolk androgen deposition (Schwabl 1993; Gil et al. 1999; reviewed in Groothuis et al. (2005)). Yolk androgen deposition may therefore be used to adjust the phenotype of sons and daughters with respect to environmental conditions. Yolk androgens stimulate the growth of muscles important for the hatching process (Lipar & Ketterson 2000) and can shorten (Eising et al. 2001) or prolong (Sockman & Schwabl 2000) the time from laying to hatching of an egg. Maternal androgens markedly affect offspring behaviour after hatching—they enhance offspring begging (Schwabl 1996; Eising & Groothuis 2003) and increase or reduce offspring growth and survival (Sockman & Schwabl 2000; Eising et al. 2001; Eising & Groothuis 2003). Sex-specific effects of yolk androgens have hardly been studied, but they differentially affected sons and daughters in some studies (Burke 1996; Henry & Burke 1999) and not in others (Lipar & Ketterson 2000).
We studied in the zebra finch Taeniopygia guttata whether experimentally elevated yolk androgens affect offspring survival, begging and growth in a sex-specific manner. Because high androgen levels are associated with more male-biased sex ratios in zebra finches (Burley 1981, 1986; Gil et al. 1999), we predicted that elevation of yolk androgens constitutes a mechanism to increase investment in sons, by one or more of its following effects: enhanced embryonic survival, advanced hatching, increased begging and faster growth of sons compared with daughters. The advantages in sibling competition may contribute to increased nestling survival of sons compared with daughters and thereby lead to male-biased secondary sex ratios.
2. Material and Methods
(a) Animals and housing
Subjects were offspring from 30 zebra finch pairs (Taeniopygia guttata) bred in the facilities of the Zoological Laboratory of the Biological Centre of the University of Groningen, the Netherlands. Pairs of adult experienced breeders were formed at random from a stock of zebra finches known to produce fertile eggs and housed in a room with a dark light cycle of 14 : 10 h with light on at 10.00 h. They were housed in standard cages (2×40×30 cm) with two perches, a wooden nest-box and hay as nesting material. They were provided with a mixed seed diet, water, cuttlefish and grit ad libitum checked and refreshed three times per week.
(b) Testosterone injections
All eggs within a clutch received the same treatment—experimental or control—and this was repeated in second clutches of the same pairs after two months with inversion of the treatment. We had 20 pairs with two successive clutches. To increase the sample size we added 10 more pairs, which produced a single clutch when the other pairs produced their second clutches. Nests were checked daily and freshly laid eggs were weighed and marked.
We injected all eggs of full clutches with 500 pg testosterone in 5 μl of sterile sesame oil. This dose corresponds to the approximate difference in androgens (testosterone+dihydrotestosterone) measured in yolks of eggs laid by females paired with unpreferred males versus females paired to preferred males (Gil et al. 1999; von Engelhardt 2004). Eggs of control clutches were injected with 5 μl of sesame oil. Eggs were injected when they had been incubated for approximately 3 days and the embryonic disk covered about a third of the yolk as ascertained by candling the egg.
Eggs were illuminated from beneath to visualize the embryo floating on top of the yolk. Injections (10 μl Hamilton syringe, equipped with a RN needle, type Z6S) were made into the middle of the egg at an angle of about 45° upwards. Needle and eggshell were wiped once with 100% ethanol. It was visually ascertained that the tip of the needle penetrated the yolk, but did not hit any blood vessels. The hole in the shell was patched with a tiny drop of paraffin and the eggs were immediately put back in their nests.
(c) Nestling begging and growth
At the time of expected hatching, nests were inspected twice daily to identify the egg from which each hatchling hatched. Hatchlings were individually marked with non-toxic pen and from day 10 onwards with numbered rings.
Begging and body weights were measured daily from hatching day until day 13 post-hatching. To ensure that all young had been food-deprived for a similar amount of time, all nests containing young were removed from their cages immediately after lights were switched on so that they had not been fed yet. Nests were placed on a table under an infrared light (25 °C) to keep the hatchlings warm. Hatchlings were individually taken out at random from the nest and placed into an empty nest. Begging was quantified by counting the time for bill-gaping after a standard gentle tactile solicitation of the bill with a metal rod. Begging was measured three times, with an interval of at least 2 min between the extinction of the previous begging bout and the next stimulation.
(d) Sexing
Molecular sex determination of all offspring (dead embryos, hatchlings and surviving young) was done by amplification of sex-specific gene sequences (Bradbury & Blakey 1998), after extraction of tissue or blood samples with Chelex (Walsh et al. 1991).
(e) Data analysis
Data were analysed in Mlwin 1.10.0006 by hierarchical linear models (Bryk & Raudenbush 1993). These models accommodate unbalanced data and allow analyses of variances and covariances, while taking the nested relationships of offspring within clutches of individual pairs into account. Model parameters were estimated by second-order penalized quasi-likelihood estimation or (when models failed to converge to a stable solution) first-order quasi-likelihood (Goldstein 1995). Parameters with α>0.1 were removed successively from the full model, starting with the least significant highest interactions, while ensuring that the amount of data used in the compared models remained the same. All factors with α<0.1 were retained in the final model. Results with α<0.05 (two-tailed) were regarded as significant. Data are shown as mean±s.e. of the mean unless stated otherwise.
Data on offspring sex, embryonic and nestling survival were analysed using a three-level model: (i) pairs, (ii) repeated clutches and (iii) individual offspring. Binary data (offspring sex and survival) were transformed by the logit link function and analysed assuming an extrabinomial error distribution at the individual level (Goldstein 1995). We tested the effect of the treatment, sex and the effect of the interaction of sex and treatment. Significance was tested using the Wald statistic, which follows a χ2 distribution.
For survival after hatching, differences in the survival pattern between the treatments were tested in a life table analysis in SPSS 11.0.1 using the Wilcoxon (Gehan) test. Because this analysis cannot take the nested relationship of young within broods into account, nestling survival was also tested in a hierarchical linear model over two 3 day periods (from hatching to day 3 and from day 3 to day 6 after which day almost no further mortality occurred). Differences in survival over one or 2 day periods could not be tested because the model did not converge to a stable solution.
Offspring weight and begging were analysed in a four-level model: (i) pairs, (ii) repeated clutches, (iii) individual offspring and (iv) age. To model the sigmoidal growth curve, we included the square of age and the cube of age as predictors in the model analysing offspring weights. For offspring weight and begging, we tested the effects of treatment, sex, age (1=day of hatching) and all interactions. The figures show the prediction lines from the models. Significance was tested using the increase in deviance (δ deviance, which follows a χ2 distribution) when a factor was removed from a model.
3. Results
(a) Survival
Embryonic survival did not differ between offspring from testosterone-treated eggs (henceforth T-young, T-females and T-males) and offspring from control eggs (henceforth C-young, C-females and C-males); there was no effect of sex and no effect of the interaction of sex and treatment (all Wald χ2<1.6, p>0.2). During the embryonic stage, C-females had on average considerably higher survival than C-males (figure 1), but this difference in survival was statistically not significant (Wald χ2=1.8, p=0.2).
Figure 1.
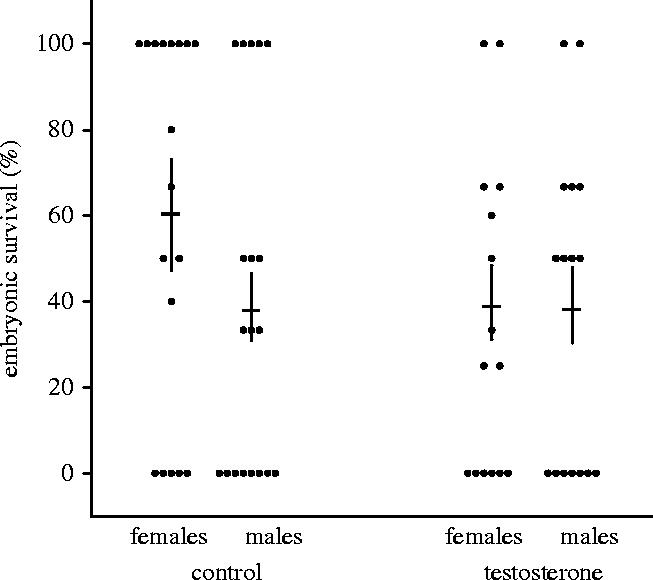
Embryonic survival (±s.e.m.) of sons and daughters from control eggs and from eggs with elevated testosterone. The dots show the mean survival of sons and daughters for each brood.
There was no significant difference in overall nestling survival between T-young and C-young and no effect of the interaction of sex and treatment (all Wald χ2<2.2, p>0.1), but the distribution of mortality of nestlings over time differed significantly between T-young and C-young (figure 2; Wilcoxon (Gehan) statistic: 6.4, p<0.05). This difference in survival pattern was due to a significantly higher survival of T-young than of C-young during the first 3 days after hatching (Wald χ2=4.5, p<0.05), while there was no difference in survival in the following 3 days (Wald χ2=0.3, p=0.6). There was no sex difference in nestling survival of C-young or T-young.
Figure 2.
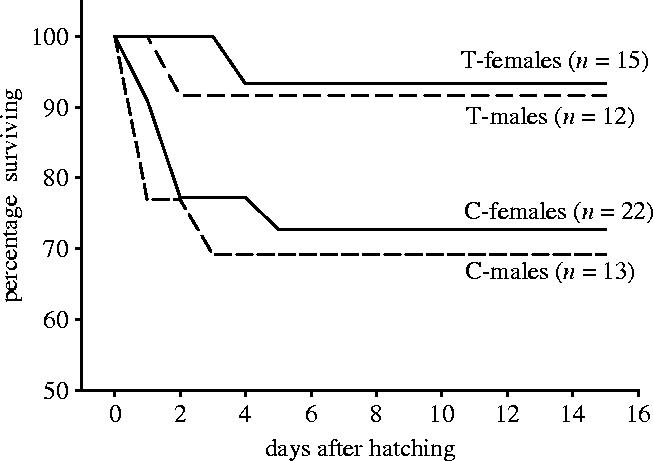
Pattern of nestling survival of sons and daughters hatching from control eggs and from eggs with elevated testosterone.
(b) Hatching time
C-young hatched after 13.3±0.1 days (females (13 broods): 13.2±0.1, males (9 broods): 13.5±0.2), half a day earlier than T-young, which hatched after 14.0±0.2 days (females (11 broods): 14.1±0.2, males (9 broods): 13.9±0.3). This difference was significant (δ deviance=11.8, p<0.001) and more pronounced in female offspring, but the effect of the interaction between treatment and sex was not significant (δ deviance=1.8, p=0.2).
(c) Offspring begging
Treatment, offspring sex, age and all interactions significantly affected offspring begging (table 1a, figure 3). In C-young, sex (δ deviance=12, p<0.001), age (δ deviance=17, p<0.001) and the interaction of sex and age (δ deviance=16, p<0.001) all significantly affected offspring begging durations, due to larger begging durations in males immediately after hatching and a stronger decrease over age in males (figure 3). In T-young, only age significantly affected begging durations (δ deviance=64, p<0.001). In females, treatment (δ deviance=10, p<0.01), age (δ deviance=17, p<0.001) and the interaction of treatment and age (δ deviance=5.5, p<0.05) all significantly affected begging durations, owing to increased begging durations in T-females immediately after hatching and a stronger decrease with age in T-females (figure 3). In males, only age significantly affected begging durations (δ deviance=64, p<0.001). The testosterone treatment thus elevated begging durations only in females during the first days after hatching, thereby abolishing the sex difference that was present in the control group.
Table 1.
Hierarchical linear model analysis of offspring begging and growth. (Treatment is coded as 0 for C-birds and 1 for T-birds and sex is coded as 0 for females and 1 for males.)
| factors | estimate | error | δ | p |
|---|---|---|---|---|
| (a) begging (s) | ||||
| constant | 9.9 | 1.2 | ||
| treatment | 4.2 | 1.6 | 7.1 | <0.01 |
| sex | 7.2 | 1.6 | 19 | <0.0001 |
| age (days) | −0.4 | 0.1 | 12.3 | <0.001 |
| treatment×sex | −6.7 | 2.3 | 8.3 | <0.01 |
| treatment×age | −0.3 | 0.17 | 4.3 | <0.05 |
| sex×age | −0.7 | 0.17 | 16.6 | <0.0001 |
| treatment×sex×age | 0.7 | 0.25 | 7.1 | <0.01 |
| (b) weight (g) | ||||
| constant | 1.32 | 0.23 | ||
| age (days) | −0.6 | 0.07 | 69.4 | <0.0001 |
| square of age | 0.2 | 0.01 | 315.7 | <0.0001 |
| cube of age | −0.007 | 0.00 | 293.9 | <0.0001 |
| treatment | −0.1 | 0.25 | 0.1 | 0.7 |
| sex | −0.28 | 0.26 | 1.1 | 0.3 |
| treatment×sex | 0.18 | 0.37 | 0.2 | 0.7 |
| treatment×age | 0.06 | 0.02 | 15.9 | <0.001 |
| sex×age | 0.12 | 0.02 | 54.9 | <0.0001 |
| treatment×sex×age | −0.103 | 0.02 | 19.7 | <0.001 |
Figure 3.
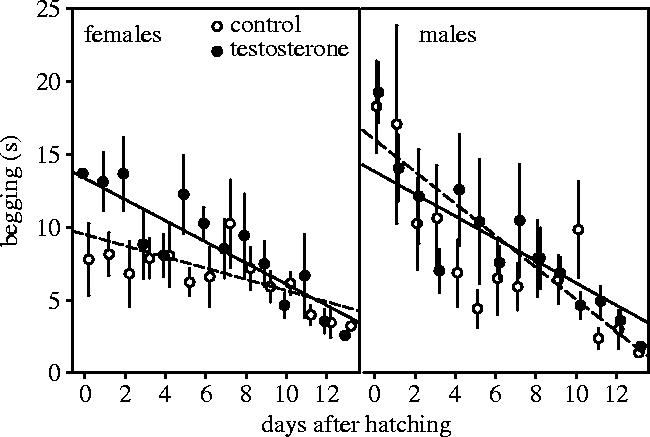
Begging (±s.e.m.) of female and male young hatched from control eggs (open symbols, injected with sesame oil) and eggs with elevated testosterone (filled symbols, injected with 500 pg testosterone in sesame oil). The lines show the predictions from the model in table 1.
(d) Offspring growth
The effect of the three-way interaction between treatment, offspring sex and age on offspring weights was highly significant (figure 4, table 1b). In C-young, there was a significant positive effect of the interaction of sex and age on offspring weights (δ deviance=50, p<0.001), indicating faster growth of males than of females (figure 4). In T-young, there was no significant effect of sex (δ deviance=0.3, p=0.6) nor an effect of the interaction of sex and age (δ deviance=2, p=0.2). We found a significant positive effect of the interaction of treatment and age (δ deviance=13, p<0.001) on the weight of females and a significant negative effect of the interaction of treatment and age (δ deviance=9, p<0.01) on the weight of males, indicating that testosterone enhanced growth of females and reduced growth of males. At hatching, there was no sex difference in weights of offspring from control eggs (δ deviance=0.67, p=0.4) or from testosterone-treated eggs (δ deviance=0.67, p=0.4).
Figure 4.
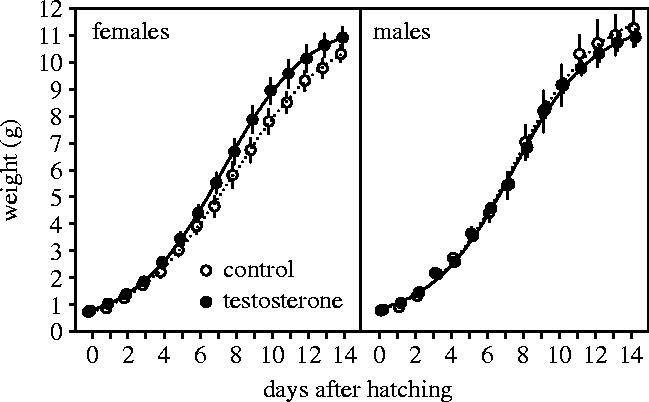
Growth (±s.e.) of female and male young hatched from control eggs (open symbols, injected with sesame oil) and eggs with elevated testosterone (filled symbols, injected with 500 pg testosterone in sesame oil). The lines show the predictions from the model in table 1.
4. Discussion
In zebra finches, females increase testosterone levels in their eggs and produce male-biased secondary sex ratios when paired to males rendered more attractive by artificial ornaments (Burley 1981, 1986; Gil et al. 1999). We therefore predicted that experimentally elevated yolk testosterone benefits sons or is detrimental for daughters, thereby possibly leading to a bias of the secondary sex ratio.
Our results contradicted these predictions: after hatching we found positive effects on begging and growth of daughters which contrast with the idea that females paired to attractive males increase yolk-testosterone levels in order to enhance the quality of sons. Because attractive male zebra finches reduce parental investment (Burley 1988) and because especially daughters suffer from suboptimal rearing conditions (de Kogel 1997; Martins 2004), we suggest the alternative hypothesis that levels of yolk testosterone are elevated in eggs of females paired to attractive males to compensate daughters for this disadvantage by stimulating begging and thereby potentially increasing paternal investment.
The sex difference in offspring begging behaviour during the first days after hatching is a very intriguing observation, which to our knowledge has not been reported previously. Longer begging bouts of male hatchlings in the control group were not due to a sex difference in size, as males were not heavier than females at hatching. Previous studies on begging behaviour in birds have either found no sex differences (Leonard et al. 1994; Monk & Koenig 1997) or differences at an age at which offspring already differ considerably in size (Saino et al. 2003; Hauber & Ramsey 2003). Balda & Balda (cited in Burley (1986)) observed that the frequency of begging calls of male and female nestling zebra finches differ, but as this study has not been published no details are known.
We found that the sex difference in begging can be modulated by yolk androgens. Testosterone treatment increased begging of female nestlings to similar levels as in male nestlings of the control group, but did not affect begging of males. These sex-specific effects of yolk testosterone on begging were closely reflected in the growth pattern of the nestlings. In the control group, males grew faster than females, while there was no sex difference in offspring of testosterone-treated eggs. The improved growth of daughters and the reduced growth of sons may be directly due to more persistent begging in female siblings, which would allow them to better compete with their male siblings. This resembles the effects of androgen treatment on sibling competition in black-headed gulls: an elevation of androgen levels in later laid eggs resulted in improved growth in the young hatching from those eggs and reduced growth of young in the same clutch that hatched earlier from untreated eggs (Eising et al. 2001).
Hatching time was slightly delayed in offspring from testosterone-treated eggs. This is consistent with a study in kestrels (Sockman & Schwabl 2000), but contrasts with the results in black-headed gulls, where elevated androgen levels led to a shortening of the hatching time (Eising et al. 2001). It also contrasts with comparative data showing that passerines with relatively high yolk androgen had relatively short hatching times (Gorman & Williams 2005). These inconsistencies suggest either that effects of yolk androgens are species-specific and/or may change direction due to an interaction with different factors influencing embryonic development, such as the incubation pattern or other egg components.
The only result consistent with our hypothesis of a beneficial effect of testosterone for sons was the finding that in the control group daughters had about 30% higher embryonic survival than did sons, while in the testosterone-treated group male embryos survived equally well as females. This difference was not significant, but the variation in survival between broods was very large (in some broods all offspring survived, in others all died), resulting in large standard errors. Given the considerable difference in mean survival and the relatively small sample size, we consider it therefore premature to conclude that yolk testosterone does not affect embryonic survival in a sex-specific manner. We did not find that the sex-specific effects on begging led to sex-specific nestling survival during the first days after hatching. At this stage, there is generally little sibling competition, because nestlings are so small that parents can easily bring sufficient food, and according to our observation early nestling mortality occurs mostly because parents fail to feed at all. Therefore, both sexes may profit from more persistent begging of female nestlings at this stage. As food was available ad libitum there was hardly any nestling mortality at later stages, but under more natural circumstances effects of elevated androgens on sibling competition may lead to sex-specific nestling mortality.
Yolk androgens may affect offspring begging and growth by the following mechanism. Androgen receptors are present in neurons and syringeal muscles of both male and female embryos and hatchlings of zebra finches (Godsave et al. 2002). Increased levels of testosterone enhance the development of the hatching muscle (Lipar & Ketterson 2000), stimulate begging and potentially change the quality of begging sounds due to effects on the syrinx (Godsave et al. 2002). Begging durations of daughters hatching from testosterone-treated eggs were elevated especially in the first days after hatching. This suggests the possibility of a direct effect of the hormone that is still available from the internalized yolk for a couple of days after hatching. In the control group, males may beg more persistently due to larger endogenous embryonic production of androgens. Males of testosterone-treated eggs may not differ from males in the control group, because their endogenous testosterone production already leads to a maximal effect. However, the hormonal organization and activation of early begging behaviour is still largely unknown (but see Schwabl & Lipar 2002; Groothuis & Ros 2005).
In addition to the sex difference, we observed a decrease of begging durations with age. Several factors probably contribute to this decline (see Zann 1996 for details on the begging behaviour of zebra finches). On the day of hatching, parents usually do not feed young and most brood reduction appears to occur in the first days after hatching, which is consistent with our observations. Begging persistency during the first days after hatching may therefore be most important for survival and help to provide a stimulus for parents to commence feeding. After the first few days, young were, in general, very well fed as food was available ad libitum so that they may have been less motivated to beg than during the first days. In addition, the decline in begging persistency may be due to habituation as the offspring's reaction to our stimulation was not rewarded by food. Finally, zebra finches open their eyes around 6–7 days of age, start to respond to visual stimuli and show from about day 10 onwards increasingly a fear response, which eventually leads to a complete extinction of the begging response to an artificial stimulus (Bischof & Lassek 1985). Therefore, the begging behaviour we observed during the first few days after hatching is probably more relevant for the natural situation than the behaviour after about day 6.
It remains puzzling that in a recent experiment (von Engelhardt 2004), in which we paired females to males they preferred or rejected in a two-way choice test, we found increased levels of androgens in eggs laid by females paired to their preferred males, but no sex difference in offspring growth and survival. In that study, females were choosing between two unmanipulated males, so that their choice was based upon the differences they perceived in the true quality between the two males. Previous studies on the effect of male attractiveness on offspring sex ratios (Burley 1981, 1986) and yolk hormones (Gil et al. 1999) manipulated male attractiveness using coloured leg bands so that the appearance of a male did not match its actual quality. If yolk androgens indeed represent an adaptive adjustment of offspring quality with respect to a context such as the quality of the male, it is to be expected that the effect of elevated yolk androgens depends upon the context in which they are acting. This should be taken into account when drawing conclusions from experiments where males truly vary in quality and experiments where male attractiveness is manipulated.
In conclusion, we find strong evidence that maternal hormone deposition affects avian sex allocation through sex-specific effects on begging behaviour and growth. Because we manipulated levels of androgens within the range that can be encountered naturally in eggs, our results suggest that also under natural circumstances mothers may modify offspring development in a sex-specific way by differential deposition of androgens in their eggs. Recently, it has been found that male and female eggs may differ in yolk androgen levels (Petrie et al. 2001; Rutstein et al. 2005). If females are able to adjust the androgen content of an egg in relation to its sex, this would give them additional flexibility by which they could benefit from the positive effects of elevated yolk androgens on offspring of one sex, while avoiding negative consequences on offspring of the other sex.
Acknowledgments
We thank Candela Rodriguez for helping with data collection and Sjoerd Veenstra and Roelie Veenstra-Wiegman for assistance with animal caretaking. We thank the referees for their suggestions. All experimental procedures were carried out according to the regulations of the Dutch law for laboratory animals and approved by the animal experimentation committee of the University of Groningen (licence DEC 2754). This study was supported by NWO grant no. 810.67.024 to Cor Dijkstra and Serge Daan.
Footnotes
Present address: Behavioural Neuroendocrinology Research Unit, University of Liege, Liege, Belgium.
References
- Anderson D.J, Reeve J, Bird D.M. Sexually dimorphic eggs, nestling growth and sibling competition in American kestrels, Falco sparverius. Funct. Ecol. 1997;11:331–335. [Google Scholar]
- Bischof H.-J, Lassek R. The gaping reaction and the development of fear in young zebra finches (Taeniopygia guttata castanotis) Z. Tierpsychol. 1985;69:55–65. [Google Scholar]
- Bradbury R.B, Blakey J.K. Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc. R. Soc. B. 1998;265:895–899. 10.1098/rspb.1998.0375 [Google Scholar]
- Bryk A.S, Raudenbush S.W. Sage; Newbury Park: 1993. Hierarchical linear models: application and data analysis method. [Google Scholar]
- Burke W.H. Effects of an in ovo injection of an anti-androgen on embryonic and posthatching growth of broiler chicks. Poult. Sci. 1996;75:648–655. doi: 10.3382/ps.0750648. [DOI] [PubMed] [Google Scholar]
- Burley N.T. Sex ratio manipulation and selection for attractiveness. Science. 1981;211:721–722. doi: 10.1126/science.211.4483.721. [DOI] [PubMed] [Google Scholar]
- Burley N.T. Sex ratio manipulation in color-banded populations of zebra finches. Evolution. 1986;40:1191–1206. doi: 10.1111/j.1558-5646.1986.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Burley N.T. The differential-allocation hypothesis: an experimental test. Am. Nat. 1988;132:611–628. [Google Scholar]
- de Kogel C.H. Long-term effects of brood size manipulation on morphological development and sex-specific mortality offspring. J. Anim. Ecol. 1997;66:168–178. [Google Scholar]
- Dijkstra C, Daan S, Buker J.B. Adaptive seasonal variation in the sex ratio of kestrel broods. Funct. Ecol. 1990;4:143–147. [Google Scholar]
- Eising C.M, Groothuis T.G.G. Yolk androgens and begging behaviour in black-headed gull chicks: an experimental field study. Anim. Behav. 2003;66:1027–1034. [Google Scholar]
- Eising C.M, Eikenaar C, Schwabl H, Groothuis T.G.G. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. 10.1098/rspb.2001.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. [DOI] [PubMed] [Google Scholar]
- Godsave S.F, Lohmann R, Vloet R.P.M, Gahr M. Androgen receptors in the embryonic zebra finch hindbrain suggest a function for maternal androgens in perihatching survival. J. Comp. Neurol. 2002;453:57–70. doi: 10.1002/cne.10391. [DOI] [PubMed] [Google Scholar]
- Goldstein H. Edward Arnold; London: 1995. Multilevel statistical models. [Google Scholar]
- Gorman K.B, Williams T. Correlated evolution of maternally derived yolk testosterone and early developmental traits in passerine birds. Biol. Lett. 2005;1 doi: 10.1098/rsbl.2005.0346. 10.1098/rsbl.2005.0346 Published online 11 July 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G, Ros A.F.H. The hormonal control of begging and early aggressive behavior: experiments in black-headed gull chicks. Horm. Behav. 2005;48:207–215. doi: 10.1016/j.yhbeh.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Kempenaers B. Parental care and adaptive brood sex ratio manipulation in birds. Phil. Trans. R. Soc. B. 2002;357:363–372. doi: 10.1098/rstb.2001.0924. 10.1098/rstb.2001.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber M.E, Ramsey C.K. Honesty in host–parasite communication signals: the case for begging by fledgling brown-headed cowbirds Molothrus ater. J. Avian Biol. 2003;34:339–344. [Google Scholar]
- Heinsohn R, Legge S, Barry S. Extreme bias in sex allocation in Eclectus parrots. Proc. R. Soc. B. 1997;264:1325–1329. 10.1098/rspb.1997.0183 [Google Scholar]
- Henry M.H, Burke W.H. The effects of in ovo administration of testosterone or an antiandrogen on growth of chick embryos and embryonic muscle characteristics. Poult. Sci. 1999;78:1006–1013. doi: 10.1093/ps/78.7.1006. [DOI] [PubMed] [Google Scholar]
- Krackow S. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 1995;70:225–241. doi: 10.1111/j.1469-185x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Leonard M.L, Theather K.L, Horn A.G, Koenig W.D, Dickinson J.L. Provisioning in western bluebirds is not related to offspring sex. Behav. Ecol. 1994;5:455–459. [Google Scholar]
- Lessells C.M. A theoretical framework for sex-biased parental care. Anim. Behav. 1998;56:395–407. doi: 10.1006/anbe.1998.0764. [DOI] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. 10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins T.L.F. Sex-specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio adjustment. Behav. Ecol. 2004;15:174–180. [Google Scholar]
- Mead P.S, Morton M.L, Fish B.E. Sexual dimorphism in egg size and implications regarding facultative manipulation of sex in mountain white-crowned sparrows. Condor. 1987;89:798–803. [Google Scholar]
- Monk D.S, Koenig W.D. Individual, brood, and sex variation in begging calls of western bluebirds. Wilson Bulletin. 1997;109:328–332. [Google Scholar]
- Petrie M, Schwabl H, Brande-Lavridson N, Burke T. Sex differences in avian yolk hormone levels. Nature. 2001;412:498–498. doi: 10.1038/35087652. [DOI] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- Rutstein A.N, Gilbert L, Slater P.J.B, Graves J.A. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav. Ecol. 2005;16:62–66. [Google Scholar]
- Saino N, Romano M, Ferrari R.P, Martinelli R, Møller A.P. Maternal antibodies but not carotenoids in barn swallow eggs covary with embryo sex. J. Evol. Biol. 2003;16:516–522. doi: 10.1046/j.1420-9101.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Yolk is source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. 1996;114A:271–276. doi: 10.1016/0300-9629(96)00009-6. [DOI] [PubMed] [Google Scholar]
- Schwabl H, Lipar J. Hormonal regulation of begging behaviour. In: Wright J, Leonard M.L, editors. The evolution of begging, competition, cooperation, and communication. Kluwer; Dordrecht: 2002. pp. 221–244. [Google Scholar]
- Sockman K.W, Schwabl H. Yolk androgens reduce offspring survival. Proc. R. Soc. B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. 10.1098/rspb.2000.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps J, Clark A, Kus B, Arrowood P. The effects of parent and offspring gender on food allocation in budgerigars. Behaviour. 1987;101:177–199. [Google Scholar]
- von Engelhardt, N. B. 2004 Proximate control of avian sex allocation. A study on zebra finches. Ph.D. thesis, University of Groningen, The Netherlands.
- Walsh J.P, Metzger D.A, Higuchi R. Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- West S.A, Reece S.E, Sheldon B.C. Sex ratios. Heredity. 2002;88:117–124. doi: 10.1038/sj.hdy.6800018. [DOI] [PubMed] [Google Scholar]
- Zann R.A. Oxford University Press; Oxford: 1996. The zebra finch. A synthesis of field and laboratory studies. [Google Scholar]


